Abstract
Objectives
This cross-sectional study aims to determine the incidence and potential risk factors associated with biofilm-producing uropathogenic Escherichia coli (UPEC) nosocomial strains from a tertiary care hospital and to examine the prospective correlation between biofilm generation and antibiotic resistance phenotypes and genotypes.
Methods
A total of 130 UPEC nosocomial isolates were identified, their biofilm formation was quantified using a modified microtiter plate assay, and their antibiotic susceptibilities were assessed utilizing the disc diffusion method. Isolates were then subjected to PCR assays targeting blaKPC, blaVIM, blaIMP, and blaOXA48 genes.
Results
Over half of the isolates (n = 76, 58.5%) were biofilm producers. Among 17 carbapenem-resistant isolates, 6 (42.9%) isolates harbored the blaOXA48 gene, and only 1 (9.1%) isolate was positive for the blaVIM gene. Prior antibiotic therapy (aOR 15.782, p 0.000) and diabetes mellitus DM (aOR 11.222, p 0.016) were the significant risk factors associated with biofilm production, as determined by logistic regression analysis of the data. In addition, gentamicin resistance was the only statistically significant antibiotic resistance pattern associated with biofilm production (aOR 9.113, p 0.02).
Conclusions
The findings of this study emphasize the significance of implementing proper infection control measures to avoid the horizontal spread of biofilm formation and associated antimicrobial resistance patterns among UPEC nosocomial strains.
Similar content being viewed by others
Introduction
Uropathogenic Escherichia coli (UPEC) is considered the most common uropathogens associated with urinary tract infections (UTIs), involving pyelonephritis, cystitis, and infectious complications that can lead to acute renal failure in both healthy individuals and renal transplant recipients (Zagaglia et al. 2022).
The capacity to generate biofilms is essential for the pathogenic role and virulence of UPEC to penetrate, proliferate, ascend, and survive within the uroepithelium (Katongole et al. 2020). Biofilms provide bacteria with a survival strategy by positioning them to use available resources efficiently and limiting their access to antimicrobial drugs, antibodies, and white blood cells (Öztürk et al. 2023). Furthermore, UPEC isolates harbor numerous antibiotic-inactivating enzymes, including ꞵ-lactamases and aminoglycosides transferases, generating an antimicrobial resistance island (Davies and Davies 2010).
This issue is exacerbated by the emergence and spread of multi-drug-resistant (MDR) bacterial strains due to diverse resistance mechanisms, severely limiting the therapeutic options available to clinicians and representing the most significant challenges associated with treating UTIs (Naber et al. 2020). Among UPEC strains harboring carbapenem-resistance genes, the prevalence of MDR is increasing, posing a severe, severe threat to global health (Katongole et al. 2020).
In terms of carbapenem hydrolysis and geographical distribution, the most effective carbapenemases are Verona integron-encoded metallo-beta lactamases (VIM)-type (representing class B β-lactamases) and oxacillin-hydrolyzing carbapenemases (OXA48)-type (representing class D β-lactamases) (Tanriverdi Cayci et al. 2021). Carbapenemase genes are chromosomally or plasmid-encoded on transmissible genetic cassettes inserted into integrons and/or associated with composite transposons, facilitating their intra- and inter-species horizontal dissemination (Reyes et al. 2020). The development of molecular approaches for genetic identification of carbapenem-resistant UPEC has progressed into a sensitive, accurate, and rapid detection technique (Codjoe and Donkor 2017). This study examines the frequency and associated risk factors of biofilm-producing UPEC nosocomial strains from a tertiary care hospital while testing the hypothesis that the presence of biofilm producers increases the risk of antimicrobial resistance (AMR) and MDR.
Materials and methods
Study design and ethical approval
This cross-sectional observational study was performed between January 2022 and March 2023 at Zagazig University Hospitals. All study procedures followed the ethical code of the World Medical Association (Declaration of Helsinki) for experiments, including human cases. The Institutional Review Board (IRB) Committee of the Faculty of Medicine, Zagazig University, approved the study protocol (approval No. 63643/29–9-2020). Informed written consent was collected from all subjects who participated in the study. Additionally, the study was conducted in accordance with the international guidelines of Strengthening the Reporting for Observational Studies in Epidemiology (STROBE) (Vandenbroucke et al. 2007).
Urine sample collection and isolation of UPEC strains
During the study period, non-duplicate UPEC nosocomial strains were collected from adult patients hospitalized for 48 h and classified according to the criteria of the Centers for Disease Control and Prevention/National Healthcare Safety Network (CDC/NHSN) (Horan et al. 2008). Demographic and clinical data of the hospitalized cases were collected utilizing a questionnaire involving age, ward/ICU admission, sex, catheterization, exitance of comorbid conditions, hospital stay's length, and a history of prior antibiotic treatment. In order to isolate UPEC, urine samples were initially screened using nutrient agar, blood agar, MacConkey agar, CLED plates, and the API 20E (bioMerieux, Mar-cy-l’Etoile, France) according to the manufacturer’s instructions (Murray et al. 2020).
Quantitative tissue culture plate method to assess biofilm formation by UPEC
The production of biofilm by UPEC was evaluated phenotypically using the quantitative tissue culture plate method (Hassan et al. 2011). Isolates from fresh agar plates were inoculated into 10 mL of trypticase soy broth with 1% glucose. Culture turbidity was adjusted to a 0.5 McFarland standard. Individual wells of sterile 96-well flat-bottom polystyrene tissue culture plates (Costar, CA, USA) were added with 200 µL of the bacterial suspension. Negative control wells contained sterile broth. The reference strain E. coli ATCC 25922 was utilized as the positive control. Plates were incubated at 37 °C for 24 h. After incubation, the content of each well was gently removed. The wells were washed three times with 200 µL of phosphate buffered saline (pH 7.2) to remove free-floating planktonic bacteria, and then the wells were air dried for 45 min. Adherence of bacteria to the culture plate was detected using crystal violet by adding 200 µL of 0.1% crystal violet to each well, and the plates were incubated at room temperature for 10 min. The excess stain was removed by rinsing with deionized water, and the plates were allowed to dry for 20 min. After drying, 200 μL of 95% ethanol was added to the wells to solubilize the incorporated dye; the plate was covered with the lid (to minimize evaporation) and left at room temperature for 30 min. The ODs of the stained adherent bacteria were measured with a microplate reader at 570 nm. Experiments were performed in triplicate (Fig. 1). The average OD values were calculated for all tested isolates and negative controls, and the cutoff value (ODc) was detected. It is defined as three standard deviations (SDs) above the mean OD of the negative control. The final OD value of a tested strain was expressed as the average OD value of the strain reduced by the ODc value. Negative values were presented as zero, whereas positive values indicated the formation of biofilm. Strains were divided into the following categories to facilitate interpretation of the results: non-biofilm producer = OD ≤ ODc; weak biofilm producer = ODc < OD ≤ 2 × ODc; moderate biofilm producer = 2 × ODc < OD ≤ 4 × ODc, and strong biofilm producer = 4 × ODc < OD (Stepanović et al. 2007; Panda et al. 2016).
Antibiotic susceptibility testing
Antibiotic susceptibility patterns were detected using the disc diffusion method on Muller-Hinton agar plates according to CLSI guidelines (CLSI 2022). The antibiotics tested included nitrofurantoin (NIT, 300 µg), doxycycline (DOX, 5 µg), tigecycline (TGC, 15 µg), trimethoprim/sulfamethoxazole (SXT, 1:19, 25 µg), levofloxacin (LVX, 30 µg), ciprofloxacin (CIP, 5 µg), amikacin (AMK, 30 µg), gentamycin (GEN, 10 µg), meropenem (MEM, 10 µg), imipenem (IPM, 10 µg), aztreonam (ATM, 30 µg), cefoxitin (FOX, 10 µg), ceftazidime (CAZ, 30 µg), cefotaxime (CTX, 30 µg), cefepime (FEP, 30 µg), piperacillin/tazobactam (TPZ, 100/10 µg), ampicillin/sulbactam (SAM, 10/10 µg), and amoxicillin/clavulanic acid (AMC, 20/10 µg) (Oxoid, England). The reference strain E. coli ATCC 25922 was utilized for quality control.
Multiplex PCR assays to detect blaKPC, blaIMP, blaVIM, and blaOXA48 genes
All carbapenem-resistant isolates were subjected to multiplex PCR to detect blaKPC, blaIMP, blaVIM, and blaOXA48 genes, as previously described (Baran and Aksu 2016). The primers utilized in this study and the amplicon sizes are listed in Table 1. PCR was performed in two multiplex reactions of a total volume of 20 µL for each run. The first reaction for detecting blaKPC and blaOXA48 contained 10 µL of 2X PCR Master mix solution (DreamTaq Green PCR Master Mix, Thermofisher Scientific, USA), 1 µL of each primer, 4 µL of template DNA, and sterile distilled water to a total volume of 20 µL. The second reaction for detecting blaIMP and blaVIM contained 10 µL 0f 2X PCR Master mix solution, 1 µL of each primer, 6 µL of template DNA, and sterile distilled water to a total volume of 20 µL. Initial denaturation (94 °C for 10 min), followed by 36 amplification cycles. Each cycle consisted of 94 °C for 30 s, 52 °C for 40 s, and 72 °C for 50 s, and the final extension step (72 °C for 5 min) was to stop the amplification. The PCR amplicons were electrophoresed on 1.5% agarose gel and were visualized by ethidium bromide staining (Poirel et al. 2011).
Statistical analysis
The Statistical Package for the Social Sciences (SPSS), version 22 (SPSS Inc., Chicago, IL, USA) was utilized for data coding, validation, and analysis. The data were expressed as frequencies and percentages. The Student’s t-test was used to contrast numeric data, whereas the Chi-square test was utilized to analyze categorical data. A binary logistic regression analysis with antecedent 95% confidence intervals (CIs) was utilized to identify relevant risk factors.
Results
Throughout the study period, all 130 non-duplicate nosocomial strains of UPEC were isolated from 75 females and 55 males. The majority of isolates (n = 72, 55.4%) were obtained from ICU patients. The ages of the patients ranged from 13 to 66 years, with a median of 40 years and a mean of 40.36.
Among all UPEC nosocomial isolates, 76 (58.5%) isolates were biofilm producers. Out of which, 17 isolates (22.4%) were moderate biofilm producers and 8 (10.5%) were strong biofilm producers, while the rest (n = 51, 67.1%) were weak biofilm producers.
Table 2 displays infected patients’ demographic and clinical features with a biofilm- and non-biofilm-producing UPEC isolates. There were no statistically significant differences between the two groups in terms of sex, age, and the majority of comorbidities. However, malignancy, diabetes mellitus (DM), duration of hospital stay, intensive care unit (ICU) admission, prior antibiotic therapy, urinary catheterization, carbapenems resistance (p ≤ 0.001 for each), and resistance patterns (p = 0.012) were statistically significant risk factors correlated with infections by biofilm-producing isolates utilizing univariate analysis.
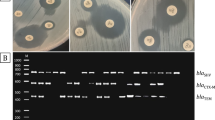
It is noteworthy that carbapenem resistance was detected in 17 UPEC nosocomial isolates (All were biofilm producers). Six isolates harbored blaOXA48 (42.9%), and only one isolate (9.1%) was positive for blaVIM (Figs. 2 and 3). Moreover, among the biofilm-producing isolates, 23 isolates (32.4%) were MDR, 37 isolates (52.1%) were extensively drug-resistant (XDR), and 11 isolates (15.5%) were pan-drug resistant (PDR).
Agarose gel electrophoresis used for separation of the amplicons produced by multiplex PCR reaction for blaKPC (498 bp) and blaOXA-48 (438bp) genes. The ladder used is 50 bp ladder, blaOXA-48 amplicon (438 bp) was detected in lanes 1, 2, 3, 5, 6, and 7 while blaKPC amplicon (498 bp) was not detected in any lane
Table 3 illustrates that after regression, prior antibiotic therapy was the most significant risk factor for biofilm production by UPEC (aOR = 5.782, p = 0.000). DM was another significant risk factor for biofilm production by UPEC (aOR = 11.222, p = 0.016).
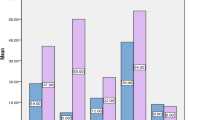
Among all UPEC nosocomial isolates, resistance rates to different antimicrobial classes were generally high and worrisome, with the highest rates reported to cefoxitin (100%) followed by cefepime, respectively aztreonam (94.6% for each). The antibiotics with the lowest resistance rates were doxycycline and tigecycline (10% for each), followed by gentamycin (18.5%). Moreover, 45 (37%) isolates were MDR, 65 (53.7%) isolates were XRD, and 11 (9.1%) isolates were PDR.
Figure 4 and Table 4 show that biofilm generation in UPEC was significantly correlated with resistance to gentamycin (p < 0.001), meropenem (p = 0.009), doxycycline, and tigecycline (p = 0.044 for each). There was no statistically significant correlation between biofilm generation and resistance to the other antimicrobials. However, after adjusting confounding factors using logistic regression analysis, the biofilm formation was only significantly linked with resistance to gentamycin (aOR = 9.113, p = 0.02) (Table 4).
Discussion
E. coli is the main etiology for UTIs, accounting for approximately 80% of community-acquired and 50% of hospital-acquired infections worldwide. In developing countries, the epidemiologic prevalence of UPEC infections ranges from 50 to 80%, compared with a rate of 3–40% in developed countries (Kot 2019). The burden of biofilm and carbapenemase production among UPEC is considered a global health crisis (Katongole et al. 2020). Furthermore, treatment of UTIs caused by UPEC, especially hospital-acquired ones, has become more challenging due to increasing resistance rates for standard antibiotics. This increase of antibiotic resistances and the emergence of MDR-UPEC are continuously associated with higher rates of inadequate empirical therapy (Kot 2019). In addition, hospitals-acquired UTIs caused by MDR-UPEC result in increased morbidity and mortality compared to those caused by susceptible strains (Flores-Mireles et al. 2015). Carbapenems have been considered the last treatment option for infections caused by MDR-UPEC. Nonetheless, the emergence of carbapenems resistance among UPEC isolates is a major concern due to their high virulence (Nasrollahian et al. 2022). Biofilm-producing UPEC is commonly associated with recurrent and complicated UTIs (Katongole et al. 2020). Due to biofilm's significance in the UPEC pathogenesis, identifying biofilm-producing strains is essential to select the most effective treatment options for these resistant strains and prevent their spread in healthcare systems.
In our study, 58.5% of all UPECs were found to produce biofilms. Recent meta-analysis research indicates that UPEC biofilm production varies globally between 13.3 and 99%. Prior epidemiological research has demonstrated that biofilm development is the most important virulence factor promoting the endemicity and chronicity of E. coli isolates in both nosocomial infections and hospital settings (Karigoudar et al. 2019). Notably, 84.2% of the biofilm-producing strains in our study were hospitalized for ≥ 7 days. Moreover, 75% of the isolates were obtained from ICU patients. This result would further clarify the application of biofilm-producing E. coli in nosocomial infections and outbreaks among high-risk ICU cases (Hu et al. 2015; Raya et al. 2019).
We reported that 52.6% of patients infected with biofilm-producing UPEC had malignancy as comorbidity. Chemotherapy and radiotherapy are commonly responsible for immunosuppression in cancer patients. Consequently, they are commonly placed on low doses of antimicrobials, which could lead to multidrug resistance. Some are catheterized, which can aid the formation of biofilms, with Rizzato et al. (2019) reporting a similar association.
Regarding urinary catheterization, 80.3% of the biofilm-producing isolates were collected from catheterized patients. Several studies agreed with our results (Zagaglia et al. 2022; Karigoudar et al. 2019). Biofilm production is the first step to catheter-associated UTI pathogenesis. Bacteria in biofilm move via the catheter into the bladder within 1 to 3 days and induce an infection (Gunardi et al. 2021; Pelling et al. 2019).
Logistic regression analysis for our data revealed that prior antibiotic therapy and DM were significant risk factors for UTIs caused by biofilm-producing isolates. Many previous studies showed that antimicrobial therapy selects antimicrobial-resistant strains commonly associated with biofilm production, providing a tough polymeric matrix impeding antimicrobial penetration (Sharma et al. 2019; Singh et al. 2017). Previous studies also indicated the relation between biofilm production and DM, as the main feature of a diabetic wound is the polymicrobial composite that modulates bacterial virulence. These microorganisms intercommunicate and build a sophisticated polymicrobial biofilm population (Zagaglia et al. 2022; Raya et al. 2019; Pouget et al. 2020; Afonso et al. 2021). Diabetes is a common risk factor for recurrent UTIs. In addition to diabetes-associated impaired host defense mechanisms resulting in an increased risk of infections, diabetic neuropathy affects the ability of the bladder to sense the presence of urine in the bladder, allowing urine stasis that favors UTIs (Nitzan et al. 2015). Moreover, the higher glucose level in the urine is known to improve the growth of bacteria in the urine and increase the probability of infection. In our study, the increased risk of UTIs caused by biofilm-producing isolates in diabetic patients could be attributed to the previously reported data that biofilm production phenotype is modulated in response to a variety of environmental signals, including available glucose levels. Additionally, quorum signaling compounds such as insulin regulate the processing of the environmental signals that regulate biofilm phenotype (Sewify et al. 2016; Raya et al. 2019; Patel et al. 2021).
In this study, 22.4% of biofilm-producing isolates were found to be carbapenem-resistant. In intensive care units, carbapenem resistance in Gram-negative bacteria, such as UPEC, is an emerging global problem. The World Health Organization (WHO) added carbapenem-resistant organisms (CRO) to its list of priority pathogens in 2017 due to their alarming spread, which could severely limit future therapeutic treatment options (Tacconelli et al. 2018). Several mechanisms contribute to carbapenem resistance, including the production of carbapenemases or a combination of porin deficiency and other β-lactamases (Karigoudar et al. 2019). Among our carbapenem-resistant isolates, six (42.9%) isolates harbored blaOXA48, and only one (9.1%) isolate was positive for blaVIM. In a study by Gurung et al. (2020), blaOXA48 was detected in 33.3% among UPEC while Bindayna et al. (2022) reported blaVIM in 8.5% of E. coli isolates. Gatya Al-Mayahie et al. (2022) reported that blaOXA-48 was the most frequent variant (57.8%), while blaVIM was detected in 10.5%. In our study, none of the isolates were positive for blaKPC and blaIMP genes, which agreed with Loqman et al. (2021).
All biofilm-producing isolates in this study displayed resistance patterns of MDR, XDR, and PDR. However, biofilm-producing organisms were more resistant to gentamycin, meropenem, doxycycline, and tigecycline than other antibiotics. Nevertheless, logistic regression analysis showed that biofilm formation was significantly linked with resistance to gentamycin (aOR = 9.113, p = 0.02), indicating a potential association between the biofilm genes and aminoglycoside resistance genes. Our findings agree with previous studies that recommend biofilms to be correlated with elevated resistance to antibiotics (Katongole et al. 2020; Singh et al. 2017).
This study has some limitations. First, the study included patients from a single center in a particular geographic region, so the findings cannot be generalized to the whole population. In addition, this study included small number of isolates and it did not investigate the biofilm-inducing genes or other carbapenem-related genes other than blaKPC, blaIMP, blaVIM, and blaOXA-48 genes at the molecular level. Further molecular-based epidemiologic multi-center studies from different regions are required. Findings from these studies can contribute to controlling the spread of highly virulent UPEC and strengthen infection prevention strategies.
Conclusions
In this study, the high prevalence of UPEC nosocomial isolates that produce bio-film is alarming and concerning. The escalating rate of antibiotic resistance is a significant cause for concern. Our findings highlight the importance of identifying potential risk factors associated with biofilm-producing UPEC nosocomial infection. These findings may aid health authorities and decision-makers in regulating the continuous selection and spread of this dangerous trait within hospitals and improving the quality of care for patients.
Data availability
No datasets were generated or analyzed during the current study.
References
Afonso AC, Oliveira D, Saavedra MJ, Borges A, Simões M (2021) Biofilms in diabetic foot ulcers: impact, risk factors and control strategies. Int J Mol Sci 22(15):8278. https://doi.org/10.3390/ijms22158278
Baran I, Aksu N (2016) Phenotypic and genotypic characteristics of carbapenem-resistant Enterobacteriaceae in a tertiary-level reference hospital in Turkey. Ann Clin Microbiol Antimicrob 15:20. https://doi.org/10.1186/s12941-016-0136-2
Bindayna KM, Joji RM, Ezzat H, Jahrami HA (2022) Antibiotic-resistance genes in E. coli strains in GCC countries: a meta-analysis. Saudi J Med Med Sci 10(1):1–11. https://doi.org/10.4103/sjmms.sjmms_638_21
CLSI (2022) Clinical and laboratory standards institute. Performance standards for antimicrobial susceptibility testing. 32nd edn, supplement M100 edn.
Codjoe FS, Donkor ES (2017) Carbapenem Resistance: a Review Med Sci (basel) 6(1):1. https://doi.org/10.3390/medsci6010001
Davies J, Davies D (2010) Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 74(3):417–433. https://doi.org/10.1128/MMBR.00016-10
Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ (2015) Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 13(5):269–284. https://doi.org/10.1038/nrmicro3432
Gatya Al-Mayahie SM, Al-Guranie DRT, Hussein AA, Bachai ZA (2022) Prevalence of common carbapenemase genes and multidrug resistance among uropathogenic Escherichia coli phylogroup B2 isolates from outpatients in Wasit Province/ Iraq. PLoS ONE 17(1):e0262984. https://doi.org/10.1371/journal.pone.0262984
Gunardi WD, Karuniawati A, Umbas R et al (2021) Biofilm-producing bacteria and risk factors (gender and duration of catheterization) characterized as catheter-associated biofilm formation. Int J Microbiol 2021:8869275. https://doi.org/10.1155/2021/8869275
Gurung S, Kafle S, Dhungel B et al (2020) Detection of OXA-48 gene in carbapenem-resistant Escherichia coli and Klebsiella pneumoniae from urine samples. Infect Drug Resist 13:2311–2321. https://doi.org/10.2147/IDR.S259967
Hassan A, Usman J, Kaleem F, Omair M, Khalid A, Iqbal M (2011) Evaluation of different detection methods of biofilm formation in the clinical isolates. Braz J Infect Dis 15(4):305–311. https://doi.org/10.1590/S1413-86702011000400002
Horan TC, Andrus M, Dudeck MA (2008) CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36(5):309–332. https://doi.org/10.1016/j.ajic.2008.03.002
Hu H, Johani K, Gosbell IB et al (2015) Intensive care unit environmental surfaces are contaminated by multidrug-resistant bacteria in biofilms: combined results of conventional culture, pyrosequencing, scanning electron microscopy, and confocal laser microscopy. J Hosp Infect 91(1):35–44. https://doi.org/10.1016/j.jhin.2015.05.016
Karigoudar RM, Karigoudar MH, Wavare SM, Mangalgi SS (2019) Detection of biofilm among uropathogenic Escherichia coli and its correlation with antibiotic resistance pattern. J Lab Physicians 11(1):17–22. https://doi.org/10.4103/JLP.JLP_98_18
Katongole P, Nalubega F, Florence NC, Asiimwe B, Andia I (2020) Biofilm formation, antimicrobial susceptibility and virulence genes of Uropathogenic Escherichia coli isolated from clinical isolates in Uganda. BMC Infect Dis 20(1):453. https://doi.org/10.1186/s12879-020-05186-1
Kot B (2019) Antibiotic resistance among uropathogenic Escherichia coli. Pol J Microbiol 68(4):403–415. https://doi.org/10.33073/pjm-2019-048
Loqman S, Soraa N, Diene SM, Rolain JM (2021) Dissemination of carbapenemases (OXA-48, NDM and VIM) producing enterobacteriaceae isolated from the Mohamed VI University Hospital in Marrakech. Morocco Antibiotics (basel) 10(5):492. https://doi.org/10.3390/antibiotics10050492
Murray PR, Rosenthal KS, Kobayashi GS, Pfaller MA (2020) Medical microbiology e-book, 9th edn. Elsevier Health Sciences
Naber KG, Bonkat G, Wagenlehner FME (2020) The EAU and AUA/CUA/SUFU guidelines on recurrent urinary tract infections: what is the difference? Eur Urol 78(5):645–646. https://doi.org/10.1016/j.eururo.2020.06.032
Nasrollahian S, Halaji M, Hosseini A et al (2022) Genetic diversity, carbapenem resistance genes, and biofilm formation in UPEC isolated from patients with catheter-associated urinary tract infection in north of Iran. Int J Clin Pract 2022:9520362. https://doi.org/10.1155/2022/9520362
Nitzan O, Elias M, Chazan B, Saliba W (2015) Urinary tract infections in patients with type 2 diabetes mellitus: review of prevalence, diagnosis, and management. Diabetes Metab Syndr Obes 8:129–136. https://doi.org/10.2147/DMSO.S51792
Öztürk FY, Darcan C, Kariptaş E (2023) The determination, monitoring, molecular mechanisms and formation of biofilm in E. coli. Braz J Microbiol 54(1):259–277. https://doi.org/10.1007/s42770-022-00895-y
Panda PS, Chaudhary U, Dube SK (2016) Comparison of four different methods for detection of biofilm formation by uropathogens. Indian J Pathol Microbiol 59(2):177–179. https://doi.org/10.4103/0377-4929.182013
Patel N, Curtis JC, Plotkin BJ (2021) Insulin regulation of Escherichia coli abiotic biofilm formation: effect of nutrients and growth conditions. Antibiotics (basel) 10(11):1349. https://doi.org/10.3390/antibiotics10111349
Pelling H, Nzakizwanayo J, Milo S et al (2019) Bacterial biofilm formation on indwelling urethral catheters. Lett Appl Microbiol 68(4):277–293. https://doi.org/10.1111/lam.13144
Poirel L, Walsh TR, Cuvillier V, Nordmann P (2011) Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis 70(1):119–123. https://doi.org/10.1016/j.diagmicrobio.2010.12.002
Pouget C, Dunyach-Remy C, Pantel A, Schuldiner S, Sotto A, Lavigne JP (2020) Biofilms in diabetic foot ulcers: significance and clinical relevance. Microorganisms 8(10):1580. https://doi.org/10.3390/microorganisms8101580
Raya S, Belbase A, Dhakal L, Govinda Prajapati K, Baidya R, Kishor Bimali N (2019) In-vitro biofilm formation and antimicrobial resistance of Escherichia coli in diabetic and nondiabetic patients. Biomed Res Int 2019:1474578. https://doi.org/10.1155/2019/1474578
Reyes JA, Melano R, Cárdenas PA, Trueba G (2020) Mobile genetic elements associated with carbapenemase genes in South American Enterobacterales. Braz J Infect Dis 24(3):231–238. https://doi.org/10.1016/j.bjid.2020.03.002
Rizzato C, Torres J, Kasamatsu E et al (2019) Potential role of biofilm formation in the development of digestive tract cancer with special reference to Helicobacter pylori infection. Front Microbiol 10:846. https://doi.org/10.3389/fmicb.2019.00846
Sewify M, Nair S, Warsame S et al (2016) Prevalence of urinary tract infection and antimicrobial susceptibility among diabetic patients with controlled and uncontrolled glycemia in Kuwait. J Diabetes Res 2016:6573215. https://doi.org/10.1155/2016/6573215
Sharma D, Misba L, Khan AU (2019) Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrob Resist Infect Control 8:76. https://doi.org/10.1186/s13756-019-0533-3
Singh S, Singh SK, Chowdhury I, Singh R (2017) Understanding the mechanism of bacterial biofilms resistance to antimicrobial agents. Open Microbiol J 11:53–62. https://doi.org/10.2174/1874285801711010053
Stepanović S, Vuković D, Hola V et al (2007) Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 115(8):891–899. https://doi.org/10.1111/j.1600-0463.2007.apm_630.x
Tacconelli E, Carrara E, Savoldi A et al (2018) WHO pathogens priority list working group. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18(3):318–327. https://doi.org/10.1016/S1473-3099(17)30753-3
Tanriverdi Cayci Y, Biyik I, Korkmaz F, Birinci A (2021) Investigation of NDM, VIM, KPC and OXA-48 genes, blue-carba and CIM in carbapenem resistant enterobacterales isolates. J Infect Dev Ctries 15(5):696–703. https://doi.org/10.3855/jidc.13345
Tenover FC, Kalsi RK, Williams PP et al (2006) Carbapenem resistance in Klebsiella pneumoniae not detected by automated susceptibility testing. Emerg Infect Dis 12(8):1209–1213. https://doi.org/10.3201/eid1208.060291
Vandenbroucke JP, von Elm E, Altman DG et al (2007) STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 4(10):e297. https://doi.org/10.1371/journal.pmed.0040297
Zagaglia C, Ammendolia MG, Maurizi L, Nicoletti M, Longhi C (2022) urinary tract infections caused by uropathogenic Escherichia coli strains-new strategies for an old pathogen. Microorganisms 10(7):1425. https://doi.org/10.3390/microorganisms10071425
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors have participated in the concept and design, analysis and interpretation of data, drafting and revising of the manuscript, and have approved the manuscript and agreed with its submission to International Microbiology.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Institutional Review Board of the Faculty of Medicine Zagazig University (protocol code 63643/29–9-2020).
Consent to participate
Informed consent was obtained from all subjects involved in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abo-alella, D., Abdelmoniem, W., Tantawy, E. et al. Biofilm-producing and carbapenems-resistant Escherichia coli nosocomial uropathogens: a cross-sectional study. Int Microbiol (2024). https://doi.org/10.1007/s10123-024-00495-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10123-024-00495-w