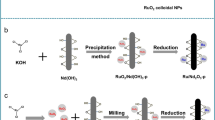
Abstract
The essential role of metal–support interaction in ammonia synthesis was investigated by using a Pr6O11 supported Ru nanoparticles (NPs) catalyst system. Loading of Ru NPs onto the Pr6O11 support with relatively low surface area yields similar metal dispersion with respect to conventional SiO2 support. The Ru NPs on the Pr6O11 was found to exhibit excellent catalytic performance in ammonia synthesis, superior to free-standing Ru NPs and Ru NPs on conventional SiO2 support. Detailed structure analysis results reveal that the superior activity of Ru/Pr6O11 can be attributed to the enhanced interaction between the Ru NPs and Pr6O11, which can modulate the electronic structure of Ru NPs and trigger catalytic ammonia synthesis over Ru NPs on Pr6O11. Furthermore, nitrogen isotope exchange experiment reveals that superior nitrogen dissociation ability is essential for the excellent activity and intrinsic activity of Ru/Pr6O11 in ammonia synthesis. These findings demonstrate that Pr6O11 can be used as a new kind of support for preparation of efficient Ru-based catalysts for ammonia synthesis, and also open up opportunities for design of Pr6O11 supported metal catalysts for other catalytic reactions.
Graphitical Abstract
Interaction between the Ru NPs and Pr6O11 can modulate the electronic structure of Ru NPs and trigger catalytic ammonia synthesis over Ru NPs on Pr6O11.








Similar content being viewed by others
References
Chu K, Luo YJ, Shen P, Li XCA, Li QQ, Guo YL (2022) Unveiling the synergy of o-vacancy and heterostructure over MoO3-x/MXene for N2 electroreduction to NH3. Adv Energy Mater 12:2103022
Li XC, Shen P, Luo YJ, Li YH, Guo YL, Zhang H, Chu K (2022) PdFe single-atom alloy metallene for N2 electroreduction. Angewandte Chemie-Int Ed 61:1–9
Li XT, Shen P, Li XC, Ma DW, Chu K (2023) Sub-nm RuOX Clusters on Pd metallene for synergistically enhanced nitrate electroreduction to ammonia. ACS Nano 17:1081–1090
Mittasch A (1950) Early studies of multicomponent catalysts. Adv Catal 2:81–104
Schlogl R (2003) Catalytic synthesis of ammonia—a “never-ending story”? Angewandte Chemie-Int Ed 42:2004–2008
Liu HZ (2014) Ammonia synthesis catalyst 100 years: practice, enlightenment and challenge. Chin J Catal 35:1619–1640
Bielawa H, Hinrichsen O, Birkner A, Muhler M (2001) The ammonia-synthesis catalyst of the next generation: barium-promoted oxide-supported ruthenium. Angew Chem Int Ed 40:1061–1063
Niwa Y, Aika K (1996) Ruthenium catalyst supported on CeO2 for ammonia synthesis. Chem Lett 25:3–4
Niwa Y, Aika K (1996) The effect of lanthanide oxides as a support for ruthenium catalysts in ammonia synthesis. J Catal 162:138–142
Sato K, Imamura K, Kawano Y, Miyahara S, Yamamoto T, Matsumura S, Nagaoka K (2017) A low-crystalline ruthenium nano-layer supported on praseodymium oxide as an active catalyst for ammonia synthesis. Chem Sci 8:674–679
Miyahara SI, Sato K, Kawano Y, Imamura K, Ogura Y, Tsujimaru K, Nagaoka K (2021) Ammonia synthesis over lanthanoid oxide-supported ruthenium catalysts. Catal Today 376:36–40
Ogura Y, Sato K, Miyahara S, Kawano Y, Toriyama T, Yamamoto T, Matsumura S, Hosokawa S, Nagaoka K (2018) Efficient ammonia synthesis over a Ru/La0.5Ce0.5O1.75 catalyst pre-reduced at high temperature. Chem Sci 9:2230–2237
Ogura Y, Tsujimaru K, Sato K, Miyahara S, Toriyama T, Yamamoto T, Matsumura S, Nagaoka K (2018) Ru/La0.5Pr0.5O1.75 catalyst for low-temperature ammonia synthesis. ACS Sustain Chem Eng 6:17258–17266
Jacobsen CJH, Dahl S, Hansen PL, Tornqvist E, Jensen L, Topsoe H, Prip DV, Moenshaug PB, Chorkendorff I (2000) Structure sensitivity of supported ruthenium catalysts for ammonia synthesis. J Mol Catal A 163:19–26
Song Z, Cai TH, Hanson JC, Rodriguez JA, Hrbek J (2004) Structure and reactivity of Ru nanoparticles supported on modified graphite surfaces: a study of the model catalysts for ammonia synthesis. J Am Chem Soc 126:8576–8584
Dahl S, Logadottir A, Egeberg RC, Larsen JH, Chorkendorff I, Tornqvist E, Norskov JK (1999) Role of steps in N2 activation on Ru(0001). Phys Rev Lett 83:1814–1817
Dahl S, Tornqvist E, Chorkendorff I (2000) Dissociative adsorption of N2 on Ru(0001): a surface reaction totally dominated by steps. J Catal 192:381–390
Rarog-Pilecka W, Miskiewicz E, Szmigiel D, Kowalczyk Z (2005) Structure sensitivity of ammonia synthesis over promoted ruthenium catalysts supported on graphitised carbon. J Catal 231:11–19
Wu Y, Li C, Fang B, Wang X, Ni J, Lin B, Lin J, Jiang L (2020) Enhanced ammonia synthesis performance of ceria-supported Ru catalysts via introduction of titanium. Chem Commun (Camb) 56:1141–1144
Wang XY, Ni J, Lin BY, Wang R, Lin JX, Wei KM (2010) Highly efficient Ru/MgO-CeO2 catalyst for ammonia synthesis. Catal Commun 12:251–254
Thangadurai V, Huggins RA, Weppner W (2001) Mixed ionic-electronic conductivity in phases in the praseodymium oxide system. J Solid State Electrochem 5:531–537
Li C, Liu H, Yang J (2015) A facile hydrothermal approach to the synthesis of nanoscale rare earth hydroxides. Nanoscale Res Lett 10:144
Borodzinski A, Bonarowska M (1997) Relation between crystallite size and dispersion on supported metal catalysts. Langmuir 13:5613–5620
Murata S, Aika K (1992) Preparation and characterization of chlorine-free ruthenium catalysts and the promoter effect in ammonia-synthesis. 1. An alumina-supported ruthenium catalyst. J Catal 136:110–117
Liang CH, Wei ZB, Xin Q, Li C (2001) Ammonia synthesis over Ru/C catalysts with different carbon supports promoted by barium and potassium compounds. Appl Catal A 208:193–201
van Deelen TW, Hernández Mejía C, de Jong KP (2019) Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat Catal 2:955–970
Lou Y, Xu J, Zhang Y, Pan C, Dong Y, Zhu Y (2020) Metal-support interaction for heterogeneous catalysis: from nanoparticles to single atoms. Mater Today Nano 12:100093
Yan L, Yu RB, Liu GR, Xing XR (2008) A facile template-free synthesis of large-scale single crystalline Pr(OH)3 and Pr6O11 nanorods. Scr Mater 58:707–710
Sinev MY, Graham GW, Haack LP, Shelef M (1996) Kinetic and structural studies of oxygen availability of the mixed oxides Pr(1–x)M(x)O(y) (M=Ce, Zr). J Mater Res 11:1960–1971
Borchert H, Frolova YV, Kaichev VV, Prosvirin IP, Alikina GM, Lukashevich AI, Zaikovskii VI, Moroz EM, Trukhan SN, Ivanov VP, Paukshtis EA, Bukhtiyarov VI, Sadykov VA (2005) Electronic and chemical properties of nanostructured cerium dioxide doped with praseodymium. J Phys Chem B 109:5728–5738
Narula CK, Haack LP, Chun W, Jen HW, Graham GW (1999) Single-phase PrOy-ZrO2 materials and their oxygen storage capacity: a comparison with single-phase CeO2-ZrO2, PrOy-CeO2, and PrOy-CeO2-ZrO2 materials. J Phys Chem B 103:3634–3639
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22179128), the National Key Research and Development Program of China (2021YFB4000401), the Dalian High-level Talents Program (2019RD09) and the Liaoning Revitalization Talents Program (XLYC2002076).
Funding
The study was supported by the National Natural Science Foundation of China (22179128), Dalian High-level Talents Program (2019RD09), National Key Research and Development Program of China (2021YFB4000401), and Liaoning Revitalization Talents Program (XLYC2002076).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, R., Liu, L., Ju, X. et al. Interaction Between Ru Nanoparticles and Pr6O11 Triggers Catalytic Ammonia Synthesis. Catal Lett (2024). https://doi.org/10.1007/s10562-024-04629-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10562-024-04629-7