Abstract
Background
We compared the efficacy, tolerability, and safety of oral sulfate tablets (OST, which contains simethicone) and 2 L-polyethylene glycol/ascorbate (2 L-PEG/Asc) with a split-dosing regimen in older individuals aged ≥ 70 years who underwent scheduled colonoscopy.
Methods
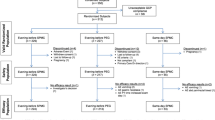
This prospective, randomized, investigator-blinded, multicenter study was conducted between June 2022 and October 2023. Participants aged ≥ 70 years were randomized at a ratio of 1:1 to the OST or 2 L-PEG/Asc groups.
Results
In total, 254 patients were evaluated using a modified full analysis set. Successful overall bowel preparation was excellent and similar between the OST and 2 L-PEG/Asc groups for the Boston Bowel Preparation Scale (BBPS) (96.5% vs. 96.6%) and Harefield Cleansing Scale (HCS) (96.5% vs. 97.4%). The overall high-quality preparation rate was higher in the OST group than in the 2 L-PEG/Asc group (BBPS: 55.7% vs. 28.4%, P < 0.001; HCS: 66.1% vs. 38.8%, P < 0.001). The overall adenoma detection rate (54.8% vs. 35.3, P = 0.003) was superior in the OST group compared to the 2 L-PEG/Asc group. Tolerability scores, including overall satisfaction, were generally higher in the OST group than in the 2 L-PEG/Asc group. The incidence of major solicited adverse events was comparable between the two groups (55.7% vs. 68.1, P = 0.051), and there were no clinically significant changes in the serum laboratory profiles on the day of or 7 days after colonoscopy.
Conclusions
OST is an effective and safe low-volume agent for colonoscopy, with better tolerance than 2 L-PEG/Asc, in older individuals aged ≥ 70 years.


Similar content being viewed by others
Abbreviations
- OST:
-
Oral sulfate tablet
- PEG:
-
Polyethylene glycol
- Asc:
-
Ascorbate
- BBPS:
-
Boston Bowel Preparation Scale
- HCS:
-
Harefield Cleansing Scale
- CRC:
-
Colorectal cancer
- OSS:
-
Oral sulfate solution
- PDR:
-
Polyp detection rate
- ADR:
-
Adenoma detection rate
- AE:
-
Adverse event
- mFAS:
-
Modified full analysis set
References
Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095–105.
Løberg M, Kalager M, Holme Ø, et al. Long-term colorectal-cancer mortality after adenoma removal. N Engl J Med. 2014;371:799–807.
Cha JM, Kozarek RA, La Selva D, et al. Risks and benefits of colonoscopy in patients 90 years or older, compared with younger patients. Clin Gastroenterol Hepatol. 2016;14:80-6.1.
Cha JM, Kwak MS, Kim HS, et al. Real-world national colonoscopy volume in Korea: a nationwide population-based study over 12 Years. Gut Liver. 2020;14:338–46.
Hassan C, Fuccio L, Bruno M, et al. A predictive model identifies patients most likely to have inadequate bowel preparation for colonoscopy. Clin Gastroenterol Hepatol. 2012;10:501–6.
Hassan C, East J, Radaelli F, et al. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline—update 2019. Endoscopy. 2019;51:775–94.
Jung YS, Lee CK, Eun CS, et al. Low-volume polyethylene glycol with ascorbic acid for colonoscopy preparation in elderly patients: a randomized multicenter study. Digestion. 2016;94:82–91.
Kwak MS, Cha JM, Yang HJ, et al. Safety and efficacy of low-volume preparation in the elderly: oral sulfate solution on the day before and split-dose regimens (SEE SAFE) Study. Gut Liver. 2019;13:176–82.
Yoon JY, Kim HG, Cho YS, et al. 1 L- versus 2 L-polyethylene glycol with ascorbic acid for bowel preparation in elderly patients: a randomized multicenter study. Surg Endosc. 2022;36:5724–33.
Di Palma JA, Bhandari R, Cleveland MV, et al. A safety and efficacy comparison of a new sulfate-based tablet bowel preparation versus a PEG and ascorbate comparator in adult subjects undergoing colonoscopy. Am J Gastroenterol. 2021;116:319–28.
Kim JH, Park YE, Kim TO, et al. Comparison of the efficacy and safety between oral sulfate tablet and polyethylene glycol for bowel preparation before colonoscopy according to age. Medicine (Baltimore). 2022;101: e29884.
Lee SE, Oh DJ, Nam JH, et al. Taking oral sulfate tablets with simethicone for bowel preparation leads to higher adenoma detection rate than polyethylene glycol: a propensity score analysis. Dig Dis Sci. 2023;68:867–76.
Jeon SR, Park SK, Yang DH, et al. Comparison of a novel mini-oral sulfate tablet and the conventional oral sulfate tablet in bowel preparation for colonoscopy: a prospective, randomized, investigator-blinded, multicenter, non-inferior, phase 3 trial. J Gastroenterol. 2023;58:1114–23.
Hsu CY, Chertow GM. Chronic renal confusion: insufficiency, failure, dysfunction, or disease. Am J Kidney Dis. 2000;36:415–8.
Calderwood AH, Schroy PC 3rd, Lieberman DA, et al. Boston Bowel Preparation Scale scores provide a standardized definition of adequate for describing bowel cleanliness. Gastrointest Endosc. 2014;80:269–76.
Halphen M, Heresbach D, Gruss HJ, et al. Validation of the Harefield Cleansing Scale: a tool for the evaluation of bowel cleansing quality in both research and clinical practice. Gastrointest Endosc. 2013;78:121–31.
Hatoum HT, Lin SJ, Joseph RE, et al. Validation of a patient satisfaction scale in patients undergoing bowel preparation prior to colonoscopy. Patient. 2016;9:27–34.
Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110:72–90.
Keswani RN, Crockett SD, Calderwood AH. AGA clinical practice update on strategies to improve quality of screening and surveillance colonoscopy: expert review. Gastroenterology. 2021;161:701–11.
Kaminski MF, Thomas-Gibson S, Bugajski M, et al. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy. 2017;49:378–97.
Baker FA, Mari A, Nafrin S, et al. Predictors and colonoscopy outcomes of inadequate bowel cleansing: a 10-year experience in 28,725 patients. Ann Gastroenterol. 2019;32:457–62.
Kim KO, Kim EY, Lee YJ, et al. Efficacy, safety and tolerability of oral sulphate tablet for bowel preparation in patients with inflammatory bowel disease: a multicentre randomized controlled study. J Crohns Colitis. 2022;16:1706–13.
Yang HJ, Park DI, Park SK, et al. Novel sulfate tablet PBK-1701TC versus oral sulfate solution for colon cleansing: a randomized phase 3 trial. J Gastroenterol Hepatol. 2020;35:29–36.
Park JH, Hong SW, Hwang SW, et al. Efficacy and safety of oral sodium sulfate tablet compared with 1-L polyethylene glycol plus ascorbate: a prospective, randomized, endoscopist-blinded trial. J Gastroenterol Hepatol. 2023;38:2090–6.
Patel V, Nicar M, Emmett M, et al. Intestinal and renal effects of low-volume phosphate and sulfate cathartic solutions designed for cleansing the colon: pathophysiological studies in five normal subjects. Am J Gastroenterol. 2009;104:953–65.
Song JH, Bae JH, Yim JY. Efficacy of oral sulfate tablets for bowel preparation and adenoma detection rate. J Gastroenterol Hepatol. 2023;38:410–5.
Yeh JH, Hsu MH, Tseng CM, et al. The benefit of adding oral simethicone in bowel preparation regimen for the detection of colon adenoma: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2019;34:830–6.
Patel SG, May FP, Anderson JC, et al. Updates on age to start and stop colorectal cancer screening: recommendations from the US multi-society task force on colorectal cancer. Gastroenterology. 2022;162:285–99.
Acknowledgements
The authors thank the clinical trial department of Pharmbio Korea for their expertise.
Funding
This study was funded by Pharmbio Korea Co., Seoul, Korea; however, clinical trial and manuscript writing were carried out independently.
Author information
Authors and Affiliations
Contributions
Concept and design: JMC; data acquisition: HSK, SYN, JYY, YJ, GSS, and JMC; statistical analysis: JYY and JMC; drafting and revision of the manuscript: HSK, and JMC. All the authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Clinical trial: Trial Registration: Clinical Trials.gov, Registration number (trial ID): NCT05249335.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, H.S., Na, SY., Yoon, J.Y. et al. Efficacy, tolerability, and safety of oral sulfate tablet versus 2 L-polyethylene glycol/ascorbate for bowel preparation in older patients: prospective, multicenter, investigator single-blinded, randomized study. J Gastroenterol 59, 402–410 (2024). https://doi.org/10.1007/s00535-024-02089-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-024-02089-9