Abstract
Reactive air brazing (RAB) is a low-cost process for joining ceramic composites in air. However, due to the comparably low strength values that can be achieved by RAB, the process is only used in special applications like solid oxide fuel cells as a sealant. The limited strength values are the result of a severe pore formation during the brazing operation that remain in the brazing fillet after the solidification of the brazing filler. In this work, the formation of the pores during RAB brazing of alumina using a paste containing Ag4CuO powder and various binders was investigated. The formation and evolution of the pores were observed and quantified in in situ X-ray measurements. It could be observed that during debinding, pores have developed in the filler metal. The pore structure depends on the binder and the heating rate in the debinding stage. With the melting of the filler metal, many pores are closed by the melt flow. But it seems that the wetting of the alumina was hindered by the pores. The change of porosity during cooling is comparatively low.
Similar content being viewed by others
1 Introduction
Reactive air brazing (RAB) is used to braze ceramics without an inert gas atmosphere [1,2,3]. This process uses metal–metal oxide filler materials. The most common filler metals are based on Ag and CuO. During the brazing process, the CuO particles diffuse to the interface of the ceramic. Due to the similar structure of the oxide particles and the ceramic base material, a bond is created. However, in brazed seams of such joints, defects usually occur in the form of pores and certain interfacial reactions, which reduce the reliability and quality of the composites. That is why reactive air brazing could not gain industrial acceptance. The formation of the undesirable reaction layers was attributed to the CuO [4,5,6]. With the aim of preventing the formation of undesirable reaction layers, investigations were carried out with various alloys. Within one approach, V2O5 was used instead of CuO to reduce the formation of the reaction layer. But the wetting behavior and strength did not meet the expectations [7,8,9]. Conversely, adding Al enhanced the wetting and strength [10, 11]. The addition of Ni or Mn led to an improvement in wetting only above the temperatures of 1350 and 1050 °C, respectively [12].
By using a Cu-coated Ag-foil, Tillmann et al. brazed Al2O3 and Crofer 22 APU. By holding the temperature at 500 °C during the heating process in an oxygen-rich atmosphere, CuO developed on the surface of the filler metal. The wetting of the base materials was brilliant and the brazing gap was free of pores and void-free joints [13].
In contrast to the composition, the influence of pores and their formation during the RAB process is still insufficiently studied. Two mechanisms are cited as the cause for the formation of the pores: One is the evaporation or burning of the binder in the commonly used brazing pastes. The other one is the outgassing of oxygen from the cooling filler material due to the reduced oxygen solubility when the liquid filler material cools or falls below the liquidus temperature [14,15,16,17]. To determine the mechanisms of pore formation, appropriate in situ analysis is necessary. In Bobzin’s work, an attempt was made to observe pore formation in in situ by brazing a light-transmissive sapphire glass as the base material. However, only pores which were open to the sapphire glass could be identified [14, 15]. To reduce the amount of pores in the brazing gap, Waetzig et al. investigated the influence of powder quality (coarse and spherical powder) of Ag0-8CuOTi on the formation of a dense and tight brazing seam [18]. When spherical powders were used for brazing, the brazing seam is interrupted by cavities and pores. The authors assumed that the reason for the defects was the gas formation at high temperatures. When the coarse powder was used, the brazing seam featured less voids, which was attributed to the wetting behavior.
In this work, the in situ X-ray transmission technique was used to observe and quantify the formation and evolution of the pores during the debinding and brazing processes of Al2O3. The aim was to determine when pores form and grow during debinding and heating, and how the developed pore structure affects the wetting during melting. Furthermore, the pore formation during cooling was investigated, too.
The findings/results show that the pore formation during debinding depends on the binder itself and on the heating rate.
2 Experimental procedure
2.1 Brazing materials
The used base material was polycrystalline Al2O3 (DEGUSSIT AL23, Kyocera, Germany), which is suitable for high-temperature applications (see Table 1). The dimensions of the specimens are 35 × 17.5 × 2 mm (H × W × D). The Al2O3 was coated with Cr (10Ah) on one side to improve the heat coupling of the laser (Interatom PVD20, Fa. Oerlikon Balzers Coating Germany GmbH, Germany). For the coating, the cathodic arc evaporation process was used.
The used brazing paste consists of a Ag4CuO powder and either an oil-based or a glycol-based binder (S, oil-based; NX, glycol-based) provided by Innobraze GmbH. The S-binder had a mass fraction of 15% and the NX-binder had one of 30%. The boiling range and the evaporation temperature of the binders are shown in Table 2. The brazing paste was applied onto the uncoated side of each alumina substrate with a stencil printer SD 300 from Paggen with a thickness of 100 µm. The total thickness of the brazing paste of the entire assembly was 200 µm. For comparison reasons, a pure Ag foil was used as a filler metal (thickness = 200 µm) as well.
2.2 Brazing procedure
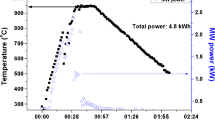
In Fig. 1, the brazing setup is shown. With the aid of the laser system, it is possible to homogeneously irradiate a total area of 35 × 35 mm at a beam angle of 35° to the surface normal. This system also has two process heads, each with a pyrometer, so that the temperatures on the sample surface (T > 500 °C) can be recorded. Temperatures below 500 °C were measured during a reference measurement with a thermocouple in the brazing gap. The performance data of the used laser is shown in Table 3. The power of the laser was controlled by adjusting the current. Two different heating rates were investigated resulting in the two different temperature profiles (T-t_1 and T-t_2) shown in Fig. 2 (heating rate 1 < heating rate 2).
To determine the in situ development of the pores in the brazing gap, radiographic testing was used. The used radiographic system consists of a microfocus X-ray tube (SXT-161B, Fa. SYBURG GmbH, Germany) and an area detector (RID 512–400, Fa. Heimann, Germany) serving as an X-ray detection system. The X-ray tube is characterized by a maximum tube voltage of Umax = 160 kV and a maximum tube current Imax = 1 mA. The investigations within the scope of this work were carried out at a tube voltage of U = 140 kV and a tube current of I = 10 µA. With the aid of the X-Ray Imaging Software XIS, a series of images was acquired for each brazing process, which is used for a subsequent evaluation of the porosity as well as the flow movement of the melted filler metal.
The temperature profile was divided into three stages. In the first stage, the sample was gradually heated so that the binder evaporates. After further heating, the filler metal melted in the second stage. In the last stage, the sample was cooled. The temperature was measured on the surface of both joining partners and reported as an arithmetic mean value. Because of the applied acquisition system for the temperature, only temperatures above 500 °C were measured. The durations of the different stages of the two temperature profiles T-t_1 and T-t_2 are shown in Table 4.
2.3 Analytical methods
The behavior of the brazing paste during heating was investigated by differential thermal analyses (DTA) and thermogravimetry (TG). At the beginning, each Al2O3 crucible is placed in the system in the empty state and a zero measurement is performed, so that the measurement data of the individual braze pastes are tared subsequently and disturbance variables are eliminated. After that, weighed brazing paste was added to a crucible and placed in the STA (LINSEIS STA PT1600, Linseis, Germany). To simulate the brazing process, a heating and cooling rate of 5 K/min and a holding time of t = 10 min at a maximum temperature of T = 1100 °C were set. The data obtained were evaluated using the Linseis TA Evaluation software.
The amount of pores, the pore size, and the porosity were determined by the software ImageJ (NIH). The software can recognize the contrast between the pores and the filler metal and can identify the number and the size of the pores. The porosity is calculated by the summed area of the pores divided by the wetted area in the beginning of the process.
By using the results of the radiographic testing, areas with different pore structures were identified. Transverse sections were made of these areas. To investigate the microstructure, the samples were analyzed using a JSM 7001F SEM (Jeol, Japan). In addition to secondary and backscattered electron detectors, the SEM has an EDS detector and composition measurements were carried out. The samples were coated in advance using cathode sputtering (Au, 3 nm) to ensure a conductive surface (CCU-010 LV, Safematic GmbH, Switzerland). The images were recorded in backscatter contrast at an accelerating voltage of 15 kV.
3 Results and discussion
3.1 DTA
Figure 3 shows the TG curves of the investigated brazing pastes in the debinding phase. A direct comparison of the mass losses of the individual peaks reveals a slightly shifted peak position as well as different peak widths and peak amplitudes. Binder S leads to a significantly lower relative mass loss. This is due to the fact that the brazing paste with binder S contains only half the amount of binder (15 m. %) as the brazing paste with binder NX (30 m. %) (Table 5). Furthermore, it can be seen that the debinding peak for both binders correlates with the most pronounced mass decrease, which corresponds to almost the entire mass fraction of the binder. Hence, it can be concluded that the brazing pastes outgas unequal amounts of binder due to the different mixing ratios. The mass loss also happens below 300 °C. Hence, the boiling of the binder seems to be the main factor for outgassing. The evaporation of the organic residues above 400 °C does not affect the mass loss that much.
Figure 4 shows the thermal voltage of the DTA measurements of the brazing pastes used during debinding. A peak can be seen, which clearly shows the difference between the binder S and NX. Binder S shows a characteristic deflection in the peak area, with a steep rise followed by a drop in slope to the reaction point. Binder NX shows peaks in a similar temperature range, but these reactions are weaker, and the peak range differs significantly from that of binder S in terms of reaction rate.
Within a temperature interval of 932.9–937.4 °C, a liquidus reaction occurred in both pastes, exhibited by a comparatively strong endothermic peak (Fig. 5 and Table 6). Thus, the melting temperatures or intervals of the brazing pastes are about 20 K lower than those of the pure Ag. An influence of the binder or its residues on the melting process is not distinctly detectable. However, within the curve of the paste containing binder NX, a small peak at about 940 °C is visible, indicating a reaction after the melting of the filler material. This might be caused by residues of the binder. Interstingly, only one peak occurs during the melting, even though the AgCuO usually shows an eutectic peak (943 °C) and a monotectic peak (967 °C) [19].
Figure 6 shows the results of the DTA measurements of the brazing pastes and the pure Ag during cooling and solidification. The difference between the onset temperature and the peak temperature is ~ 1 K (Table 7). The peak of the Ag is more pronounced than the peaks of the pastes. Both pastes, NX and S, exhibit an exothermic reaction occurring immediately before solidification at temperatures above 910 °C. It can be assumed that these are reactions of the binder residues.
3.2 Development of pores during heating
Figure 7a exemplarily shows the development of pores within the debinding stage of a Ag4CuO/S sample with the low heating rate (T-t_1). From the beginning, there are pores in the assembly in the filler metal paste. With increasing laser power and time, the pores grow. In addition, new pores are formed, which also grow with increasing process times. After 900 s, the porosity reaches a saturation. Hence, it can be assumed that the binder has completely outgassed. In the sample which was heated at the lower heating rate, small and significantly fewer pores can be seen at the beginning (Fig. 7b). With increasing laser power and time, the pores grow faster than with the higher heating rate (T-t_2). In addition, significantly more pores are formed.
Figure 8 shows the amount of pores and the average pore size of the samples for both heating rates and binders after debinding. For the Ag4CuO/S filler metal, the number of pores rises initially until it reaches its maximum, before it drops again down to 195 at the end of the debinding stage (Fig. 8a). At the same time, the size of the pores steadily increases (Fig. 8b) and some pores coalesce, causing the number of pores to decrease again. The higher heating rate led to the formation of 611 small pores (0.37 mm2), which can be seen in Fig. 8c and d, whereas the lower heating rate led to the formation of 195 larger pores (1.4 mm2) (see Fig. 8a and b). The difference in porosity for the Ag4CuO/S at the different heating rates is 9% (49 and 58%). This low difference in porosity indicates that the different heating rates do not affect the volume of outgassed binder but the outgassing behavior. One possible explanation for the different behavior could be that the outgassing in an existing cavity requires a lower amount of energy than creating a new cavity. With increasing heating rate, the binder has less time to diffuse to an existing cavity, which is why more pores develop.
The application of the glycol-based paste Ag4CuO/NX leads to different trends in the pore formation and pore growth. The higher heating rate (T-t_2) results in only 183 pores with an average size of 0.57 mm2. This corresponds to a porosity of 24%. The lower heating rate, conversely, leads to 298 pores with a mean diameter of 0.26 mm2 (porosity = 17%). Since a higher heating rate has a positive effect on the number of pores in the case of the Ag4CuO/S, but a negative effect in the case of the Ag4CuO/NX, a general statement about the influence of the heating rate and the binder on the pore formation during the debinding stage cannot be made. However, it could be shown that both parameters have an influence on the pore formation. In addition, it could be shown that the size of the pores is not proportional to the amount of binder [9].
Figure 9 shows the wetted area of the specimens after debinding (black) and melting (red). It can be seen that the pore structure did not change after the debinding and before the melting. This results match with the results of Bobzin et al. [20]. Regardless of the heating rate and the binder, the wetted area of the filler metal decreases after melting. The samples with the Ag4CuO/S filler metal already show a high porosity after debinding. After melting, the wetted area decreases by 35.5% for T-t_1 and by 52.9% for T-t_2 (see Fig. 10). For the Ag4CuO/NX, the wetted area decreases by 84.7 (T-t_1) and 85.5%.
The high porosity resulting from the S-binder promotes a homogeneous distribution of the molten material within the brazing joint. In contrast, the NX-binder leads to a concentration of the molten material primarily in the edge regions of the brazing gap. The excessive flow of the molten material can be attributed to the vertically oriented configuration, which allows gravity to facilitate its movement. Additionally, the insufficient contact pressure exerted on the specimen impedes the development of a capillary force, which would be strong enough to counteract gravitational effects.
Interestingly, areas where pores have occured after debinding seem to be less often wetted after the melting. This could indicate that reactions between the filler metal and the Al2O3 take place during heating (before melting), which improve the wettability. The results of Bobzin et al. match with this assumption. They found out that a holding time during heating (before melting) improves the wetting of the alumina [20].
In Fig. 10, the absolute wetted areas after debinding, melting, and solidifiacation are plotted. It can be seen that the wetted area of the samples with NX binder decreases relatively to the debinded state as well as the initial state dramatically. In case of the S binder, the wetted area in the debided state is significantly smaller compared to the NX binder, but the relative drop in the following stages, melting, and solidification is less prononounced. This leads to higher values of the wetted area in the final state. Interestingly, it can be seen that the wetted area partially increases during solidification, which leads to higher values of the wetted area when comparing the melted and solidified states. The results show that there is no clear correlation between the porosity after debinding and after melting.
In order to clarify the increase of the wetted area during cooling and the development of pores, the solidification behavior of the pure Ag (foil) was investigated (see Fig. 11). In the molten state, the Ag shows isolated small pores within the area wetted by the molten filler metal. With the beginning of the cooling stage (0 s), pores grow slightly. Already after 16 s, a strong pore growth can be seen, which initiates at the left side. Previously, existing pores serve as the starting point for this development. The pores increase in size and also distort in the direction of the temperature gradient or towards the point of highest temperature. After 18 s, the individual pores rapidly increase in size and the pore band serves as a starting point for the formation of additional pores. Especially in the central region of the molten filler metal, the pores coalesce and move towards the top of the specimen, until they reach the surface. On the left side of the sample, the melt is partially driven out by the increasing porosity (20 s). It can be confirmed that the use of pure Ag leads to a comparatively severe porosity, which is formed during solidification. The outgassing during the cooling can be explained with the solubility of gases being higher in the melt than in the solid filler metal. Interestingly, the outgassing during cooling is far stronger in the pure Ag melt than in the melt of the Ag4CuO. These results were observed by Bobzin et al., too [20]. They assumed that Ag was deoxidized, which avoided the emitting of oxygen.
3.3 Microstructure
Since X-ray transmission is limited to the study of the distribution of the filler metal in one plane, SEM analyses of the transverse sections were performed additionally to determine the microstructure in the filler metal, especially with regard to the phase distribution at the interfaces. A representative micrograph is shown in Fig. 12a, which displays the microstructure of the sample Ag4CuO/S manufactured with the low heating rate T-t_1. It is characterized by an irregular distribution of CuO at the interfaces (Fig. 12b, c) in the brazing seam.
This contradicts with the findings of Bobzin et al. [12], who observed a continuous CuO phase band at the interface of alumina and filler metal. In contrast to the setup used in this work, the brazing process of Bobzin was carried out with horizontally placed samples, so that the influence of gravity on the distribution of the CuO in the molten filler metal is much smaller.
Furthermore, no reaction layer between the base material and the filler metal material could be detected by EDS analysis. A possible explanation could be the fact that the achieved temperature of the depicted filler metal amounted Tmax = 951 °C, which is outside or below the critical temperature interval (980–1050 °C), in which a reaction layer of copper aluminate between AgCuO filler metal and Al2O3 substrate takes place [21, 22]. Another reason could be the limited resolution of the EDS detector, which is based on the diameter of the electron beam (dE = 4 µm).
In Fig. 13, the formation of melt beads is shown (Ag), which is visible in mostly non-wetted areas. The beads are visible on both alumina specimens and predominantly consist of pure Ag.
Figure 14 displays a cavity inside the brazing gap. Residues of Ag and CuO are clearly visible at the surfaces of the alumina substrates. The unaffected surface within the cavity is visible, showing isolated CuO phases in the Ag matrix. This shows that pores and cavities in the brazed seam do not have to be attributed to a lack of wetting, but that effects such as gravity or cohesion can have an influence, too.
4 Conclusions and outlook
In this work, it could be shown that the chosen experimental setup allows the investigation of pore formation and evolution within the brazed seam during the joining process. With the aid of the used setup, it was also possible to obtain further findings that are summarized as follows:
-
1.
The type of binder and the heating rate have an influence on the pore formation during debinding.
-
a.
The application of the S-binder resulted in a higher porosity in the brazing seam than by using the NX-binder.
-
b.
For the S-binder, a high heating rate resulted in many small pores, whereas a low heating rate results in few large pores.
-
c.
The opposite effect was observed with the NX-binder.
Since the heating rate and the binder affect the pore structure but the reasons for that are unclear, further investigations are necessary.
-
2.
Areas where pores developed during debinding are less likely wetted during melting.
-
3.
Within the melting stage, a reduction of the porosity could be observed due to the movement of the melt that led to the disappearance of single pores.
-
4.
The pore structure only changed slightly during solidification without a clear tendency to increase or decrease the pores size, even though the change in the solubility of oxygen causes a movement of the melt.
In future work, the effect of the pore structure on the joint strength should be investigated.
Data availability
Data sets generated during the current study are available from the corresponding author on reasonable request.
References
Kim JY, Weil KS (2006) Development of a copper oxide-silver braze for ceramic joining. Advances in joining of ceramics. John Wiley & Sons, Inc, Hoboken, NJ, USA, pp 119–132
Kim JY, Hardy JS, Scott Weil K (2005) Effects of CuO content on the wetting behavior and mechanical properties of a Ag-CuO braze for ceramic joining. J Am Ceram Soc 9:2521–2527
Erskine KM, Meier AM, Pilgrim SM (2002) Brazing perovskite ceramics with silver/copper oxide braze alloys. J Mater Sci 8:1705–1709
Bobzin K, Schläfer T, Schlegel A et al (2009) Reactive Air brazing – optimizing parameters and analysing of reaction mechanisms. In: 4th International Brazing and Soldering Conference, April 26–29
Kuhn B, Wetzel FJ, Malzbender J et al (2009) Mechanical performance of reactive-air-brazed (RAB) ceramic/metal joints for solid oxide fuel cells at ambient temperature. J Power Sources 1:199–202
Kim JY, Hardy JS, Weil KS (2005) Novel metal-ceramic joining for planar SOFCs. J Electrochem Soc 6:J52
Zink, N. M.; Meier, A. M.; Weil, K. S. et al.: Reactive air brazing of LSCoF and alumina with Ag-V2O5 alloys for SOFC applications. In Advances in Ceramic Coatings and Ceramic-Metal Systems: Ceramic Engineering and Science Proceedings, Volume 26, Number 3, John Wiley & Sons, Inc, Hoboken, NJ, USA (2005), S. 341–348.
Lamb MC, Camardello SJ, Meier AM et al (2005) Brazing of porous alumina to monolithic alumina with Ag-CuO and Ag-V2O5 alloys. In: Advances in ceramic coatings and ceramic-metal systems: Ceramic engineering and science proceedings, volume 26, number 3. John Wiley & Sons, Inc, Hoboken, pp 391–398
Pavlina EJ, Meier AM, Monteleone P et al (2007) Development of silver–metal oxide reactive air braze alloys for electroding PZT ceramics. J Mater Sci 2:705–713
Kuhn B (2008) Bruchmechanische Untersuchung von Metall/Keramik-Verbundsystemen für die Anwendung in der Hochtemperaturbrennstoffzelle (SOFC). Dissertation, Technische Universität Clausthal
Reichle MS, Koppitz T, Federmann D et al (2011) Anwendung Dilatometrischer Messungen bei der Entwicklung von Gasloten und Reaktiven Metallloten zum Fügen von Hochtemperaturbrennstoffzellen. Materialwiss Werkstofftech 3:188–199
Bobzin K, Schläfer T, Kopp N (2010) Konturtreue Lote zum Fügen von Keramik an Luft. DVS-Berichte 263:64–69
Tillmann W, Anar N, Wojarski L (2020) Mechanical behavior of reactive air brazed (RAB) Crofer 22 APU-Al2O3 joints at ambient temperature. SN Appl Sci 2:809
Bobzin K (2012) Industrielle Nutzung des Reactive Air Brazings zum Fügen von leckdichten Keramik-Keramik- und Keramik-Metallverbunden. RWTH-Aachen
Bobzin K (2012) Industrielle Nutzung des Reactive Air Brazings zum Fügen von leckdichten Keramik-Keramik- und Keramik-Metallverbunden. RWTH-Aachen
Kopp N (2012) Reaktive Air Brazing - Löten von Keramik-Keramik und Keramik-Metall-Verbunden an Luft. Dissertation, RWTH Aachen
Pönicke A (2013) Löten von Keramik-Metall-Verbunden mit Reaktivloten an Luft und deren Alterungsverhalten bei hohen Temperaturen. Dissertation, Fraunhofer Verlag, Stuttgart
Waetzig K, Schilm J, Mosch S, Tillmann W, Eilers A, Wojarski L (2020) Influence of the brazing paste composition on the wetting behavior of reactive air brazed metal–ceramic joints. Adv Eng Mater
Si X, Cao J, Song X (2017) Qu; Y, Feng, J: Reactive air brazing of YSZ ceramic with novel Al2O3 nanoparticles reinforced Ag-CuO-Al2O3 composite filler: microstructure and joint properties. Mater Des 114:176–184
Bobzin K, Kopp N, Wiesner S (2014) Influence of filler and base material on the pore development during reactive air brazing. Adv Eng Mater
Bobzin K, Schläfer T, Zhao L, Kopp N, Schlegel A (2010) Brazing of ceramic-to-ceramic and ceramic-to-metal joints in air. Front Mech Eng China 5:125–129
Kim JY, Engelhard M, Pyungchoi J, Weil KS (2008) Effects of atmospheres on bonding characteristics of silver and alumina. Int J Hydrogen Energy 33:4001–4011
Acknowledgements
The authors gratefully acknowledge the financial support of the German Research Foundation (DFG) for the project Ti343/147-1, within which these findings were obtained.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Recommended for publication by Commission XVII - Brazing, Soldering and Diffusion Bonding
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wojarski, L., Ulitzka, H., Geldmacher, A. et al. In situ observation of the pore formation during laser-based reactive air brazing of alumina. Weld World (2024). https://doi.org/10.1007/s40194-024-01733-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40194-024-01733-5