Abstract
Due to the limitations of conventional ultrafiltration/microfiltration-based membrane bioreactors (UF/MF-MBRs) in removing trace organic compounds (TrOCs), the concept of high-retention membrane bioreactors (HR-MBRs) was introduced. Despite the benefits, HR-MBRs still suffer several drawbacks. Therefore, this paper critically reviews the effectiveness and feasibility of the proposed strategies to alleviate fouling, salinity build-up and incomplete biodegradation of TrOCs during wastewater treatment by HR-MBRs. The severity of each challenge is compared amongst the various configurations together with the associated capital and operational expenditure to determine the most cost-effective set-up. Guidance is provided on strategies and/or lessons that could be adopted from well-established processes used at municipal scale. Chemical cleaning as mitigation for fouling degrades membranes leading to poor TrOCs removal, while pre-treatment and membrane surface modification increase operational expenditure (OpEX). However, there are other environmentally-friendly pretreatment and cleaning options which hold great potential for future application. These options such as advanced oxidation processes (AOPs) are critically discussed in this work. Further, in-depth discussion is made on the pros and cons of the various approaches (such as frequent sludge withdrawal, intermittent UF/MF filtration and using organic salts) to alleviate salt build-up. Finally, incomplete biodegradation of rejected TrOCs in the bioreactor transfers problems of toxic pollutants from wastewater treatment to sludge management. Herein mitigation strategies including using stronger biological agents and coupling HR-MBRs with other techniques are debated. Despite the challenges, HR-MBRs are a promising solution for clean water production from TrOCs impaired wastewater. Therefore, more research is needed to improve the performance of HR-MBRs.
Similar content being viewed by others
Conventional and high-retention membrane bioreactors
Conventional membrane bioreactors (MBR) combine biological processes as well as membrane filtration, utilizing microfiltration (MF) and ultrafiltration (UF) membranes for wastewater treatment and reclamation. However, MF and UF membranes are ineffective in retaining Trace Organic Compounds (TrOCs), including pharmaceuticals, pesticides, hormones and antibiotics1,2. Therefore, high-retention membranes such as nanofiltration (NF), reverse osmosis (RO), forward osmosis (FO), and membrane distillation (MD) have been introduced in biological treatment to achieve high removal of TrOCs. The coupled process known as a high-retention membrane bioreactor (HR-MBR) has been the research focus in recent years. The high-retention processes ensure prolonged retention of the TrOCs in the bioreactor for further biodegradation.
The most investigated high-retention membrane bioreactor (HR-MBR) processes to date are nanofiltration membrane bioreactor (NF-MBR)3,4,5, reverse osmosis membrane bioreactor (RO-MBR)6,7,8, membrane distillation bioreactor (MDBR)9,10,11 as well as an osmotic membrane bioreactor (OMBR)12,13,14. Figure 1 shows the number of publications on the different configurations on HR-MBR. The graphs were drawn from the number of peer-reviewed scientific papers related to HR-MBR technologies in Scopus (abstract and citation database of peer-reviewed literature). RO-MBR remains the highly investigated HR-MBR process.
The main differences between conventional MBRs and high-retention membrane bioreactors (HR-MBRs) are the type of membranes used, driving force, solute rejection properties by the membranes and flux (Table 1), which have been reviewed by Luo et al.15.
HR-MBRs were proposed to mitigate several challenges associated with MF/UF-MBRs, which originate from the inability of MF/UF-MBRs to remove slowly degradable small molecular weight compounds16. The poor removal of slowly biodegradable (recalcitrant) organics leads to the following consequences, as noted by Phattaranawik et al.16: (i) poor permeate quality that negatively affects polishing and disinfection steps, limiting direct reuse potential of the permeate17; (ii) since poorly biodegradable organics permeate through MF/UF-MBRs, there is inadequate time for microorganisms to acclimatize to achieve acceptable permeate quality and (iii) effective biodegradation of slowly biodegradable organic compounds require longer Hydraulic Retention Time (HRT), and this implies requirements of larger MF/UF-MBRs tanks which would increase capital and operational costs.
The removal of organic substances by MF/UF-MBRs depends mainly on the biological activity because MF/UF membranes have poor TrOCs removal. As noted before, there are several reasons for using HR-MBRs, including the better removal of dissolved solids, organic compounds, nutrients and pathogens18,19,20,21 by facilitating the biodegradation of pollutants22. In this paper, we focus on the rejection of TrOCs by HR-MBRs, the challenges associated with HR-MBRs and their effects on TrOCs removal, and mitigation approaches and effectiveness.
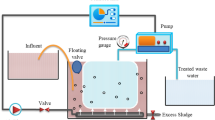
HR-MBRs may be operated under aerobic or anaerobic conditions, although most of the studies to date have focused on aerobic HR-MBR. Further, the high-retention membranes may be submerged or applied as a side stream mode. Figure 2 shows a schematic representation of aerobic HR-MBRs. Anaerobic systems have a similar set-up without air bubbling.
In an NF/RO membrane bioreactor (NF/RO-MBR), hydraulic pressure serves as the driving force for the permeation of feed from the bioreactor (Fig. 2a, b). The membrane rejects low molecular weight TrOCs and salts in this process through various mechanisms discussed later. In OMBR (Fig. 2c, d), osmotic pressure is used to drive raw water from the bioreactor through FO membranes. An osmotic pressure develops across the membrane interface because of the high salt concentration in the draw solution. Low molecular weight salts such as NaCl and MgCl2 are often used as draw solutes. Due to the dilution of the draw solution, an additional RO or MD process is used to produce desalinated water and reconcentrate the draw solution15. In MDBR (Fig. 2e, f), the MD membrane allows water vapor to permeate through the hydrophobic membrane. The driving force here is the temperature gradient between the feed and permeate side of the membrane. Hydrophobic MD membranes reject non-volatile TrOCs, while aeration stripping and biological degradation eliminate volatile organic compounds that would otherwise pass through the hydrophobic membrane15.
Asif et al.23 reviewed the fate of TrOCs during wastewater treatment by HR-MBRs. The authors discussed different removal mechanisms as well as influencing factors. However, the authors did not evaluate the different challenges in HR-MBR, their mitigation strategies, and their effectiveness. A 2014 review by Luo et al.15 highlighted some challenges in HR-MBR, including salinity build-up, low permeate flux and membrane stability. However, a significant number of new relevant studies have made it necessary to present the current state of understanding on the topic.
Despite the potential, HR-MBRs have several challenges, including membrane fouling, salt build-up in the bioreactor and incomplete biodegradation (and therefore accumulation) of the rejected TrOCs. Further, although the removal of TrOCs is higher than in conventional MBRs, some TrOCs are still detected in the permeates of high-retention membranes. This review seeks to discuss, in detail, the major challenges associated with HR-MBRs and their effects on the overall performance of the removal of TrOCs, highlight the remediation strategies and assess their effectiveness and feasibility. A brief outline of conventional MBRs followed by HR-MBRs and their configurations are presented. Because most of the available studies only reported the overall TrOCs removal, our review focuses on that; however, the relative contribution of biological and membrane processes will be presented whenever available. The major challenges in HR-MBRs are identified, and their impacts on the gross performance of HR-MBRs in removing TrOCs are discussed. Mitigation strategies are provided for each problem, and their efficacy and feasibility are assessed. Based on the extensive literature review, this work provides knowledge gaps and recommendations for future research to mitigate challenges associated with TrOCs removal by HR-MBRs.
Rejection of trace organic compounds (TrOCs) by HR-MBRs
In recent years, Trace Organic Compounds (TrOCs), including pharmaceuticals, hormones, personal care products, per- and poly-fluoroalkyl substances, disinfection by-products, flame retardants, and plasticisers have been detected in several water sources such as river streams, wastewater, tap water, groundwater, and marine water across the globe24,25,26,27,28. TrOCs may have unforeseen physiological consequences for humans and other organisms, and their toxicity level depends on the compound’s class. Such negative consequences include liver damage, infant weight loss, respiratory problems, neurological disorders, immunodeficiency and reproduction disorders29,30. While some TrOCs degrade quickly and easily, other TrOCs are persistent31. The recalcitrant TrOCs resist biodegradation, sunlight photolysis, and other abiotic degradation processes, and thus the compounds, are expected to be in the biosphere permanently since most of the water treatment process cannot remove them25. Similarly, conventional MBR processes utilizing MF and UF membranes fail to remove TrOCs from wastewater1,2, hence the introduction of HR-MBRs, which have better TrOCs removal efficiency than the individual biological and membrane filtration processes32 and conventional MBRs17. For example, TrOCs have been detected in the draw solution of MF-MBRs at concentrations of 3.8–100.4 ng/L33.
In HR-MBR, TrOCs are removed in the biological reactor as well as in the membrane filtration step and this makes properties of the bioreactor, the high-retention membranes, and the solute properties important parameters. TrOCs removal in the bioreactor is through biodegradation by microbes34 and adsorption of TrOCs onto the sludge35, subsequently retained by the high-retention membranes. Adsorption and biodegradation are influenced by hydraulic retention time (HRT), sludge retention time (SRT), dissolved oxygen (DO), pH, addition of sorbent materials and solids’ concentrations, amongst others. In addition, the physicochemical properties of TrOCs such as charge, molecular structure, hydrophobicity, and molecular interaction force amongst others also play a role in adsorption and biodegradation.
For example, Song et al.36 found that the removal of ketoprofen, primidone, ibuprofen, diclofenac, carbamazepine, gemfibrozil, simazine, atrazine, and diuron in the bioreactor was poor (<30%) and this was attributed to the presence of electron-withdrawing groups, such as chloro, amide, and nitro in their molecular structures37. Further, these compounds were relatively hydrophilic with Log D values lower than 3.2 at neutral pH, thus they poorly adsorbed to the sludge phase. Therefore, their removal mechanism was mainly due to biodegradation. Compounds with low hydrophobicity such as caffeine, sulfamethoxazole, trimethoprim, and amitriptyline were effectively removed in the biological process because of their high intrinsic biodegradability due to the presence of electron donation groups (amine and hydroxyl) in their molecular structure38. Their removal in the biological reactor could reach 70%36. Contrary, highly hydrophobic compounds such as linuron, clozapine, diazinon, triclosan, and triclocarban highly absorb on the sludge and therefore are biodegraded highly. Degradation rates and adsorption of TrOCs in the bioreactor have been recently reviewed by Gu et al.39, where both processes vary for the different TrOCs and are influenced by the properties of TrOCs as well as the bioreactor. In another review, Semblante et al.40 highlighted the role and mechanisms of sorption, biodegradation, and abiotic transformation of TrOCs in conventional wastewater treatment. In the membrane filtration step, the TrOCs are removed through size exclusion41,42, electrostatic interactions43,44 and non-electrostatic interactions42,45, which include hydrophobic interactions, van der Waals interactions and hydrogen bonding46. To achieve high TrOCs removal, the properties of both the high-retention membranes and TrOCs are important. In addition, the chemistry of the feed water and operational parameters also play a role.
The removal of TrOCs by HR-MBRs was recently reviewed by Asif et al.23, who focused on the retention mechanisms and influencing factors. Other publications on the removal of TrOCs by MBRs and HR-MBRs, together with their removal mechanisms, include the works of Zhang et al.32, Lim et al.47, Luo et al.48 and Criscuoli et al.49. Using antibiotics as model TrOCs, Haffiez et al.50 focused on specific bioreactor parameters that affect the biodegradation of organic waste. In another work, Mahlangu et al.51 reviewed the removal of TrOCs by high-retention membrane processes and focused on the removal mechanisms, challenges and opportunities for further improvement.
TrOCs removal by MDBR
The removal of TrOCs by membrane distillation bioreactors (MDBR) is controlled by interactions between the membrane and TrOCs; and this makes the characteristics of TrOCs and membrane important. The organics are retained through charge exclusion as well as exclusion based on the volatility and hydrophobicity of the compounds. MD membranes have pore sizes larger than that of NF/RO membranes and this makes TrOCs removal by sieving irrelevant52. In addition, the membrane porosity play no role in the retention of TrOCs53. Therefore, volatility and adsorption (due to the hydrophobicity of the membrane) are the major TrOCs removal mechanisms. Due to the negative surface charge of MD membranes, negatively charged TrOCs are highly rejected than positive compounds through charge repulsions54. The volatility of TrOCs is the main determining factor for their removal by MD because the mass transfer occurs in the vapor phase. Thus, non-volatile compounds are completely rejected, while volatile TrOCs permeate the membrane leading to poor removal55. Regarding hydrophobicity, it has been reported that hydrophilic TrOCs with low volatility are rejected more than hydrophobic TrOCs with high volatility56. The degradation of TrOCs due to the high feed temperatures (thermal degradation) in MD is another feasible TrOCs removal mechanism. However, this depends on the characteristics of TrOCs and operating conditions57.
TrOCs removal by NF/RO-MBR
According to the solution-diffusion model, the removal of TrOCs by NF/RO membranes is mainly through size exclusion and adsorption where the solutes first adsorb onto the membrane surface, partition into the membrane, and diffuse through the membrane to the permeate side58. Regarding size exclusion, TrOCs that are larger than the membrane molecular weight cut-off (MWCO) are well rejected by the membrane. The size of the TrOCs determines their diffusion through the membrane, and this parameter has been represented by molecular size such as molecular weight, Stokes diameter, molecular width and length, minimum projection area and van der Waals volume59,60. When the TrOCs size is relatively smaller than the pore size of the membrane, adsorption becomes the important mechanism to achieve removal. In this case, TrOCs with high affinity for the membrane are adsorbed onto the membrane surface and partition to the permeate side61. Membrane-TrOCs affinity interactions include electrostatic, polar, hydrophobic, hydrogen bonding and π–π interactions62. Therefore, adsorption is affected by the TrOCs charge, polarity, hydrophobicity, and functional groups. Surface carboxylic functional groups give NF/RO membranes a negative surface charge. Therefore, negatively charged TrOCs are well rejected due to electrostatic repulsions, while positively charged TrOCs are attracted to the membrane surface resulting in poor retention63. TrOCs with high dipole moment are aligned such that their dipole charge is opposite to that of the membrane surface charge, and this promotes their adsorption and lower removal64,65. Hydrophilic TrOCs are rejected more than hydrophobic TrOCs due to the high affinity of hydrophobic TrOCs to adsorb onto the membrane surface66,67. Finally, hydrogen bonding and π–π interactions promote the adsorption of TrOCs onto the membrane surface leading to poor rejection44,68.
TrOCs removal by OMBR
In FO, the transport of water through the membrane is in opposite direction to reverse salt flux (from the draw solution). As a result, reverse salt flux prevents diffusion of TrOCs from the feed side to the draw side leading to higher rejection than in NF/RO69. The removal of non-ionic TrOCs is largely dictated by size exclusion but some deviations have been reported70. Charge interactions also play a role where repulsive membrane-TrOCs interactions results in higher removal. However, at higher draw solute concentration, the effects of charge interactions are canceled because to the suppression of the double layer surrounding a charged solute71. This effect is caused by increase in ionic strength in the feed side due to reverse salt flux at high ionic strength69 For uncharged TrOCs, their interaction with the membrane is not influenced by ionic strength; therefore, size exclusion becomes the important removal mechanism72. Other important TrOCs removal mechanisms in FO are adsorption or dipolar interactions where TrOCs with high dipole moment (e.g., carbamazepine with 3.6 Debye) orientate towards the membrane pore resulting in poor removal73. The removal of TrOCs by MDBR, NR-MBR, OMBR and RO-MBR is presented on Tables 2–5, respectively.
Figure 3 presents a summary of TrOCs removal efficacy by HR-MBR processes. The data is drawn from Tables 2–5 which present detailed information on studies that have reported on the removal efficiency of TrOCs by HR-MBRs. The chemical structures of the TrOCs are presented in Fig. 4. TrOCs removal is based on the removal rates reported by different studies, and the TrOCs are ranked according to biodegradation and/or sorption in the bioreactor (from lowest to highest). Most of the TrOCs are poorly removed in the bioreactor, however, they are well retained by the various membrane processes.
Fouling of high-retention membranes
Classification of fouling types and fouling mechanisms
Fouling remains a major challenge in conventional MBRs and HR-MBRs, whereas, in the former, it is primarily attributed to the deposition of macromolecules that are biopolymeric as well as colloidal and particulate in nature. In the latter, fouling is even more complex due to the additional effects of elevated salinity (a challenge discussed in a separate section). Membrane fouling is classified based on the type of foulants accumulated on the membrane surface. Accordingly, the major fouling types are organic fouling, colloidal fouling, inorganic fouling, biofouling and combined fouling (i.e., fouling by more than one type of foulant). Colloidal, organic, and inorganic foulants in the bioreactor result in colloidal fouling, organic fouling and scaling, respectively; biofilm formation on the high-retention membranes results in biofouling that degrades membrane performance18,19,20,74.
Organic fouling is a term used to describe membrane fouling by natural organic matter (NOM) and polysaccharides omnipresent in raw water. Organic fouling has been widely reported, and the flux decline has been ascribed to increased hydraulic resistance exerted by the fouling layer. Organic fouling is exacerbated by the presence of divalent cations (e.g., calcium), which result in the formation of compact foulant-divalent cation complexes (Fig. 5a) that resemble an egg-box75,76. Although wastewater has lower salt content compared to seawater and brackish water, the high rejection properties of membranes used in HR-MBR result in salinity build-up in the feed. Therefore, the accumulation of divalent cations may exacerbate organic fouling during wastewater treatment by HR-MBRs.
a Organic fouling in the presence and absence of divalent cations and b salt concentration polarization profile in virgin and fouled membranes, adapted from Ju and Hong79. Cf is the concentration in the feed, Cm is the concentration on the membrane surface, and Cp is the concentration in the permeate.
Inorganic fouling is described as precipitation and crystallization or scaling of rejected salts (e.g., magnesium sulfate (MgSO4), calcium carbonate (CaCO3), and calcium sulfate (CaSO4) dehydrate or gypsum) on the membrane surface leading to flux decline77. Due to the high-retention of sparingly soluble salts, their concentration on the membrane surface increases over time, and this raises the osmotic pressure gradient across the membrane leading to flux loss78. This concept, called concentration polarization, has been dubbed the major cause of flux decline in inorganic fouling.
Colloidal fouling refers to membrane fouling by colloids such as silica and aluminum oxide. Interactions between the colloids and membranes control the deposition of colloidal particles on the membrane surface. Colloidal fouling results in flux decline due to hydraulic resistance and cake-enhanced concentration polarization (CECP—a phenomenon where the fouling layer prevents back-diffusion of salts from the vicinity of the membrane surface to the feed leading to accelerated salt build-up on the membrane surface and subsequently a rise in osmotic pressure gradient and decline in driving force (Fig. 5b))79,80,81,82. The cake layer exacerbates concentration polarization effects on flux.
Biofouling occurs through five major steps (Fig. 6), namely: (a) conditioning of the membrane surface, (b) attachment of microbes on the membrane surface, (c) production of extracellular polymeric substances (EPS), which help adhere cells and facilitate attachment of cells onto the membrane surfaces, (d) cell growth and multiplication, and (e) detachment of microbes83.
Stages of biofilm formation on membrane surfaces during waste and wastewater treatment by MD338: a formation of conditioning film, b attachment of microorganisms, c excretion of extracellular polymeric substances (EPS), d biofilm formation, and e detachment of microorganisms. Similar fouling mechanisms occur in FO, NF, and RO processes.
A review by Meng et al.84 discusses the biofouling of low-pressure membranes by polysaccharides and proteins in conventional MBRs and fouling mitigation strategies. However, fouling in low-pressure membrane filtration may differ from that of high-pressure filtration processes due to the lack of hydraulic compaction of the fouling layer85; thus, the work of Meng and co-workers may be relevant to biofouling in OMBR and MDBR but not NF/RO-MBR. Further, the work of Meng and co-workers was centered on biofouling. Wastewater constitutes a mixture of foulants which have different fouling mechanisms and effects. Therefore, this work further elaborates on the different fouling types. However, the work of Meng et al. is still important in understanding biofouling in HR-MBRs when biofoulants predominate in the feed.
Membrane fouling is controlled by membrane properties, foulant properties, and operating conditions discussed by Tang et al.86. Foulant characteristics include foulant type, size and charge82,87; membrane properties comprise surface roughness22,88, charge, hydrophilicity and functional groups, while feed composition and operational parameters involve the concentration of foulant82,89, solution pH90, ionic strength90,91, presence and concentration of divalent cations82,87,90,91,92, initial permeate flux90, cross-flow velocity87,90,93, feed temperature89,93 as well as module design and spacers94,95. The roles of the various factors have largely been investigated using synthetic water. However, there are studies based on real wastewater samples. However, the findings from fouling based on synthetic water are still relevant and provide insights into membrane fouling mechanisms during wastewater treatment by HR-MBRs. In MD, flux decline is mainly due to pore wetting by the contacting solutions, increase in salt concentration in the feed solution and cake layer formation36, while in NF and RO, fouling is mainly due to pore blocking and concentration polarization by rejected salts which lower the driving force. In FO, the reduction in flux is predominantly due to increased hydraulic resistance by the formed cake layer and reverse salt flux which drops the concentration gradient between the feed and draws the side of the FO membrane. Fouling in FO is more reversible than that in high-pressure-driven processes due to the negligible compression of the cake layer85. Figure 7 shows the four classic filtration models (complete pore blocking, standard blocking, intermediate blocking, and cake filtration) widely used to explain flux decline in membrane fouling96,97.
Fouling degrades the membrane and leads to a shorter lifespan. Previous studies have reported that when organic and colloidal foulants co-exist in the feed, fouling is mainly dominated by organic fouling, regardless of the concentration ratio between the different fouling types82. Table 6 summarizes the major fouling or flux decline mechanisms in membrane filtration.
Variation in fouling mechanisms and fouling propensity
From Fig. 2, it can be noted that NF/RO-MBR are pressure-driven processes while OMBR and MDBR are not. Previous studies have reported on variations in the fouling mechanisms and propensity of the different processes. From the different configurations, the fouling propensity to some extent is controlled by membrane surface properties, operating pressure, temperature as well as water transport mechanisms. In MD, the occurrence of large pores containing air and vapor affect fouling due to the effects of the liquid-air interface on the adhesion of foulants and crystal nucleation98,99. Contrary, FO and RO membranes have sub-nanometer pores which are smaller than most foulants. Therefore, foulants do not accumulate inside the membrane pores like in MD99. Although some studies have claimed that MD and FO are less susceptible to fouling than RO due to their low operating pressure100,101, there are some findings that have demonstrated that the effect of pressure on fouling is negligible102. Temperature plays a role in membrane fouling because it affects solubility, crystallization kinetics and concentration polarization of inorganic salts. For organic fouling, high temperatures may affect fouling through denaturation proteins or depolymerization of polysaccharides103. Hydraulic-osmotic pressure difference in FO and RO transports water across the membrane interface. On the other hand, water permeation is driven by vapor pressure difference between the feed and permeate side. In FO and RO, fouling lowers flux through cake-enhanced concentration polarization (CECP). Further, it causes hydraulic drag which reduces the hydraulic pressure. For MD fouling, both CECP and to a lesser extent hydraulic drag reduce vapor pressure. In addition, MD is also prone to cake-enhanced temperature polarization due to the temperature difference in the permeate and feed side of the membrane104.
A study by Siddiqui et al.105 compared organic fouling propensity in FO and RO mode using sodium alginate as model foulant. The authors found that FO was more prone to fouling than RO due to the reduction in the intensity of internal concentration polarization (ICP) and increase in the effective osmotic driving force during fouling in FO mode. However, their findings were contrary to common claim in the literature where conclusions on fouling are based only on the flux profiles. The researchers also found that the specific fouling resistance for FO was greater than that of RO and this was due to the contribution of reverse solute diffusion from the draw solution. Further, there was no evidence of hydraulic pressure compressing the fouling layer and there was greater foulant accumulation in FO than in RO. In another study, the fouling propensity between RO, FO and MD was compared under identical hydrodynamic conditions using calcium sulfate and sodium alginate as model representatives of inorganic and organic foulants, respectively106. There was more flux decline in MD due to scaling after 36 h, while FO resisted scaling and no flux decline was observed. Contrary, there was lesser flux decline in MD due to organic fouling (14%) compared to FO and RO (46–47%). This was ascribed to the high operating temperature raising the diffusion coefficient of foulants, augmenting transport away from the membrane and elevating the critical flux. The results presented in this review indicate that the different process may resists fouling by one foulants while getting fouled by another. Therefore, the extent of fouling depends on the foulant type as well as filtration process.
Tables 7–10 present studies that have reported the fouling of high-retention membranes (MDBR, NF-MBR, OMBR, and RO-MBR, respectively) during wastewater treatment by HR-MBRs and the findings indicate that the type of fouling is the same for the different HR-MBR processes and mainly depends on the composition of the feed. For instance, organic, colloidal, scaling and biological fouling has been reported for all the configurations. However, there are noticeable differences in the flux decline mechanisms and implications of fouling to the process. For example, flux decline in MDBR is associated with membrane wetting as well as thermal and mass transfer resistance. For OMBR, flux decline is ascribed to internal concentration polarization of salts from the draw side. Due to the high pressure applied in NF-MBR and RO-MBR, the flux decline is due to additional hydraulic resistance imparted by the compacted cake layer (for compressible foulants). In addition, cake-enhanced concentration polarization is one major contributor to reduction in flux during wastewater treatment using NF-MBR and RO-MBR configurations.
Fouling has negative effects on the typical design/performance values in the HR-MBR application. Besides the decline in flux, fouling in NF/RO leads to increase in the specific energy consumption in kWh per m3 (kWh/m3) of permeate production due to additional energy required to drive permeation through the fouled membrane. The increase in energy consumption is also due to the resistance to fluid flow through the membrane (because of cake resistance), the friction losses in the retentate and permeate channels of membrane modules and the non-conventional operation of high pressure pumps and energy recovery devices107. For FO and MD, permeation is due to concentration gradient as well as temperature gradient, respectively. Therefore, the effects of fouling on specific energy consumption are less severe in the vapor phase separation process (MD) compared to the liquid phase separation processes (NF and RO). Furthermore, fouling in MD results in immediate failure in membrane separation. Fouling may promote membrane pore wetting which subsequently leads to direct permeation of feed water into the distillate stream and remarkably undermine pollutant removal rate108.
Influence of fouling on TrOCs removal by high-retention membranes
In HR-MBR, the feed from the biological process will impact the membrane filtration step mainly because wastewater contains a blend of microorganisms, organic foulants, colloids and salts. Additionally, the presence of foulants in the feed (wastewater) may either decrease or improve the biodegradation of TrOCs by the microbes in the reactor. For instance, 1 mg/L of humic acid was found to promote the degradation of bisphenol A and oxybenzone (phenolic compounds) while inhibiting the biodegradation of sulfamethoxazole, carbamazepine, and diclofenac (non-phenolic compounds) in direct contact membrane distillation (DCMD) coupled with photolysis109. Further, fouling can influence the performance of high-retention membranes where the removal of TrOCs can either be enhanced, remain unchanged or even decrease (Fig. 8).
Influence of fouling on the rejection of TrOCs by high-retention membranes: a TrOCs removal by NF270 and NF90 membranes before and after fouling with effluent organic matter (ACT acetaminophen, CBZ carbamazepine, ATL atenolol and DTZ diatrizoate)161; b Rejection of primidone by different membranes before and after fouling by effluent organic matter316; error bars present standard deviations.
The effects of fouling on the retention of TrOCs are controlled by the properties of the organics. Hajibabania et al.110 studied the effects of fouling on the removal of organic compounds by NF membranes and found that the consequences were dependent on the properties of the compounds. The removal of ionic compounds including diclofenac (85–90%), naproxen (90–92%) and ibuprofen (92–94%) was not affected by membrane fouling and this was ascribed to electrostatic repulsions between the ionic solutes and the negatively charged membrane surface which prevented adsorption of the compounds on the fouling layer111. Contrary, fouling generally decreased the removal of nonionic hydrophobic TrOCs. This was due to the formation of loose cake layers (because of the vast range of molecular weight of organic matter) where the hydrophobic compounds (e.g., risperidone (95–65%) and fluoxetine (96–80%)) gradually increased in concentration leading to a reduction in their retention. However, the extent of decrease in the rejection of hydrophobic compounds was less severe for highly hydrophobic compounds due to their adsorption onto the foulants in the feed. Fouling greatly reduced the removal of nonionic hydrophilic compounds (caffeine (65–50%), sulfamethoxazole (90–30%) and trimethoprim (98–46%)) due to cake-enhanced concentration polarization which was promoted by the formation of porous fouling layers112.
In another study investigating the removal of TrOCs using a unique ultrafiltration-osmotic MBR, the removal of nonionic and hydrophobic TrOCs by FO membrane was lower compared to the charged compounds. Contrary, the RO membrane rejected almost all nonionic compounds, while hydrophobic nonionic compounds were poorly rejected113. The removal of positively charged and nonionic TrOCs by fouled membranes has been reported to be higher than that of the virgin membrane and this has been ascribed to the fouling layer (organic, colloidal and biological) preventing partitioning of the compounds through the membrane (pore blocking) and adsorption of TrOCs onto the organic fouling layer. Therefore, the presence of foulants in the feed and on the membrane surface can enhance the removal of TrOCs by providing additional sites for adsorption thereby prolonging their time for biodegradation.
In this work, the effect of fouling on TrOCs removal is centered on the membrane filtration step because foulants from the biological process accumulate on the membrane and change the membrane surface properties. However, it is acknowledged that biological activity is affected by the presence of foulants, and the performance of the biological process will have a role in the removal of TrOCs by membranes. For instance, poor degradation of TrOCs in the bioreactor will imply high TrOCs concentration in the feed of high-retention membranes, and this can lead to poor removal due to concentration polarization. The role of the concentration of TrOCs in the feed on their removal by high-retention membranes has been investigated, and both improvements and decline in rejection have been reported for different membranes114, while some researchers did not observe any correlation between the retention of TrOCs and their concentration in the feed115. Reduction in TrOCs removal at high concentrations has been ascribed to charge shielding effects, a concept whereby elevated TrOCs concentration increases adsorption on the membrane surface and raises the TrOCs concentration in the electric double layer. This shields the membrane’s surface charge and blocks membrane-solute repulsive interactions, lowering rejection116. To be more relevant to HR-MBRs with respect to feed water type, the removal of TrOCs from fouling studies investigated using wastewater and synthetic wastewater samples are referenced (Table 11).
Strategies to mitigate fouling in HR-MBRs
Several fouling mitigation strategies have been adopted, with some focusing on fouling prevention, whereas others are applied as corrective measures to restore membrane flux after the membrane has fouled. The following fouling alleviation approaches have been reported: pre-treatment of the feed, membrane cleaning, membrane surface modification to make membrane surfaces resistant, and optimization of operating conditions to delay fouling.
Pre-treatment of feed as mitigation for fouling
In conventional MBR, pre-treatment is performed to lower the levels of total suspended solids. However, pre-treatment is limited to sedimentation or chemical dosage of coagulants, flocculants, or adsorbents, implying that similar pre-treatment approaches would be used in HR-MBR. Chemical pre-treatment involves using coagulants or flocculants such as ferric chloride (FeCl3), aluminum sulfate (alum), or adsorbent agents such as activated carbon. At the municipal scale, sedimentation is normally followed by other pre-treatment approaches such as: i) dissolved air flotation (DAF) then dual media filter (DMF); ii) DMF followed by cartridge filter or iii) DAF followed by sand filtration then cartridge filter117. Regardless of the processes used, coagulation is done before the influent enters the first unit of the treatment process. Subsequently, anti-scalants are added to prevent membrane fouling by minimizing the deposition of scale forming ions on the membrane118. Combining sedimentation with DAF is recommended to obtain desirable water quality especially when the turbidity of the influent increases unexpectedly. Similarly, DMF is preferred over single media filter because it can produce water of high quality. These pre-treatment approaches (which are effective in wastewater treatment at municipal scale) can be integrated in HR-MBR to mitigate fouling challenges. Furthermore, lessons learned during conventional wastewater treatment can be applied in HR-MBR for process improvement.
Due to the excessive fouling propensity of high-retention membranes, sedimentation and chemical treatment may not be sufficient, and therefore other pre-treatment options, such as MF or UF membrane, may be necessary. Different studies, including the works of Uzal et al.119, have shown that MF and UF membranes can remove macromolecules that can potentially foul high-retention membranes, thus reducing membrane fouling120. Nonetheless, due to their large pore sizes, MF and UF membranes do not remove all fouling agents from feed solutions121. This implies that fouling may still occur even with MF/UF pre-treatment. Further, adding MF/UF as a pre-treatment step increases the capital and operational cost of HR-MBR, but the reduction in membrane cleaning and longer membrane lifespan can compensate for this cost. Nonetheless, several UF membranes have been successfully installed and integrated in full-scale water treatment plants since the mid-2000s in various countries such as Singapore, Spain, China, Saudi Arabia and United Arab Emirates122. Therefore, UF membranes could be used as pre-treatment in HR-MBR to mitigate fouling.
Advanced oxidation processes (e.g., pre-ozonation123 and UV-oxidation124) may also be used as pre-treatment for HR-MBR because advanced oxidation processes can break down organic macro- and micro-pollutants. However, such processes are not only high-cost but also deactivate the microorganisms responsible for the biodegradation of TrOCs in the reactor. Pramanik et al.125 found that pre-treatment of anaerobically treated dairy effluent with ultraviolet/persulfate oxidation reduced fouling of FO membranes by decreasing reversible and irreversible fouling, resulting in over 95% flux recovery after three cycles. The use of persulfate as fouling mitigation has also been demonstrated by Asif et al.11, who illustrated a reduced fouling layer on the membrane surface in the persulfate-assisted direct contact membrane distillation system used to treat secondary effluent spiked with a mixture of TrOCs. Persulfate remarkably degraded total organic carbon (70%) and total nitrogen (40%), leading to membrane fouling during wastewater treatment by HR-MBR. Although advanced oxidation processes and other advanced pre-treatment methods are used for dense membrane processes, their practical application in MBR may not be feasible17, thus most of the processes have only been demonstrated at laboratory scale. Figure 9 shows the classification of the reviewed pre-treatment methods based on the cost, efficiency, and associated challenges. From the image, AOPs have been classified as costly, and efficient but have high challenges which are linked to the formation of disinfection by-products and destruction of the microorganisms in the biological process.
Due to the low fouling propensity and energy efficiency of FO processes, fouling of OMBR coupled with RO has been investigated, where the FO served as pre-treatment. The coupled processes are robust, resist fouling and perform better than RO-MBR without the FO process126. The inclusion of the FO step induces additional costs. However, the advantages of incorporating FO compensate for the additional capital and operational costs. Firstly, membrane cleaning is minimized, while membrane lifespan increases due to the reduction in fouling. Secondly, the coupled process can ensure simultaneous wastewater treatment and reconcentration of FO draw, thus maintaining a zero liquid discharge—a benefit for water-stressed countries. Treating diluted FO draw-streams with RO will reduce fouling due to concentration polarization and save the RO process energy because low-pressure NF/RO membranes can be used as the final treatment.
Cleaning to restore membrane flux and performance
Depending on the membrane process, foulant type and extent of fouling, membrane flux (and performance) can be restored by cleaning with deionized water. For instance, Liu et al.93 used deionized water to clean a hydrophobic polytetrafluoroethylene (PTFE) membrane fouled by lithium chloride (LiCl) in direct contact membrane distillation. In another study, Asif et al.2 restored UF and NF membrane fluxes by backwashing with permeate water. The membranes were reversibly fouled by synthetic wastewater containing TrOCs. To ensure a longer membrane lifespan and achieve high cumulative permeate volume while saving energy, periodic cleaning with water is advised than continuous membrane application until substantial fouling is observed127.
Chemical cleaning may be required for compact fouling layers. Membranes fouled by inorganic foulants are cleaned with acidic solutions, while alkaline solutions are used to clean membranes fouled by organic foulants. Chemical cleaning still needs to be optimized to reduce operational costs. Underdosing chemicals and using ineffective chemicals decreases membrane lifespan and increases membrane replacement costs. Similarly, overdosing on chemicals may result in rapid membrane deterioration because most membrane materials are intolerant of high concentrations of cleaning agents128. The cleaning strategies, which include the time for flushing and backwashing, frequency of backwashing, the type and amount of chemicals and chemical cleaning, may depend on the chemistry of the wastewater. Therefore, constant monitoring of the wastewater characteristics becomes key so that the cleaning strategies may be modified accordingly. Observations can also be done by profiling the autopsy of a fouled membrane to determine the key foulants.
There are reports of chemical cleaning changing the physico-chemical properties of polymeric membranes and their separation efficiency. Simon et al.129 investigated the influence of cleaning nanofiltration (NF270) membrane with citric acid, sodium hydroxide, ethylenediaminetetraacetic acid and sodium dodecyl sulfate. Caustic cleaning resulted in a remarkable increase in the membrane surface hydrophobicity and permeability due to the temporal enlargement of the membrane pores. Cleaning with citric acid and sodium dodecyl sulfate resulted in a slight increase in the rejection of carbamazepine, while the rejection of sulfamethoxazole remained unchanged. However, a decline in sulfamethoxazole rejection was noted at pH less than 8 following chemical cleaning. Nevertheless, this effect has been labeled temporary and could be restored by applying acidic cleaning immediately after caustic cleaning130. In another study, Zhou et al.131 found that membrane cleaning with urea-based chemicals resulted in decrease in the rejection of neutral and negatively charged TrOCs by NF270 membrane. However, TrOCs removal by the NF90 membrane was not affected by chemical cleaning (Fig. 10). The effects of chemical cleaning are less significant for negative TrOCs because their rejection is predominantly governed by electrostatic repulsion between the compound and the negatively charged membrane surface. Therefore, their removal is not influenced by any enlargement of the membrane pores132.
Effects of urea-based chemical cleaning on the rejection of TrOCs by NF membranes: removal of neutral (a) and negatively charged TrOCs (b) by NF270 membrane; and removal of neutral (c) and negatively charged TrOCs (d) by NF90 membrane131; error bars present standard deviations.
Membrane surface modification
Recent studies have focused on modifying membrane surface properties to make the membranes repellent to fouling while maintaining high fluxes and TrOCs retention properties42,133,134,135. Depending on the design requirements, the membranes can be more hydrophilic or hydrophobic (for MD purposes). One of the membrane modification techniques is the addition of nanoparticles, including graphene oxide (GO), graphitic boron, zinc oxide (ZnO), titanium dioxide (TiO2), copper oxide (CuO), silica dioxide (SiO2), and many others. For example, Mahlangu et al.42 modified UF polyethersulfone (PES) membranes with GO-ZnO and noted improvement in rejecting 23 TrOCs. Other reports on membrane modification to improve TrOCs removal are the works of Patala et al.136, Ojajuni et al.137 and Kong et al.138, who demonstrated improvement in the rejection of TrOCs. Although the nanoengineered membranes perform better than the pristine membranes, the nanomaterials may leach from the polymer matrix. For example, the leaching of CuO139 and silver nanoparticles140,141,142 has been reported during filtration and membrane cleaning. Nanoparticle leaching may limit the application of nanoengineered membranes because nanoparticles such as Pb, Cd and Hg have been reported to inhibit the growth and enzyme production of white-rot fungus143, while silver is well known for its antimicrobial activity. Tan et al.144 reported that silver nanoparticles caused a remarkable decrease in nitrifying efficiency from 98% to 15% and increased the extracellular polymeric substance content in the activated sludge by 77.8%. Extracellular polymeric substances could promote membrane fouling and lead to more issues with membrane cleaning.
Further, the leaching of nanoparticles has been associated with a decrease in rejection of MgSO4, while hydrophobicity, permeability, surface roughness and porosity increased139. This was in agreement with the findings of Simon et al.129, who studied the influence of chemical cleaning on the physico-chemical properties of an NF270 membrane. Such changes in physico-chemical properties are expected to affect the removal of TrOCs during wastewater treatment.
There are other membrane modification techniques used to improve membrane properties, and they include cross-linking modification145, tuning interlayer spacing146, and graft polymerization147. Adding nanomaterials improves the membrane’s structural and chemical properties resulting in thin-film nanocomposite membranes with enhanced properties compared to their respective pristine thin-film nanocomposite membranes148. In graft polymerization, polyethylene glycol is used as a hydrophilic filler to improve the membrane surface for fouling resistance. The main challenge with polyethylene glycol is its stability in complex media where polyethylene glycol degrades via auto-oxidation149. Therefore, it may be suggested that studies on graft polymerization utilize polymers with both positively and negatively charged functional groups to obtain membranes with a net neutral surface charge. Other emerging strategies to build multifunctional coatings and engineered materials include biomimetic coating with mild and versatile merits, sequential infiltration synthesis as well as atomic layer deposition with precise and controllable features. Yang et al.150 prepared catalytic-cleaning antifouling membranes using a prebiotic-chemistry-inspired aminomalononitrile (AMN)/Mn2+-mediated biomimetic mineralization technique. The biomimetic mineralized membranes fouled less during oil-in-water emulsion separation (with 99.8% flux recovery rate) and exhibited excellent in situ regeneration efficacy. Atomic layer deposition and/or sequential infiltration synthesis allows for the regulation of material properties to obtain thin films of desirable thickness, stoichiometry as well as physical and chemical properties using atomic engineering151. In a more recent work, Yang et al.152 demonstrated unprecedented formation of acid-tolerant ultrathin membranes with finely tuned sub-nanopores for energetic-efficient molecular sieving. The membranes were prepared from polyurethane (PU)-based materials and the highly crosslinked structure and unique electron-absorbing ability enabled the novel membranes to withstand strong acidic conditions. The membranes have great potential for energy efficient environmental remediation and resource recovery. Although a lot of work has been done to improve membrane properties, there is still little information regarding the performance of the modified membranes using real wastewater. For commercial membranes, there is sufficient information on the ideal/optimal operating conditions that has helped to build consumer confidence, while such information is still lacking for the newer generation membranes. Further, some modification techniques are costly, not green (i.e., use toxic chemicals) and hard to reproduce.
System optimization and operating conditions
Optimizing the design configuration and operating conditions can also reduce membrane fouling. For instance, Hai et al.153 placed a bundle of hollow fibers within a non-woven coarse pore to prevent the direct deposition of macromolecules onto the membrane surface. Further, the authors optimized operational parameters such as temperature, pH, applied Hydraulic Retention Time (HRT) and flux to reduce fouling and maintain high performance over a prolonged time. When tuning operational temperature to alleviate fouling, it must be considered that the performance of MBR regarding removing TrOCs is influenced by temperature. For example, Hai et al.154 studied the removal of TrOCs by MBR under temperature variation and found that operating under 45 °C significantly influenced the removal of certain less hydrophobic micro-pollutants possessing strong electron-withdrawing functional groups.
On the one hand, the removal of most hydrophobic compounds was stable when the temperature was maintained between 10–35 °C. On the other hand, their removal deteriorated when the temperature increased to 45 °C. Plattner et al.56 studied the role of temperature (40, 55 and 70 °C) on the removal of pesticides by a commercial hydrophobic PTFE flat sheet membrane in membrane distillation and observed a decrease in rejection at high feed temperature. This was more noticeable for dichlorvos, while the removal of atrazine and parathion-methyl declined slightly. Furthermore, elevated temperatures are believed to result in the thermal expansion of the active membrane layer and an increase in both membrane pore size and the diffusion coefficient of the TrOCs155. Some studies have reported probable distortion of the microstructure of MD membranes at high temperatures. Saffarini et al.156 studied the effect of temperature variation (25–70 °C) on microstructure and stability of commercial PTFE membranes in relation to the liquid entry pressure (LEP) under MD conditions. Differential scanning calorimetry (DSC) measurements displayed potential structural modifications resulting in the relaxation of internal stresses in the interconnected continuous fibrils of the expanded PTFE membranes. This microstructural evolution was due to the distortion of fibril, gap and node as the temperature was elevated.
Further, it was found that the membrane pore diameter increased with temperature. Similar observations of the pore size of NF membranes becoming larger as the feed temperature was raised were reported by Xu et al.157. Their work investigated the influence of temperature on the retention of pharmaceuticals and personal care products (PPCPs) by NF membranes. In another work, the removal of TrOCs decreased with an increase in temperature158, and the authors reported that the pore size of an NF membrane depended on the feed solution temperature. The effective pore radius increased from 0.39 to 0.44 nm when the feed temperature was raised from 20 to 40 °C. Long-term effects of the temperature variations on pore or void structure, membrane flux and TrOCs removal have not been well investigated and require systematic investigations.
Therefore, there must be a balance where fouling is kept at a minimum while high removals are prioritized because fouling can be mitigated by simple backwash (depending on the fouling type), while poor removals will require additional treatment steps that may be costly. Studies have already shown the role of operating conditions on the removal of TrOCs, and the governing mechanisms explaining the removal trends have been reviewed by Mahlangu et al.51 and Khanzada et al.52, amongst other researchers. For example, the following has been reported: i) elevated calcium concentration reduced the removal of halogenated acetic acid by NF270 membranes159. The presence of divalent cations in the feed (calcium concentrations of 5 and 10 mM) improved the rejection of positively charged solutes and decreased the rejection of negatively charged TrOCs by NF membranes due to shielding of the surface charge, while the removal of neutral TrOCs was not affected63; ii) NF270 membrane had poor removal of perfluoro carboxylic acid at low pH, while the removal of the same compound was improved for NF90, XLE, BW30, and SW30XLE membranes160. The removal of sulfamethoxazole, carbamazepine and ibuprofen by NF270 decreased when the pH was changed from pH 10.5 to pH 3.5161; iii) increasing the pressure from 10–20 bar resulted in a 15% decline in the removal of estrone162, while other studies noted improved rejection with increasing pressure but up to a limit163; iv) the rejection of TrOCs increase with flux but at high flux rejection may decline due to the effects of concentration polarization164; v) the rejection of neutral and positively charged solutes (benzotriazole, hydrochlorothiazide, carbamazepine, metoprolol, trimethoprim, and sulpiride) by NF membranes (VNF1 and VNF2) decreased with an increase in temperature from 5 °C to 25 °C, while the removal of negatively charged TrOCs was not affected due to the presence of stronger electrostatic repulsions between the negatively charged compounds and NF membrane surface157; and vi) the presence of macromolecules (foulants) may result in membrane fouling and have various effects on TrOCs retention (Table 11).
The choice of HR-MBR process and enzyme in the bioreactor can also be used as a fouling mitigation strategy. Regarding the choice of the HR-MBR process, the idea is to adopt a system that would have an inherently low membrane fouling tendency, in this case, OMBR. Concerning the option of enzymes, Tufail et al.165 used horseradish peroxide enzymes to reduce fouling in an enzymatic membrane distillation bioreactor coupled with a membrane distillation process. The authors observed lesser fouling of the MD membrane (28%) after 240 h for the enzyme-assisted MBR compared to the conventional MBR without adding horseradish peroxidase (50%) and emphasized the importance of the choice of enzyme in enzymatic MBR. To the best of our knowledge (on writing this review), a comparison of different enzymes’ efficiency in reducing membrane fouling in HR-MBRs has not been reported.
The onset of fouling can be delayed by operating below the critical flux, a benchmark flux at which membranes can be operated without remarkable fouling, whereas filtration above the critical flux results in aggravated fouling. The critical flux concept has been used to control membrane fouling in MF166, UF167, NF168,169, RO170 and FO171. Therefore, the same idea can be applied to HR-MBRs to prevent membrane fouling. However, operating at critical flux may not produce enough permeate to meet consumer demand. Further, the rejection of TrOCs at lower fluxes could be compromised due to the dependency of the rejection of TrOCs to flux172,173,174. Table 12 presents studies that have used various strategies to mitigate the fouling of high-retention membranes. These approaches include pre-treatment, introduction of advanced oxidation processes, cleaning of the fouled membranes, modification of the membrane surface and optimizing operational parameters. Some of the pre-treatment methods are filtration using MF/UF membranes175 and activated carbon adsorption176. Advanced oxidation processes such as Fenton processes177, photooxidation178, electrochemical coagulation179 and sonication-assisted filtration180,181 have shown great potential in reducing membrane fouling. In photooxidation, UV irradiation is used to degrade compounds that can potentially foul the membrane. Electrochemical coagulation involves the application of an electric field coupled to a low voltage to reduce membrane fouling through repulsive interactions between the membrane and charged foulants such as natural organic matter, colloids, and bacteria. Sonication or ultrasound-assisted filtration involves the irradiation of ultrasound (cavitation) to detach foulants from the membrane surface. Membrane cleaning can be performed through chemical cleaning or physical cleaning (e.g., hydraulic flushing and osmotic backwashing). Membrane fouling can also be delayed by fine-tuning the operational conditions. Optimization of operational conditions ensures that the conditions are not favorable for fouling to take place. For example, intermittent aeration removes loose foulants on the membrane surface thus preventing cake build-up182. Membrane surface modification is another method adopted to reduce the affinity of foulants for the membrane surface (i.e., reduction in hydrophobic interactions). Furthermore, the modification aims at making membrane surfaces smoother thus preventing attachment and accumulation of foulants on the membrane surface. Adoption of the fouling mitigation strategies (currently implemented in municipal wastewater treatment) can improve the technical feasibility and the economic viability of HR-MBR which is envisaged to produce high quality effluent compared to conventional biological treatment coupled with MF/UF and advanced oxidation processes.
The different cleaning strategies have various effects on NF/RO, FO, and MD processes. For example, repeated chemical cleaning to remove deposited sediments on the surface of MD membranes can lead to surface wetting and polymer degradation183. Polymer degradation is also possible for pressure-driven membranes, however, the effect on membrane performance may not be as severe as the effects of pore wetting which could lead to immediate failure in MD. Residual deposits may remain after cleaning. In NF/RO and FO, the effects would be lower flux recovery, while in MD the residual deposits may lead to premature wetting creating hydrophilic bridges and/or becoming nucleation sites thus promoting scaling. Cyclic cleaning has different effects to MD compared to pressure-driven membranes, where in NF/RO high permeate quality and module performance are restored after each cleaning cycle whereas in MD there is degradation of permeate quality with each cycle due to pore wetting.
Generally, FO has more fouling propensity than NF/RO and MD due to cake-enhanced concentration polarization. However, physical cleaning is more efficient in FO and less efficient in MD due to lesser reversible fouling in MD. Jang et al. found that the flux recovery after cyclic cleaning was higher for FO and RO and lowest for MD184. This observation was ascribed to stronger hydrophobic MD membrane-foulant interactions, whereas FO and RO membrane surfaces are hydrophilic.
Several pre-treatment steps are needed to prevent fouling in NF/RO processes. Contrary, a single pretreatment step may be enough to maintain stable operations in MD. This implies that although MD has higher fouling propensity, it could still be more easier, cost-effective and environmentally friendly to manage than NF/RO and FO185.
Salinity build-up in HR-MBRs
Salinity build-up: causes and effects on the removal of TrOCs
HR-MBRs are severely affected by salinity build-up in the bioreactor due to the membranes’ high salt rejection capabilities and reverse salt flux in OMBR. The impact of salinity on the performance of HR-MBR has been reviewed by Lay et al.17. The effects of salt accumulation were not linked to the removal of TrOCs but to physiological, microbial and membrane aspects, which are mutually engaged in the removal of TrOCs.
Elevated salinity in the bioreactor affects the physical and biochemical properties of microbes, and this is detrimental to the microbial community as it deteriorates or inhibits biological activity186,187,188 by reducing the diversity of microbial colony189 and cell plasmolysis48. In OMBR, salinity build-up decreases the effective driving force due to increased osmotic pressure in the feed solution, resulting in low permeate flux. Further, the high salt concentration in the bioreactor can damage membranes and reduce performance and lifespan190. Salinity inhibits or slows down microbial kinetics leading to lower growth yield and higher endogenous decay of halophobic bacteria in the bioreactor17,189. However, it is believed that the bacterial population may acclimatize to the elevated salinity environment by rapidly reproducing halotolerant or halophilic bacteria126,191.
There are contradictory reports regarding the influence of increasing salinity on microbial diversity. Luo et al.191 found similar α-diversity metrics in the bioreactor of the control and saline-MBRs implying that salinity did not affect microbial diversity. This was attributed to the succession of halophobic microorganisms by halotolerant or halophilic bacteria as the bioreactor salinity increased. However, hierarchical clustering showed noticeable differences in bacterial community structure between the control and the saline-MBR systems. The elevated salinity was believed to promote the development of different bacterial communities.
Further, there was a reduction in the abundance of various bacterial phyla (including Planctomycetes, Verrucomicrobia, Bacteroidetes, Armatimonadetes and Gemmatimonadetes) in the saline-MBRs. The effect of salinity accumulation on microbial activity depends on the type of microbes and anions in the feed. Chapple et al.192 used 15 cations and anions from common inorganic salts to show that the enzymatic activity of laccase was not inhibited by monovalent cations such as Na+, NH4+ and K+. In contrast, multivalent cations (e.g., Mg2+) had variable effects from negligible to complete inhibition of both enzyme activity and the degradation of TrOCs.
Reports of high salinity increase the concentration of soluble microbial by-products and extracellular polymeric substances193,194. Extracellular polymeric substances result in inefficient oxygen transfer and elevated viscosity126. Thus salinity build-up may aggravate membrane fouling, leading to poor fluxes195 and shorter membrane lifespan, resulting in membrane replacement when the membrane life is spent187. Salinity build-up may not only change the microbial diversity and aggravate fouling, but it may also decrease the overall removal of TrOCs. Wijekoon et al.10 noted a decrease in the biodegradation of TrOCs with electron-withdrawing functional groups (carbamazepine, triclosan and atrazine) due to salinity build-up in a membrane distillation thermophilic reactor. This was attributed to the unfavorable physical and chemical parameters for oxygen transfer, density, turbidity, viscosity, salt precipitation, solute interaction, and colloid chemistry at elevated salt concentrations. Such changes affect the biological activity of nitrification bacteria17 and lead to lower degradation of carbamazepine and other nitrogenous compounds that nitrifying bacteria remove48,196.
There are more constraints of salinity build-up on removing biological nutrients (i.e., nitrogen and phosphorus removal by nitrifiers, denitrifiers and phosphorous accumulating organisms). These microorganisms are affected by increasing salinity, with denitrifiers being the most salt tolerant, while phosphorus-accumulating organisms are the least tolerant197. Uygur and Kargi197 observed a continuous decline in phosphorus removal efficiency with salt accumulation, and this was attributed to the increase in the microbial cells’ osmotic pressure, which diminishes their phosphate accumulating capability. Similarly, nitrifiers are also impacted by salinity build-up. However, it is not clear whether nitrite oxidizers are more susceptible to salt effects than ammonia oxidisers198,199. A decrease in ammonia removal due to salinity build-up has been reported and ascribed to a loss in biological activity200,201.
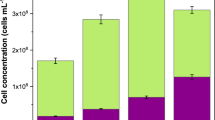
The effects of salinity build-up on the rejection of TrOCs during wastewater treatment have not been well investigated. However, the performance of biological treatment for wastewater under salty conditions (30–200 mg/L NaCl) has been reviewed by Luo et al.15, who linked salt build-up to the removal of total Kjeldahl nitrogen and chemical oxygen demand. Halophilic microbes removed 63–95% of chemical oxygen demand and 20–96% of total Kjeldahl nitrogen from high-salinity wastewater. In another study, Tay et al.202 did not find any impact of salinity build-up on the performance of NF-MBR as the system had similar biodegradation efficiency as well as excellent organic (>97%) and ammonia removal (>98%) at low and high salinity conditions. However, salt accumulation aggravated membrane fouling. Although these studies did not investigate TrOCs removal, the performance of the halotolerant microbes under high salt concentrations encountered in these works still provides useful and relevant information for our objective of relating high salt effects on removal of TrOCs by HR-MBRs. Salinity build-up may reduce the removal of TrOCs by promoting fouling, thus decreasing flux due to cake-enhanced concentration polarization effects81. To some extent, the rejection of solutes, including TrOCs by RO and NF membranes, is controlled by flux as predicted by the solution-diffusion model46,58. Based on the the van’t Hoff equation (\(\pi =\sum ({v}_{i}{c}_{i}{RT}/{M}_{{wi}}\); where π is osmotic pressure, \({v}_{i}\) is factor for mole increase due to dissociation of the dissolved salts, \({c}_{i}\) is concentration in g/L, R is resistance, T is temperature and \({M}_{{wi}}\) is molecular weight), at 50 g/L NaCl, the osmotic pressure is greater than 40 bar17, and this will affect flux in RO (flux decline) unless the applied pressure is increased, leading to more operational costs. Further, this salt concentration and osmotic pressure would be problematic in OMBR, which operates on an osmotic pressure driving force. Linking the retention of TrOCs to membrane flux, Mahlangu et al.163 found that carbamazepine and NaCl rejection by fouled NF270 membranes decreased with flux due to fouling and cake-enhanced concentration polarization. However, fouling may also promote the removal of TrOCs by high-retention membranes (Table 11), but if the biological activity is compromised, the problems with TrOCs in our ecosystem will be transferred from water treatment to sludge management (unless halotolerant microbes are used). In OMBR, the removal of low molecular weight TrOCs may be affected by a decline in microbial activity because their elimination depends mostly on biological degradation186. Luo et al.48 investigated the impact of salinity build-up on MBR’s performance regarding removing TrOCs, nutrients and biomass characteristics. They noted that salt accumulation decreased the removal of hydrophilic TrOCs, organic carbon and nutrients by MBR, while the removal of hydrophobic TrOCs was unaffected (Fig. 11).
Effects of salinity build-up on the removal of TrOCs by MBRs48; error bars present standard deviations.
In OMBR, salinity build-up is also due to the reverse salt flux of solutes from the draw solution across the membrane interface to the feed side (bioreactor)203. Reverse salt flux results in the loss of the draw solute. Draw solutions made from divalent cations such as MgCl2 and MgSO4 result in lesser reverse salt flux compared to draw solutions prepared from monovalent ions such as NaCl and KCl. This is due to divalent ions’ lower diffusion coefficient than monovalent ions. In addition, to reverse salt flux, OMBRs have problems associated with the contamination and dilution of draw solution, and this reduces the osmotic driving force leading to lower fluxes. Dilute draw solutions are reconcentrated using desalination techniques such as RO and MD, but this adds to the capital and operational costs. However, the reconcentration of draw solutions eliminates the challenges associated with brine disposal in NF/RO and MD systems. Thus, coupling OMBR with NF/RO or MD would be more beneficial, except that desalination processes also reject other solutes besides salts, and these concentrated solutes may contaminate the draw solution and change its chemistry.
Besides the effects of salt build-up on the biological process, the membrane processes can also be affected. In pressure-driven processes (NF/RO), salt build-up leads to permeate flux decline through concentration polarization. Similarly, in FO the accumulation of salts in the feed (due to reverse salt flux) may lower the concentration gradient leading to the reduction in permeate flux. However, unlike NF/RO processes, salinity build-up can be prevented by changing the draw solute. In MD, salt build-up could lead to rapid flux decline due to crystal deposition and scale formation on the membrane surface and this reduces membrane permeability. Generally, the main issue in MD is temperature polarization which affects crystallization. Therefore, controlling temperature polarization may reduce the effects of salt build-up on the performance of the membrane. In MD, there are two main challenges associated with the treatment of high salinity feed streams. The first problem is attachment of fouling agents onto the hydrophobic membrane surface leading to blockage of the membrane pores, and consequently cause significantly reduced water vapor flux. Secondly, when MD is used to treat saline water containing amphiphilic molecules, the hydrophobic tails of the amphiphilic molecules attach onto the hydrophobic membrane pore surface, leaving the hydrophilic head exposed and eventually rendering the membrane pores hydrophilic108. This eventually promotes wetting of the membrane pores leading to immediate failure in the separation performance of the membrane. Salinity build-up together with membrane fouling impact energy efficiency in NF/RO processes, while they result in immediate failure in membrane separation in MD.
4.2. Alleviating salinity build-up and reverse salt flux in HR-MBRs
4.2.1. Maintaining optimum concentration factor
The concentration factor can be loosely defined as the concentration increase of inorganic salts in the bioreactor and can be used to control and optimize salinity build-up in HR-MBRs17. Since the concentration factor is related to the sludge retention time and hydraulic retention time, maintaining a lower concentration factor can reduce salinity build-up, which also implies lower water recovery15. As Lay et al.17 recommended, the optimum concentration factor should be 10–30, corresponding to 5–15 g/L total dissolved solids (assumed for wastewater with total dissolved solids of 500 mg/L). These values were estimated for 10–30 days of sludge retention time and hydraulic retention time lower than 1 day.
The frequent withdrawal of the sludge can also control salinity build-up. However, this may not be ideal for HR-MBRs, which need to operate at longer sludge retention time. Further, periodic sludge withdrawal may disturb the biological media and lead to other problems associated with sludge management. Sludge treatment is not a focus of this work. However, details on sludge treatment and handling can be found in the work of Zhang et al.204.
Intermittent filtration with MF or UF membranes
Intermittent filtration with MF/UF membranes may be performed to control salinity build-up in HR-MBRs. An additional MF/UF is incorporated in this process, and liquid media is ejected periodically through the MF/UF membranes205. This process not only removes salts (due to poor salt removal capabilities of MF/UF membranes) but also reconcentrates the microbes rejected by the MF/UF membranes, which is expected to maintain high biodegradation activity. Holloway et al.206 used a unique strategy to mitigate salt accumulation where an ultrafiltration membrane was applied parallel to a forward osmosis membrane in the same OMBR. This approach ensured low salinity was maintained in the activated sludge (implying uninterrupted microbial activity).
The recovery of nutrients was not compromised, and membrane fouling was prevented (by reducing the concentration of cations that exacerbate fouling). These benefits may outweigh the additional capital, operational and maintenance costs associated with incorporating MF/UF process in HR-MBRs to control salt concentrations in the bioreactor. A similar approach of periodic MF extraction for nutrient recovery and salinity maintenance in OMBR coupled with RO was adopted by Luo et al.207. The authors made similar conclusions to the works of Holloway and co-workers. Since acclimation of microorganisms is sensitive to fluctuations in salt concentration, intermittent MF ensures operation at constant salt concentration, thus allowing the system to be operated at longer sludge retention time. The gradual accumulation of extracellular polymeric substances must be monitored under such circumstances to ensure negligible fouling. However, a remarkable increase in extracellular polymeric substances is not envisaged under low salinity conditions.
Optimization of draw solute in OMBRs
Reverse salt flux in OMBRs can be reduced by using divalent ions in draw solutions, bearing in mind that water fluxes from such solutions would be lower than those of draw solutions prepared from monovalent ions at similar osmotic pressure188,208. Similarly, salts containing sulfate ion (SO42−) may produce hydrogen sulfide and sulfuric acid, resulting in scaling that fouls the membrane209. Exacerbated fouling with the sulfur build-up was reported by Song et al.210, and this was ascribed to the release of carbohydrates and proteins (constituents of soluble microbial by-products and extracellular polymeric substances, respectively) at elevated sulfur concentrations. As a result, membrane fouling occurred through cake formation and pore blocking211. Further, the build-up of sulfur has been associated with a decrease in the removal of hydrophilic TrOCs210. The extent of reverse salt flux can be reduced by adding small concentrations of salts having divalent or organic ions (e.g., magnesium chloride and sodium acetate) to draw solutions prepared from salts having monovalent ions (e.g., NaCl)208,212.
Using ammonium carbonate (NH4HCO3) is another option to reduce salinity build-up in OMBRs because nitrification and denitrification processes in the bioreactor remove both ammonia (NH3) and carbon dioxide (CO2). Although this may be a solution, a decrease in the pH of the activated sludge may occur due to the fast diffusion of CO2 across the membrane interface to the bioreactor, affecting nitrifying microorganisms at lower pH213. Organic draw solutions such as glucose, glycine and sodium acetate have been proposed to reduce the intoxication of microorganisms and fouling due to inorganic scaling. Organic draw solutions have lower reverse solute flux compared to inorganic salts. Further, their escape from the draw solution to the bioreactor would not affect microbial activity since they are biodegradable214. However, their main challenge is lower fluxes due to lower osmotic pressure.
Alleviating losses in biological activity
Losses in biological activity due to salinity build-up can be mitigated or compensated for by adopting one of the two strategies: acclimation and inoculation of halophilic or halotolerant microorganisms, where the former is suggested for salt concentrations up to 30 g/L. At the same time, the latter is recommended for salt concentrations of 30–150 g/L17. Due to the extremely longer time required for acclimation, the inoculation of halotolerant microorganisms may be a better option215. However, the challenge here is to get the right type of microbe according to the salt range17. Luo et al.189 found that halotolerant bacteria (such as Methylibium, belonging to the family Comamonada ceae) proliferated and became more abundant with the increase in salinity from 0.4 to 13.3 mS/cm in the bioreactor permitting continuous biodegradation of pollutants. It must be noted that some halotolerant microbes have a threshold salt concentration limit17. For instance, some genus from the family Cytophagaceae increased up to 45.5% when the feed electrical conductivity was elevated to 11 mS/cm but decreased to 32.6% as the conductivity of the mixed liquor was raised further189. The concept of halotolerant microbes in HR-MBRs has not been well investigated. Therefore, more research is needed to understand all the influencing factors better.
Incomplete biodegradation and removal of TrOCs
Incomplete degradation of TrOCs in the bioreactor
Conventional MBRs use bacteria which have limitations to degrade TrOCs completely. This leads to the build-up of rejected TrOCs in the bioreactor and transfers the problem to sludge management. In HR-MBRs, partial biodegradation may increase TrOCs concentration in the feed, potentially leading to their poor rejection by high-retention membranes114. Insufficient biodegradation of TrOCs can be mitigated by making biological agents stronger. This has motivated the introduction of the concept of enzymatic membrane bioreactors where enzymes such as laccase have shown better performance in degrading TrOCs than conventional bacteria216,217,218,219. Although complete degradation of some compounds is achieved, recalcitrant TrOCs still resist complete degradation by enzymes hinting at the need for further improvement. This has prompted redox-mediators’ inclusion in enzymatic MBR to enhance biodegradation further220,221,222.
Another option to improve the biodegradation of TrOCs is to choose microorganisms with high activity in degrading the target TrOCs. In a recent study, horseradish peroxide enzyme was used in an enzymatic MDBR and degradation greater than 99% was achieved for some antibiotics165. Horseradish activity was improved further when hydrogen peroxide (H2O2) was added. The performance of different microorganisms on the same feed may need to be compared for the accurate selection of appropriate microorganisms. Newer research studies have investigated the use of an additional concentrate treatment step utilizing advanced oxidation processes. The works of Tufail et al.109 investigated the removal of TrOCs by an enzymatic MBR incorporating a membrane distillation process coupled with UV photolysis. The photolysis step was used to degrade the concentrate from the MD process, and some TrOCs, such as sulfamethoxazole, bisphenol A and diclofenac, were completely degraded, thus solving the challenges of incomplete degradation in the bioreactor.
The enzymatic activity of ligninolytic enzymes and their catalytic efficiency is inhibited by some organic and inorganic compounds derived from wastewater. For example, oxalic acid and ethylenediaminetetraacetic acid at 1 mM inhibit white-rot fungus and their extracellular enzymes223. A detailed review of the mechanisms and factors controlling the interactions of interfering compounds with microbes and their enzymes can be found in the literature143. It is important for future research to fully characterize all the parameters that affect enzymatic activity so that complete biodegradation of TrOCs can be achieved in enzymatic MBRs without the need for additional expensive processes.
Incomplete rejection of TrOCs by membranes
Although HR-MBRs perform better than conventional activated sludge and MBRs, incomplete removal of TrOCs has been reported47,210,216,224. TrOCs in the permeate of the high-retention membranes (though at a lower concentration) and biodegradation by-products may pose a health risk to humans. To ensure the complete removal of toxic TrOCs, the latest research trend has focused on coupling HR-MBRs with other techniques, such as advanced oxidation processes. The advantage of such techniques is that they enhance the biodegradability index of pollutants by decomposing complex compounds, which in turn increases.
García–Gómez et al.225 coupled MBR with electro-oxidation (an advanced oxidation process). They achieved almost complete removal of carbamazepine (a recalcitrant compound) through the oxidation of carbamazepine with the hydroxyl radicals (*OH) formed near the high voltage O2-overvoltage anode. Electrocoagulation processes have also been combined with MBR processes to enhance the removal of TrOCs while reducing membrane fouling by increasing floc size and particle polarization due to electrocoagulation and the applied electric field. Improvement in removing acetaminophen, clarithromycin, and carbamazepine was achieved in an MBR-electrocoagulation coupled process226. Another example of utilizing an advanced oxidation process is incorporating a bio-electrochemical system to target TrOCs that are not easily degraded by microorganisms. In this process, the TrOCs are transformed into intermediate products that are then biodegraded by the microorganisms. The concept of coupled membrane bio-electrochemical reactor has already been reported to improve the removal of sulfamethoxazole by 21%227. Ultraviolet (UV) photolysis is another advanced oxidation process that can be incorporated into HR-MBRs. A study on removing TrOCs by an enzymatic MBR preceding UV photolysis reported complete degradation of the contaminants while producing fewer by-products9. These findings demonstrated that advanced oxidation processes could be used as a polishing step for TrOCs that permeate through the membrane during wastewater treatment by HR-MBRs. The addition of persulfate in the bioreactor seems to improve the degradation of TrOCs. A study focusing on the persulfate oxidation-assisted membrane distillation process found that persulfate-assisted direct contact membrane distillation removed more than 99% of all the investigated TrOCs. In comparison, the control without adding persulfate had lower removals (86–99%)11.
Although advanced oxidation processes successfully degrade TrOCs, their effectiveness may be negatively impacted by the presence of halogens in the feed. For instance, bicarbonate ion inhibits the photodegradation of sulfamethoxazole, while diclofenac degradation is reduced in the presence of chloride and nitrate ions228. Further, most advanced oxidation processes are costly and may produce degradation by-products that may be more toxic than the parent compound109.
Capital and Operational cost
Another challenge with HR-MBR is high cost associated with capital, operational and maintenance of the biological and membrane processes. The cost of each process is controlled by several parameters such as plant capacity, power requirements, plant configuration, chemistry of the feed, labor and treatment requirements amongst others229. There is limited information on the peer-reviewed information on capital expenditure (CAPEX) or cost of biological processes as well as high-retention membrane processes, while there is focus on energy demand. In wastewater treatment, energy demands contribute to 27–34% of the operational expenditure (OPEX) for treatment and aeration energy230 in a large MBR (19–49 megaliters per day, capacity). Working at full capacity helps lower OPEX associated with aeration by minimizing the specific aeration demand while maximizing flux. Further, the energy for mixing and biological aeration can be reduced by operating at low solid concentration. Based on the design capacity, the capital cost of MBR with flow capacity of 100, 500 and 2 500 m3/d is estimated at 0.6, 0.48 and 0.38 $/L/d, respectively; while the operational costs are approximately 0.58, 0.45 and 0.4 $/m3 for 100, 500 and 2500 m3/d plants, respectively230. Interestingly, small plants have higher energy demands. For example, a 100 m3/d plant has specific energy demand of about 2 kWh/m3 while a 2500 m3/d plant requires half of that. DeCarolis et al.231 performed cost analysis of MBR plants supplied by Koch Membrane Systems, Huber Technologies Inc., Parkson Corporation and Kruger Inc with design capacities of 4 000 to 20 000 m3/d. The estimated CAPEX ranges from $7 990–$9 850 for a 4 000 m3/d plant and $32 270–$38 790 for a 20 000 m3/d plant. The yearly total OPEX was estimated at $3 350–$4 649 and $14 974–$20 344 for plants with design capacities of 4 000 and 20 000 m3/d, respectively.
High-retention membrane processes are also associated with high CAPEX and OPEX. In the literature, the cost of high-retention membranes is presented for desalination technologies, while this work focuses on wastewater treatment where the salt content is lower. Therefore, the operational costs are expected to be lower than those of brackish and/or seawater desalination plants using high-retention membranes. The CAPEX for RO plants with treatment capacity of 1 000 m3/d to 290 000 000 m3/d ranges between 0.1 and 293 million US$, while the OPEX for the same plants is between 0.2 and 1.2 US$/m3. Such plants have total energy requirements between 0.5 and 4.0 kWh/m3. NF plants with design capacity of 2 400 to 100 000 m3/d require 1–17 million US$ to install and OPEX of 0.16 – 0.30 US$/m3. Their energy requirements range from 0.01–2.4 kWh/m3. MD plants are assumed to have lower capital cost than NF/RO due to the use of inexpensive plastics as construction material232. Khan et al.233 performed a techno-economic assessment of MD integrated to an anaerobic digestion biogas plant. The plant with design capacity of 190 m3/d in full-scale operation had CAPEX of about 375 000 US$ with thermal energy demand of 22 MWh/d. Further, the maintenance and operating cost was 375 700 US$/year for the MD. External thermal energy costs contributed about 90% of the total maintenance and operating expenditure. Installation of FO is also costly. However, CAPEX makes up a large part while operating and maintenance costs contribute a small fraction to the total cost234. According to Le and Nunes235, high CAPEX is associated with membranes that have low power density due to the requirements for huge membrane area and high membrane installation costs. A membrane with a power density of 1 W/m2 has a capital cost of 20 000 US$/kW but the capital cost of a membrane with power density of 5 W/m2 would be 4 000 US$/kW236.
Feasibility assessment and effectiveness of control strategies
Table 13 summarizes the challenges and mitigation strategies in HR-MBRs. The feasibility and effectiveness of the control strategies are also assessed. The proposed solutions appear effective in mitigating problems associated with HR-MBRs; however, their main common drawback is the high cost which would make them unsustainable. Further, some of the processes still need further investigation for improvement before commercialization.
Research gaps and future directions
This work highlighted the major challenges associated with HR-MBRs and their respective potential corrective measures. In the past few years, substantial work has been conducted to enhance the performance of HR-MBRs. However, challenges and research gaps still need further investigation for improvement.
There is a need to improve cleaning approaches to ensure that the membrane integrity is not compromised after repeated cycles of cleaning. Where possible, environmentally-friendly cleaning techniques must be adopted to ensure longer membrane service life-span. Ideally, fouling prevention methods must be utilized to reduce fouling and avoid chemical cleaning. Amongst these methods, advanced oxidation processes (AOPs) such as sonication-assisted filtration and electrochemical coagulation hold great potential for future application. Therefore, more research is needed for further optimization before commercialization of these methods.
More research ought to be conducted on the use of more economical yet effective polymers or their mixtures with more established membrane materials to lower capital costs. A blending of hydrophobic polymers could enhance hydrophobicity in MD membranes. In this case, a hydrophobic polymer is mixed with a small amount of a more hydrophobic polymer. For example, Su et al.237 fabricated PTFE hollow fiber membranes for MD application. PTFE was chosen because of its high chemical resistance, thermal stability, and hydrophobicity. The fabricated membrane was highly hydrophobic (water contact angle of 164°) and had high flux. The membrane flux was stable over long-term experiments and elevated salinity, indicating its potential for MD application and treatment of high-salinity wastewater. In a recent study, Burnwal et al.238 prepared a polyvinylidene fluoride–polytetrafluoroethylene (PVDF–PTFE) hybrid flat sheet membrane to separate oil-in-water emulsions through MD. PVDF was used as the base polymer while PTFE was added as a copolymer in varying mass between 1 and 2 wt%. Mixing the polymers made the membranes more hydrophobic, with contact angles increasing from 88° for the pure PVDF membrane to 117° for the blended membrane, making it more suitable for MD operation. These findings hint at the possibility of combining polymers to enhance membrane properties. However, more research is still needed to evaluate the cost and applicability of membranes with polymer blends in wastewater treatment. There are preliminary studies in line with these concepts, but these are still far from commercialization.
In OMBR processes, the regeneration of the draw solution is energy intensive and reduces the advantage of energy saving by FO. The use of alternative energy sources, such as harvesting and applying geothermal and low-grade heat energy (e.g., industrial waste heat and solar), needs further investigation to reduce the total energy costs of OMBR and MDBR processes189. Another issue with OMBR is severe reverse salt flux when monovalent salt ions are used. Using monovalent salt as a draw solution produces higher flux than divalent salt, but higher reverse salt flux for monovalent salt decreases the driving force across the membrane leading to lower permeate flux. Holloway et al.208 lowered reverse salt flux and maintained high flux by adding small concentrations of salts having divalent or organic ions (e.g., magnesium chloride and sodium acetate) to NaCl in the draw solution. This was attributed to the low diffusivity of divalent ions, which inhibited sodium and chloride ions from diffusing through the membrane pores at a high rate. The water flux was also enhanced slightly when a low concentration of MgCl2 was introduced into a draw solution of NaCl. In another study, Nguyen and co-workers212 kept the reverse salt flux to a minimum of 0.13 g/m2h by combining a surfactant (Triton X100) and sodium phosphate in a forward osmosis desalination system. Further, the mixture of Triton X100 and sodium phosphate was easily recovered through a UF–NF system that rejected 98% of the solutes. The use of draw solutions combining various solutes in OMBR needs further investigation, and the effects on the performance of the bioreactor and the high-retention membranes must be evaluated.
Salt can gradually build up in all HR-MBR due to salt retention in raw wastewater and deteriorate biological activity186,187. Intermittent withdrawal of bioreactor media through MF/UF membrane can be done to prevent the build-up of salt205,239. Further, halotolerant or halophilic microorganisms can be used to ensure that biodegradation is not compromised215. Frequent sludge withdrawal has been proposed as another option to prevent salinity build-up by maintaining a low concentration factor. However, this approach may not be suitable as it implies a lower water recovery15.
Incomplete degradation of rejected recalcitrant TrOCs can be mitigated by using microbes of special degrading capacity—this has led to the concept of using enzymes from suitable microbes in the bioreactor (enzymatic membrane bioreactors)9,217,218,222,240. Redox mediators are added in the bioreactor to strengthen the biological activity of enzymes further; however, some reports show that the mediator-enhanced enzymatic process may degrade the parent compound but produce toxic effluent streams. For example, Nguyen et al.221 studied laccase-syringaldehyde meditated degradation of TrOCs in an enzymatic bioreactor: the treated effluent showed remarkable toxicity at syringaldehyde concentrations greater than 10 µM. Therefore, more research is needed to establish safe working concentrations for the redox mediator-enhanced enzymatic process. On the other hand, membrane bioreactor processes can be coupled to other treatment techniques, such as AOPs; however, such concepts are still in their infancy. Further, incomplete biodegradation of TrOCs retained within HR-MBR shifts the problem from wastewater treatment to sludge treatment and management. The option of using advanced oxidation processes (AOPs) to treat the concentrated TrOCs-sludge may be considered; however, some inherent limitations of AOPs, such as high cost and the potential production of disinfection by-products, need to be tackled.
The application of separation membranes is influenced by the material surface wettability and microstructure amongst other parameters. The membrane vapor flux is controlled by microstructural properties including average pore size and pore size distribution, pore structure, and porosity. In addition to desirable microstructure, hydrophobicity is another feature determining the application of separation membranes. For potential MD applications, hydrophobic materials with high water contact angles and small maximum pore size are required to avoid wettability of the membrane pores. However, this often leads to low MD permeability. Contrarily, high-pressure-driven (NF/RO) and osmotic pressure-driven (FO) processes require hydrophilic materials with small pore sizes to achieve high flux and separation performance. The challenges in HR-MBR (pore wetting in MD, membrane fouling and incomplete retention of TroOCs amongst other challenges) are associated with membrane properties (material wettability, microstructure, thickness etc). Therefore, there is a need for research to further fine-tune the membranes to achieve the desirable properties to advance the separation efficiency (targeting poorly removed TrOCs like carbamazepine) and fouling prevention. This could also be achieved through developing novel membrane materials with the desirable physicochemical properties for the respective application.
References
Luo, Y. et al. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 473–474, 619–641 (2014).
Asif, M. B. et al. Impact of simultaneous retention of micropollutants and laccase on micropollutant degradation in enzymatic membrane bioreactor. Bioresour. Technol. 267, 473–480 (2018).
Racar, M. et al. Challenges of municipal wastewater reclamation for irrigation by MBR and NF/RO: physico-chemical and microbiological parameters, and emerging contaminants. Sci. Total Environ. 722, 137959 (2020).
Asif, M. B. et al. Removal of trace organic contaminants by enzymatic membrane bioreactors: role of membrane retention and biodegradation. J. Membr. Sci. 611, 118345 (2020).
Harb, M., Wei, C. H., Wang, N., Amy, G. & Hong, P. Y. Organic micropollutants in aerobic and anaerobic membrane bioreactors: changes in microbial communities and gene expression. Bioresour. Technol. 218, 882–891 (2016).
Martí-Calatayud, M. C. et al. Transients of micropollutant removal from high-strength wastewaters in PAC-assisted MBR and MBR coupled with high-retention membranes. Sep. Purif. Technol. 246, 116863 (2020).
da Costa Fonseca, M. J., Silva, J. R. P., da, Borges, C. P. & da Fonseca, F. V. Ethinylestradiol removal of membrane bioreactor effluent by reverse osmosis and UV/H2O2: a technical and economic assessment. J. Environ. Manag. 282, 111948 (2021).
Dolar, D. et al. Removal of emerging contaminants from municipal wastewater with an integrated membrane system, MBR-RO. J. Hazard. Mater. 239–240, 64–69 (2012).
Tufail, A. et al. Combining enzymatic membrane bioreactor and ultraviolet photolysis for enhanced removal of trace organic contaminants: degradation efficiency and by-products formation. Process Saf. Environ. Prot. 145, 110–119 (2021).
Wijekoon, K. C. et al. A novel membrane distillation-thermophilic bioreactor system: Biological stability and trace organic compound removal. Bioresour. Technol. 159, 334–341 (2014).
Asif, M. B. et al. Persulfate oxidation-assisted membrane distillation process for micropollutant degradation and membrane fouling control. Sep. Purif. Technol. 222, 321–331 (2019).
Luo, W., Hai, F. I., Price, W. E., Elimelech, M. & Nghiem, L. D. Evaluating ionic organic draw solutes in osmotic membrane bioreactors for water reuse. J. Membr. Sci. 514, 636–645 (2016).
Zhang, B., Song, X., Nghiem, L. D., Li, G. & Luo, W. Osmotic membrane bioreactors for wastewater reuse: performance comparison between cellulose triacetate and polyamide thin film composite membranes. J. Membr. Sci. 539, 383–391 (2017).
Pathak, N. et al. Assessing the removal of organic micropollutants by a novel baffled osmotic membrane bioreactor-microfiltration hybrid system. Bioresour. Technol. 262, 98–106 (2018).
Luo, W. et al. High retention membrane bioreactors: challenges and opportunities. Bioresour. Technol. 167, 539–546 (2014).
Phattaranawik, J., Fane, A. G., Pasquier, A. C. S. & Bing, W. A novel membrane bioreactor based on membrane distillation. Desalination 223, 386–395 (2008).
Lay, W. C. L., Liu, Y. & Fane, A. G. Impacts of salinity on the performance of high retention membrane bioreactors for water reclamation: a review. Water Res 44, 21–40 (2010).
Dialynas, E. & Diamadopoulos, E. Integration of a membrane bioreactor coupled with reverse osmosis for advanced treatment of municipal wastewater. Desalination 238, 302–311 (2009).
Comerton, A. M., Andrews, R. C. & Bagley, D. M. Evaluation of an MBR-RO system to produce high-quality reuse water: microbial control, DBP formation and nitrate. Water Res 39, 3982–3990 (2005).
Qin, J. J. et al. New option of MBR-RO process for production of NEWater from domestic sewage. J. Membr. Sci. 272, 70–77 (2006).
Shannon, M. A. et al. Science and technology for water purification in the coming decades. Nature 452, 301–310 (2008).
Lay, W. C. L. et al. Study of integration of forward osmosis and biological process: membrane performance under elevated salt environment. Desalination 283, 123–130 (2011).
Asif, M. B. et al. Understanding the mechanisms of trace organic contaminant removal by high retention membrane bioreactors: a critical review. Environ. Sci. Pollut. Res. 26, 34085–34100 (2019).
Ademollo, N. et al. Occurrence, distribution and pollution pattern of legacy and emerging organic pollutants in surface water of the Kongsfjorden (Svalbard, Norway): environmental contamination, seasonal trend and climate change. Mar. Pollut. Bull. 163, 111900 (2021).
Borrull, J., Colom, A., Fabregas, J., Borrull, F. & Pocurull, E. Presence, behaviour and removal of selected organic micropollutants through drinking water treatment. Chemosphere 276, 130023 (2021).
Grung, M. et al. Occurrence and trophic transport of organic compounds in sedimentation ponds for road runoff. Sci. Total Environ. 751, 141808 (2021).
Liu, Q. et al. Occurrence and removal of organic pollutants by a combined analysis using GC-MS with spectral analysis and acute toxicity. Ecotoxicol. Environ. Saf. 207, 111237 (2021).
Schulze, S. et al. Occurrence of emerging persistent and mobile organic contaminants in European water samples. Water Res 153, 80–90 (2019).
Haarstad, K., Bavor, H. J. & Mæhlum, T. Organic and metallic pollutants in water treatment and natural wetlands: a review. Water Sci. Technol. 65, 76–99 (2012).
Li, A. J., Pal, V. K. & Kannan, K. A review of environmental occurrence, toxicity, biotransformation and biomonitoring of volatile organic compounds. Environ. Chem. Ecotoxicol. 3, 91–116 (2021).
Yamamoto, H. et al. Persistence and partitioning of eight selected pharmaceuticals in the aquatic environment: laboratory photolysis, biodegradation, and sorption experiments. Water Res 43, 351–362 (2009).
Zhang, S. et al. Removal of trace organic contaminants in municipal wastewater by anaerobic membrane bioreactor: efficiencies, fates and impact factors. J. Water Process Eng. 40, 101953 (2021).
Qiu, G., Chen, H., Srinivasa Raghavan, D. S. & Ting, Y. P. Removal behaviors of antibiotics in a hybrid microfiltration-forward osmotic membrane bioreactor for real municipal wastewater treatment. Chem. Eng. J. 417, 129146 (2021).
De Wever, H. et al. Comparison of sulfonated and other micropollutants removal in membrane bioreactor and conventional wastewater treatment. Water Res. 41, 935–945 (2007).
Urase, T., Kagawa, C. & Kikuta, T. Factors affecting removal of pharmaceutical substances and estrogens in membrane separation bioreactors. Desalination 178, 107–113 (2005).
Song, X. et al. An anaerobic membrane bioreactor—membrane distillation hybrid system for energy recovery and water reuse: removal performance of organic carbon, nutrients, and trace organic contaminants. Sci. Total Environ. 628–629, 358–365 (2018).
Wijekoon, K. C. et al. Development of a predictive framework to assess the removal of trace organic chemicals by anaerobic membrane bioreactor. Bioresour. Technol. 189, 391–398 (2015).
Tadkaew, N., Hai, F. I., McDonald, J. A., Khan, S. J. & Nghiem, L. D. Removal of trace organics by MBR treatment: the role of molecular properties. Water Res 45, 2439–2451 (2011).
Gu, Y. et al. Fate of pharmaceuticals during membrane bioreactor treatment: status and perspectives. Bioresour. Technol. 268, 733–748 (2018).
Semblante, G. U. et al. Trace organic contaminants in biosolids: Impact of conventional wastewater and sludge processing technologies and emerging alternatives. J. Hazard. Mater. 300, 1–17 (2015).
Fujioka, T., Aizawa, H. & Kodamatani, H. Fouling substances causing variable rejection of a small and uncharged trace organic chemical by reverse osmosis membranes. Environ. Technol. Innov. 17, 100576 (2020).
Mahlangu, O. T., Nackaerts, R., Thwala, J. M., Mamba, B. B. & Verliefde, A. R. D. Hydrophilic fouling-resistant GO-ZnO/PES membranes for wastewater reclamation. J. Membr. Sci. 524, 43–55 (2017).
Maryam, B., Buscio, V. & Ustun, S. A study on behavior, interaction and rejection of Paracetamol, Diclofenac and Ibuprofen (PhACs) from wastewater by nanofiltration membranes. Environ. Technol. Innov. 18, 100641 (2020).
Fujioka, T. et al. Assessing the passage of small pesticides through reverse osmosis membranes. J. Membr. Sci. 595, 117577 (2020).
Chen, L., Xu, P. & Wang, H. Interplay of the factors affecting water flux and salt rejection in membrane distillation: a state-of-the-art critical review. Water (Switz.) 12, 2841 (2020).
Verliefde, A., Van der Meeren, P. & Van der Bruggen, B. Solution-diffusion processes. in In: Encyclopedia of Membrane Science and Technology (ed. E. M. V. Hoek and V. V. Tarabara (Eds.)) 4013 (Wiley and Sons, 2013). https://doi.org/10.1002/9781118522318.emst017
Lim, M., Patureau, D., Heran, M., Lesage, G. & Kim, J. Removal of organic micropollutants in anaerobic membrane bioreactors in wastewater treatment: critical review. Environ. Sci. Water Res. Technol. 6, 1230–1243 (2020).
Luo, W. et al. Effects of salinity build-up on biomass characteristics and trace organic chemical removal: Implications on the development of high retention membrane bioreactors. Bioresour. Technol. 177, 274–281 (2015).
Criscuoli, A. et al. Integration of membrane bioreactors with reverse osmosis and membrane distillation units for wastewater treatment: recent developments and future perspectives. Sep. Purif. Rev. 00, 1–13 (2022).
Haffiez, N. et al. A critical review of process parameters influencing the fate of antibiotic resistance genes in the anaerobic digestion of organic waste. Bioresour. Technol. 354, 127189 (2022).
Mahlangu, O. T., Motsa, M. M., Nkambule, T. I. & Mamba, B. B. Rejection of trace organic compounds by membrane processes: mechanisms, challenges, and opportunities. Rev. Chem. Eng. https://doi.org/10.1515/revce-2021-0046 (2022).
Khanzada, N. K. et al. Removal of organic micropollutants using advanced membrane-based water and wastewater treatment: a review. J. Membr. Sci. 598, 117672 (2020).
Hamzah, N. & Leo, C. P. Fouling prevention in the membrane distillation of phenolic-rich solution using superhydrophobic PVDF membrane incorporated with TiO2 nanoparticles. Sep. Purif. Technol. 167, 79–87 (2016).
Ramos, R. L. et al. Direct contact membrane distillation as an approach for water treatment with phenolic compounds. J. Environ. Manag. 303, 114117 (2022).
Couto, C. F., Lange, L. C. & Amaral, M. C. S. Occurrence, fate and removal of pharmaceutically active compounds (PhACs) in water and wastewater treatment plants—a review. J. Water Process Eng. 32, 100927 (2019).
Plattner, J., Kazner, C., Naidu, G., Wintgens, T. & Vigneswaran, S. Removal of selected pesticides from groundwater by membrane distillation. Environ. Sci. Pollut. Res. 25, 20336–20347 (2018).
Tufail, A., Price, W. E. & Hai, F. I. A critical review on advanced oxidation processes for the removal of trace organic contaminants: a voyage from individual to integrated processes. Chemosphere 260, 127460 (2020).
Paul, D. R. Reformulation of the solution-diffusion theory of reverse osmosis. J. Membr. Sci. 241, 371–386 (2004).
Fujioka, T., Khan, S. J., Mcdonald, J. A. & Nghiem, L. D. Nanofiltration of trace organic chemicals: A comparison between ceramic and polymeric membranes. Sep. Purif. Technol. 136, 258–264 (2014).
Sharma, R. R., Agrawal, R. & Chellam, S. Temperature effects on sieving characteristics of thin-film composite nanofiltration membranes: pore size distributions and transport parameters. J. Membr. Sci. 223, 69–87 (2003).
Semião, A. J. C. & Schäfer, A. I. Removal of adsorbing estrogenic micropollutants by nanofiltration membranes. Part A—experimental. Evid. J. Membr. Sci. 431, 244–256 (2013).
Semião, A. J. C., Foucher, M. & Schäfer, A. I. Removal of adsorbing estrogenic micropollutants by nanofiltration membranes: Part B—model development. J. Membr. Sci. 431, 257–266 (2013).
Verliefde, A. R. D. et al. The role of electrostatic interactions on the rejection of organic solutes in aqueous solutions with nanofiltration. J. Membr. Sci. 322, 52–66 (2008).
Van der Bruggen, B., Schaep, J., Wilms, D. & Vandecasteele, C. Influence of molecular size, polarity and charge on the retention of organic molecules by nanofiltration. J. Membr. Sci. 156, 29–41 (1999).
Van der Bruggen, B., Schaep, J., Maes, W., Wilms, D. & Vandecasteele, C. Nanofiltration as a treatment method for the removal of pesticides from ground waters. Desalination 117, 139–147 (1998).
Plakas, K. V. & Karabelas, A. J. Removal of pesticides from water by NF and RO membranes—a review. Desalination 287, 255–265 (2012).
Albergamo, V. et al. Removal of polar organic micropollutants by pilot-scale reverse osmosis drinking water treatment. Water Res 148, 535–545 (2019).
Schäfer, A. I., Akanyeti, I. & Semião, A. J. C. Micropollutant sorption to membrane polymers: a review of mechanisms for estrogens. Adv. Colloid Interface Sci. 164, 100–117 (2011).
Alturki, A. A. et al. Removal of trace organic contaminants by the forward osmosis process. Sep. Purif. Technol. 103, 258–266 (2013).
Zheng, L. et al. New insights into the relationship between draw solution chemistry and trace organic rejection by forward osmosis. J. Membr. Sci. 587, 117184 (2019).
Nghiem, L. D., Schafer, A. I. & Elimelech, M. Role of electrostatic interactions in the retention of pharmaceutically active contaminants by a loose nanofiltration membrane. J. Membr. Sci. 286, 52–59 (2012).
Nghiem, L. D., Schaffer, A. I. & Elimelech, M. Nanofiltration of hormone mimicking trace organic contaminants nanofiltration of hormone mimicking trace organic contaminants. Sep. Sci. Technol. 40, 2633–2649 (2005).
Darvishmanesh, S. et al. Physicochemical characterization of solute retention in solvent resistant nanofiltration: the effect of solute size, polarity, dipole moment, and solubility parameter. J. Phys. Chem. B 115, 14507–14517 (2012).
Gur-Reznik, S., Katz, I. & Dosoretz, C. G. Removal of dissolved organic matter by granular-activated carbon adsorption as a pretreatment to reverse osmosis of membrane bioreactor effluents. Water Res. 42, 1595–1605 (2008).
Grant, G. T., Morris, E. R., Rees, D. A., Smith, P. J. C. & Thom, D. Biological interactions between polysaccharides and divalent cations: the egg-box model. FEBS Lett. 32, 195–198 (1973).
Lu, H., Butler, J. A., Britten, N. S., Venkatraman, P. D. & Rahatekar, S. S. Natural antimicrobial nano composite fibres manufactured from a combination of alginate and oregano essential oil. Nanomaterials 11, 1–17 (2021).
Karabelas, A. J., Karanasiou, A. & Sioutopoulos, D. C. Experimental study on the effect of polysaccharides on incipient membrane scaling during desalination. Desalination 416, 106–121 (2017).
Goh, P. S., Lau, W. J., Othman, M. H. D. D. & Ismail, A. F. Membrane fouling in desalination and its mitigation strategies. Desalination 425, 130–155 (2018).
Ju, Y. & Hong, S. Nano-colloidal fouling mechanisms in seawater reverse osmosis process evaluated by cake resistance simulator-modified fouling index nanofiltration. Desalination 343, 88–96 (2014).
Chong, T. H., Wong, F. S. & Fane, A. G. Enhanced concentration polarization by unstirred fouling layers in reverse osmosis: detection by sodium chloride tracer response technique. J. Membr. Sci. 287, 198–210 (2007).
Hoek, E. M. & Elimelech, M. Cake-enhanced concentration polarization: a new fouling mechanism for salt-rejecting membranes. Environ. Sci. Technol. 37, 5581–5588 (2003).
Mahlangu, O., Thwala, J. M., Mamba, B. B., D’Haese, A. & Verliefde, A. R. D. Factors governing combined fouling by organic and colloidal foulants in cross-flow nanofiltration. J. Membr. Sci. 491, 53–62 (2015).
Knoell, T. et al. Biofouling potentials of microporous polysulfone membranes containing a sulfonated polyether-ethersulfone/polyethersulfone block copolymer: correlation of membrane surface properties with bacterial attachment. J. Membr. Sci. 157, 117–138 (1999).
Meng, F. et al. Fouling in membrane bioreactors: an updated review. Water Res 114, 151–180 (2017).
She, Q., Wang, R., Fane, A. G. & Tang, C. Y. Membrane fouling in osmotically driven membrane processes: a review. J. Membr. Sci. 499, 201–233 (2016).
Tang, C. Y., Chong, T. H. & Fane, A. G. Colloidal interactions and fouling of NF and RO membranes: a review. Adv. Colloid Interface Sci. 164, 126–143 (2011).
Arkhangelsky, E. et al. Combined organic–inorganic fouling of forward osmosis hollow fiber membranes. Water Res 46, 6329–6338 (2012).
Vrijenhoek, E. M., Hong, S. & Elimelech, M. Influence of membrane surface properties on initial rate of colloidal fouling of reverse osmosis and nanofiltration membranes. J. Membr. Sci. 188, 115–128 (2001).
Mohammadi, T., Kazemimoghadam, M. & Saadabadi, M. Modeling of membrane fouling and flux decline in reverse osmosis during separation of oil in water emulsions. Desalination 157, 369–375 (2003).
Tang, C. Y., Kwon, Y. N. & Leckie, J. O. The role of foulant-foulant electrostatic interaction on limiting flux for RO and NF membranes during humic acid fouling-theoretical basis, experimental evidence, and AFM interaction force measurement. J. Membr. Sci. 326, 526–532 (2009).
You, X. et al. New insights into membrane fouling by alginate: Impacts of ionic strength in presence of calcium ions. Chemosphere 246, 125801 (2020).
Mahlangu, O. T., Mamba, B. B. & Verliefde, A. R. D. Effect of multivalent cations on membrane-foulant and foulant-foulant interactions controlling fouling of nanofiltration membranes. Polym. Adv. Technol. 31, 2588–2600 (2020).
Liu, J., Albdoor, A. K., Lin, W., Hai, F. I. & Ma, Z. Membrane fouling in direct contact membrane distillation for liquid desiccant regeneration: effects of feed temperature and flow velocity. J. Membr. Sci. 642, 119936 (2022).
Rahmawati, R. et al. Engineered spacers for fouling mitigation in pressure-driven membrane processes: progress and projection. J. Environ. Chem. Eng. 9, 106285 (2021).
Liu, X., Li, W., Chong, T. H. & Fane, A. G. Effects of spacer orientations on the cake formation during membrane fouling: quantitative analysis based on 3D OCT imaging. Water Res. 110, 1–14 (2017).
Aliasghari Aghdam, M., Mirsaeedghazi, H., Aboonajmi, M. & Kianmehr, M. H. Effect of ultrasound on different mechanisms of fouling during membrane clarification of pomegranate juice. Innov. Food Sci. Emerg. Technol. 30, 127–131 (2015).
Pramanik, B. K., Shu, L., Jegatheesan, V. & Bhuiyan, M. A. Effect of the coagulation/persulfate pre-treatment to mitigate organic fouling in the forward osmosis of municipal wastewater treatment. J. Environ. Manag. 249, 109394 (2019).
Warsinger, D. M. et al. Combining air recharging and membrane superhydrophobicity for fouling prevention in membrane distillation. J. Membr. Sci. 505, 241–252 (2016).
Rezaei, M., Warsinger, D. M., Lienhard, V. J. H. & Samhaber, W. M. Wetting prevention in membrane distillation through superhydrophobicity and recharging an air layer on the membrane surface. J. Membr. Sci. 530, 42–52 (2017).
Xie, M., Lee, J., Nghiem, L. D. & Elimelech, M. Role of pressure in organic fouling in forward osmosis and reverse osmosis. J. Membr. Sci. 493, 748–754 (2015).
Kim, Y., Elimelech, M., Shon, H. K. & Hong, S. Combined organic and colloidal fouling in forward osmosis: fouling reversibility and the role of applied pressure. J. Membr. Sci. 460, 206–212 (2014).
Tow, E. W. & Lienhard, V. J. H. Unpacking compaction: effect of hydraulic pressure on alginate fouling. J. Membr. Sci. 544, 221–233 (2017).
Kim, Y., Lee, S., Shon, H. K. & Hong, S. Organic fouling mechanisms in forward osmosis membrane process under elevated feed and draw solution temperatures. Desalination 355, 169–177 (2015).
Warsinger, D. M., Swaminathan, J., Guillen-Burrieza, E., Arafat, H. A. & Lienhard, J. H. Scaling and fouling in membrane distillation for desalination applications: a review. Desalination 356, 294–313 (2015).
Arsalan, F., She, Q., Fane, A. G. & Field, R. W. Exploring the differences between forward osmosis and reverse osmosis fouling. J. Membr. Sci. 565, 241–253 (2018).
Tow, E. W. et al. Comparison of fouling propensity between reverse osmosis, forward osmosis, and membrane distillation. J. Membr. Sci. 556, 352–364 (2018).
Karabelas, A. J., Koutsou, C. P., Kostoglou, M. & Sioutopoulos, D. C. Analysis of specific energy consumption in reverse osmosis desalination processes. Desalination 431, 15–21 (2018).
Wang, Z. & Lin, S. Membrane fouling and wetting in membrane distillation and their mitigation by novel membranes with special wettability. Water Res. 112, 38–47 (2017).
Tufail, A., Price, W. E. & Hai, F. I. Impact of inorganic ions and organic matter on the removal of trace organic contaminants by combined direct contact membrane distillation–UV photolysis. Membranes 10, 1–15 (2020).
Hajibabania, S. et al. Effect of fouling on removal of trace organic compounds by nanofiltration. Drink Water Eng. Sci. 4, 71–82 (2011).
Verliefde, A. R. D. et al. Influence of membrane fouling by (pretreated) surface water on rejection of pharmaceutically active compounds (PhACs) by nanofiltration membranes. J. Membr. Sci. 330, 90–103 (2009).
Verliefde, A. R. D. et al. Fate of trace organic compounds during treatment by nanofiltration. J. Membr. Sci. 373, 130–139 (2011).
Holloway, R. W., Regnery, J., Nghiem, L. D. & Cath, T. Y. Removal of trace organic chemicals and performance of a novel hybrid ultrafiltration-osmotic membrane bioreactor. Environ. Sci. Technol. 48, 10859–10868 (2014).
Nikbakht Fini, M., Madsen, H. T. & Muff, J. The effect of water matrix, feed concentration and recovery on the rejection of pesticides using NF/RO membranes in water treatment. Sep. Purif. Technol. 215, 521–527 (2019).
Zhang, Y., Van der Bruggen, B., Chen, G. X., Braeken, L. & Vandecasteele, C. Removal of pesticides by nanofiltration: effect of the water matrix. Sep. Purif. Technol. 38, 163–172 (2004).
Verliefde, A. R. D. et al. Influence of electrostatic interactions on the rejection with NF and assessment of the removal efficiency during NF/GAC treatment of pharmaceutically active compounds in surface water. Water Res 41, 3227–3240 (2007).
Badruzzaman, M., Voutchkov, N., Weinrich, L. & Jacangelo, J. G. Selection of pretreatment technologies for seawater reverse osmosis plants: a review. Desalination 449, 78–91 (2019).
Lee, W. J. et al. Fouling mitigation in forward osmosis and membrane distillation for desalination. Desalination 480, 114338 (2020).
Uzal, N., Yilmaz, L. & Yetis, U. Microfiltration/ultrafiltration as pretreatment for reclamation of rinsing waters of indigo dyeing. Desalination 240, 198–208 (2009).
Lee, S. & Lee, C. H. Microfiltration and ultrafiltration as a pretreatment for nanofiltration of surface water. Sep. Sci. Technol. 41, 1–23 (2006).
Hacıfazlıoğlu, M. C., Parlar, İ., Pek, T. & Kabay, N. Evaluation of chemical cleaning to control fouling on nanofiltration and reverse osmosis membranes after desalination of MBR effluent. Desalination 466, 44–51 (2019).
Suárez, J., Salgado, B., Casañas, A., González, J. C. & Pordomingo, J. One-year operational experience with ultrafiltration as pretreatment of seawater reverse osmosis desalination system (Maspalomas-I Plant). Desalin. Water Treat. 55, 2813–2821 (2015).
Amadou-Yacouba, Z., Mendret, J., Lesage, G., Zaviska, F. & Brosillon, S. Impact of pre-ozonation during nanofiltration of MBR effluent. Membranes 12, 341 (2022).
Nguyen, L. N., Hai, F. I., Kang, J., Price, W. E. & Nghiem, L. D. Removal of emerging trace organic contaminants by MBR-based hybrid treatment processes. Int. Biodeterior. Biodegrad. 85, 474–482 (2013).
Pramanik, B. K., Hai, F. I. & Roddick, F. A. Ultraviolet/persulfate pre-treatment for organic fouling mitigation of forward osmosis membrane: possible application in nutrient mining from dairy wastewater. Sep. Purif. Technol. 217, 215–220 (2019).
Luo, W. et al. Osmotic versus conventional membrane bioreactors integrated with reverse osmosis for water reuse: biological stability, membrane fouling, and contaminant removal. Water Res 109, 122–134 (2017).
Ansari, A. J., Hai, F. I., He, T., Price, W. E. & Nghiem, L. D. Physical cleaning techniques to control fouling during the pre-concentration of high suspended-solid content solutions for resource recovery by forward osmosis. Desalination 429, 134–141 (2018).
Ang, W. S., Yip, N. Y., Tiraferri, A. & Elimelech, M. Chemical cleaning of RO membranes fouled by wastewater effluent: achieving higher efficiency with dual-step cleaning. J. Membr. Sci. 382, 100–106 (2011).
Simon, A., Price, W. E. & Nghiem, L. D. Effects of chemical cleaning on the nanofiltration of pharmaceutically active compounds (PhACs). Sep. Purif. Technol. 88, 208–215 (2012).
Fujioka, T. et al. N-nitrosamine rejection by reverse osmosis: effects of membrane exposure to chemical cleaning reagents. Desalination 343, 60–66 (2014).
Zhou, Z., Yan, Y., Li, X., Zeng, F. & Shao, S. Effect of urea-based chemical cleaning on TrOCs rejection by nanofiltration membranes. Sep. Purif. Technol. 315, 123662 (2023).
Simon, A., Price, W. E. & Nghiem, L. D. Changes in surface properties and separation efficiency of a nanofiltration membrane after repeated fouling and chemical cleaning cycles. Sep. Purif. Technol. 113, 42–50 (2013).
Ma, C. et al. Graphene oxide-polyethylene glycol incorporated PVDF nanocomposite ultrafiltration membrane with enhanced hydrophilicity, permeability, and antifouling performance. Chemosphere 253, 126649 (2020).
Ngo, T. H. A. et al. Surface modification of polyamide thin film composite membrane by coating of titanium dioxide nanoparticles. J. Sci. Adv. Mater. Devices 1, 468–475 (2016).
Ali, M. E. A., Hassan, F. M. & Feng, X. Improving the performance of TFC membranes: via chelation and surface reaction: applications in water desalination. J. Mater. Chem. A 4, 6620–6629 (2016).
Patala, R., Mahlangu, O. T., Nyoni, H., Mamba, B. B. & Kuvarega, A. T. In situ generation of fouling resistant Ag/Pd modified PES membranes for treatment of pharmaceutical wastewater. Membranes 12, 762 (2022).
Ojajuni, O., Holder, S., Cavalli, G., Lee, J. & Saroj, D. P. Rejection of caffeine and carbamazepine by surface-coated PVDF hollow-fiber membrane system. Ind. Eng. Chem. Res. 55, 2417–2425 (2016).
Kong, F. et al. Rejection of pharmaceuticals by graphene oxide membranes: role of crosslinker and rejection mechanism. J. Membr. Sci. 612, 118338 (2020).
Kajau, A., Motsa, M., Mamba, B. B. & Mahlangu, O. Leaching of CuO nanoparticles from PES ultrafiltration membranes. ACS Omega 6, 31797–31809 (2021).
Andrade, P. F., de Faria, A. F., Oliveira, S. R., Arruda, M. A. Z. & Gonçalves Mdo, C. Improved antibacterial activity of nanofiltration polysulfone membranes modified with silver nanoparticles. Water Res. 81, 333–342 (2015).
Liu, Y., Rosenfield, E., Hu, M. & Mi, B. Direct observation of bacterial deposition on and detachment from nanocomposite membranes embedded with silver nanoparticles. Water Res. 47, 2949–2958 (2013).
Zhang, M., Zhang, K., De Gusseme, B. & Verstraete, W. Biogenic silver nanoparticles (bio-Ag0) decrease biofouling of bio-Ag0/PES nanocomposite membranes. Water Res. 46, 2077–2087 (2012).
Asif, M. B., Hai, F. I., Hou, J., Price, W. E. & Nghiem, L. D. Impact of wastewater derived dissolved interfering compounds on growth, enzymatic activity and trace organic contaminant removal of white rot fungi—a critical review. J. Environ. Manag. 201, 89–109 (2017).
Tan, J. M., Qiu, G. & Ting, Y. P. Osmotic membrane bioreactor for municipal wastewater treatment and the effects of silver nanoparticles on system performance. J. Clean. Prod. 88, 146–151 (2015).
Zhao, J., Yang, Y., Jiang, J., Takizawa, S. & Hou, L. Influences of cross-linking agents with different MW on the structure of GO/CNTs layers, membrane performances and fouling mechanisms for dissolved organic matter. J. Membr. Sci. 617, 118616 (2021).
Jiang, Y. et al. Two dimensional COFs as ultra-thin interlayer to build TFN hollow fiber nanofiltration membrane for desalination and heavy metal wastewater treatment. J. Membr. Sci. 635, 119523 (2021).
Straub, A. P., Asa, E., Zhang, W., Nguyen, T. H. & Herzberg, M. In-situ graft-polymerization modification of commercial ultrafiltration membranes for long-term fouling resistance in a pilot-scale membrane bioreactor. Chem. Eng. J. 382, 122865 (2020).
Chien, Z., Jye, W., Chun, K., Al-ghouti, M. A. & Fauzi, A. Improving properties of thin film nanocomposite membrane through polyethyleneimine intermediate layer: a parametric study. Sep. Purif. Technol. 274, 119035 (2021).
Cheng, G., Xue, H., Zhang, Z., Chen, S. & Jiang, S. A switchable biocompatible polymer surface with self-sterilizing and nonfouling capabilities**. Angew. Chem. Int. Ed. 47, 8831–8834 (2008).
Yang, X. et al. Engineering in situ catalytic cleaning membrane via prebiotic‐chemistry‐inspired mineralization. Adv. Mater. 35, 2306626 (2023).
Yang, X., Martinson, A. B. F., Elam, J. W., Shao, L. & Darling, S. B. Water treatment based on atomically engineered materials: atomic layer deposition and beyond. Matter 4, 3515–3548 (2021).
Yang, F. et al. Unprecedented acid-tolerant ultrathin membranes with finely tuned sub-nanopores for energetic-efficient molecular sieving. Sci. Bull. 68, 29–33 (2023).
Hai, F. I., Yamamoto, K. & Fukushi, K. Development of a submerged membrane fungi reactor for textile wastewater treatment. Desalination 192, 315–322 (2006).
Hai, F. I. et al. Removal of micropollutants by membrane bioreactor under temperature variation. J. Membr. Sci. 383, 144–151 (2011).
Doederer, K., Farré, M. J., Pidou, M., Weinberg, H. S. & Gernjak, W. Rejection of disinfection by-products by RO and NF membranes: influence of solute properties and operational parameters. J. Membr. Sci. 467, 195–205 (2014).
Saffarini, R. B., Mansoor, B., Thomas, R. & Arafat, H. A. Effect of temperature-dependent microstructure evolution on pore wetting in PTFE membranes under membrane distillation conditions. J. Membr. Sci. 429, 282–294 (2013).
Xu, R., Zhou, M., Wang, H., Wang, X. & Wen, X. Influences of temperature on the retention of PPCPs by nanofiltration membranes: experiments and modeling assessment. J. Membr. Sci. 599, 117817 (2020).
Quang, H. D., Procr, W. E. & Nghiem, L. D. The effects of feed solution temperature on pore size and trace organic contaminant rejection by the nanofiltration membrane NF270. Sep. Purif. Technol. 125, 43–51 (2014).
Yang, L. et al. Role of calcium ions on the removal of haloacetic acids from swimming pool water by nanofiltration: mechanisms and implications. Water Res. 110, 332–341 (2017).
Soriano, Á., Gorri, D. & Urtiaga, A. Selection of high flux membrane for the effective removal of short-chain perfluorocarboxylic acids. Ind. Eng. Chem. Res. 58, 3329–3338 (2019).
Azaïs, A. et al. Nanofiltration for wastewater reuse: counteractive effects of fouling and matrice on the rejection of pharmaceutical active compounds. Sep. Purif. Technol. 133, 313–327 (2014).
Nghiem, L. D., Manis, A., Soldenhoff, K. & Schäfer, A. I. Estrogenic hormone removal from wastewater using NF/RO membranes. J. Membr. Sci. 242, 37–45 (2004).
Mahlangu, T. O., Hoek, E. M. V., Mamba, B. B. & Verliefde, A. R. D. Influence of organic, colloidal and combined fouling on NF rejection of NaCl and carbamazepine: role of solute–foulant–membrane interactions and cake-enhanced concentration polarisation. J. Membr. Sci. 471, 35–46 (2014).
Azaïs, A., Mendret, J., Petit, E. & Brosillon, S. Evidence of solute-solute interactions and cake enhanced concentration polarization during removal of pharmaceuticals from urban wastewater by nanofiltration. Water Res. 104, 156–167 (2016).
Tufail, A. et al. Coupling horseradish peroxidase based bioreactor with membrane distillation: elucidating antibiotics degradation and membrane fouling mitigation. Desalination 542, 116039 (2022).
Wicaksana, F., Fane, A. G., Pongpairoj, P. & Field, R. Microfiltration of algae (Chlorella sorokiniana): critical flux, fouling and transmission. J. Membr. Sci. 387–388, 83–92 (2012).
Xu, J. et al. Ultrafiltration as pretreatment of seawater desalination: critical flux, rejection and resistance analysis. Sep. Purif. Technol. 85, 45–53 (2012).
Stoller, M. Effective fouling inhibition by critical flux-based optimization methods on a NF membrane module for olive mill wastewater treatment. Chem. Eng. J. 168, 1140–1148 (2011).
Lan, Y., Groenen-Serrano, K., Coetsier, C. & Causserand, C. Fouling control using critical, threshold and limiting fluxes concepts for cross-flow NF of a complex matrix: membrane BioReactor effluent. J. Membr. Sci. 524, 288–298 (2017).
Chong, T. H., Wong, F. S. & Fane, A. G. Implications of critical flux and cake enhanced osmotic pressure (CEOP) on colloidal fouling in reverse osmosis: experimental observations. J. Membr. Sci. 314, 101–111 (2008).
Nguyen, T. T., Kook, S., Lee, C., Field, R. W. & Kim, I. S. Critical flux-based membrane fouling control of forward osmosis: Behavior, sustainability, and reversibility. J. Membr. Sci. 570–571, 380–393 (2019).
Mahlangu, T. O. et al. Role of permeate flux and specific membrane-foulant-solute affinity interactions (∆Gslm) in transport of trace organic solutes through fouled nanofiltration (NF) membranes. J. Membr. Sci. 518, 203–215 (2016).
Zhao, Y. et al. Role of membrane and compound properties in affecting the rejection of pharmaceuticals by different RO/NF membranes. Front. Environ. Sci. Eng. 11, 20 (2017).
Wang, X., Li, B., Zhang, T. & Li, X. Performance of nanofiltration membrane in rejecting trace organic compounds: experiment and model prediction. Desalination 370, 7–16 (2015).
Uyanik, I., Özkan, O. & Koyuncu, I. NF-RO membrane performance for treating the effluent of an organized industrial zone wastewater treatment plant: effect of different UF types. Water 9, 506 (2017).
Chen, G. Q. et al. Performance of different pretreatment methods on alleviating reverse osmosis membrane fouling caused by soluble microbial products. J. Membr. Sci. 641, 119850 (2022).
Brasil, Y. L., Moreira, V. R., Lebron, Y. A. R., Moravia, W. G. & Amaral, M. C. S. Combining yeast MBR, Fenton and nanofiltration for landfill leachate reclamation. Waste Manag. 132, 105–114 (2021).
Marszałek, A. & Puszczało, E. Effect of photooxidation on nanofiltration membrane fouling during wastewater treatment from the confectionery industry. Water 12, 793 (2020).
Hosseinzadeh, M., Bidhendi, G. N., Torabian, A., Mehrdadi, N. & Pourabdullah, M. A new flat sheet membrane bioreactor hybrid system for advanced treatment of effluent, reverse osmosis pretreatment and fouling mitigation. Bioresour. Technol. 192, 177–184 (2015).
Naji, O. et al. Ultrasound-assisted membrane technologies for fouling control and performance improvement: a review. J. Water Process Eng. 43, 102268 (2021).
Aktij, S. A., Taghipour, A., Rahimpour, A., Mollahosseini, A. & Tiraferri, A. A critical review on ultrasonic-assisted fouling control and cleaning of fouled membranes. Ultrasonics 108, 106228 (2020).
Tam, L. S., Tang, T. W., Lau, G. N., Sharma, K. R. & Chen, G. H. A pilot study for wastewater reclamation and reuse with MBR/RO and MF/RO systems. Desalination 202, 106–113 (2007).
Gryta, M. Effect of iron oxides scaling on the MD process performance. Desalination 216, 88–102 (2007).
Jang, Y. et al. Comparison of fouling propensity and physical cleaning effect in forward osmosis, reverse osmosis, and membrane distillation. Desalin. Water Treat. 57, 24532–24541 (2016).
Abdel-Karim, A. et al. Membrane cleaning and pretreatments in membrane distillation—a review. Chem. Eng. J. 422, 129696 (2021).
Alturki, A. et al. Performance of a novel osmotic membrane bioreactor (OMBR) system: flux stability and removal of trace organics. Bioresour. Technol. 113, 201–206 (2012).
Li, K. et al. Effects of returning NF concentrate on the MBR-NF process treating antibiotic production wastewater. Environ. Sci. Pollut. Res. 23, 13114–13127 (2016).
Ansari, A. J. et al. Selection of forward osmosis draw solutes for subsequent integration with anaerobic treatment to facilitate resource recovery from wastewater. Bioresour. Technol. 191, 30–36 (2015).
Luo, W. et al. An osmotic membrane bioreactor-membrane distillation system for simultaneous wastewater reuse and seawater desalination: performance and implications. Environ. Sci. Technol. 51, 14311–14320 (2017).
Tomasini, H. R. et al. Concentrate management for integrated MBR-RO process for wastewater reclamation and reuse-preliminary tests. J. Water Process Eng. 29, 100455 (2019).
Luo, W. et al. Effects of salinity build-up on the performance and bacterial community structure of a membrane bioreactor. Bioresour. Technol. 200, 305–310 (2016).
Chapple, A. et al. Impact of inorganic salts on degradation of bisphenol A and diclofenac by crude extracellular enzyme from Pleurotus ostreatus. Biocatal. Biotransform. 37, 10–17 (2019).
Qiu, G. & Ting, Y. P. Short-term fouling propensity and flux behavior in an osmotic membrane bioreactor for wastewater treatment. Desalination 332, 91–99 (2014).
Lee, W. L. et al. Probing the key foulants and membrane fouling under increasing salinity in anaerobic osmotic membrane bioreactors for low-strength wastewater treatment. Chem. Eng. J. 413, 127450 (2021).
Siddique, M. S., Khan, S. J., Shahzad, M. A., Nawaz, M. S. & Hankins, N. P. Insight into the effect of organic and inorganic draw solutes on the flux stability and sludge characteristics in the osmotic membrane bioreactor. Bioresour. Technol. 249, 758–766 (2018).
Hai, F. I., Li, X., Price, W. E. & Nghiem, L. D. Removal of carbamazepine and sulfamethoxazole by MBR under anoxic and aerobic conditions. Bioresour. Technol. 102, 10386–10390 (2011).
Uygur, A. & Kargi, F. Salt inhibition on biological nutrient removal from saline wastewater in a sequencing batch reactor. Enzym. Microb. Technol. 34, 313–318 (2004).
Campos, J. L., Mosquera-Corral, A., Sánchez, M., Méndez, R. & Lema, J. M. Nitrification in saline wastewater with high ammonia concentration in an activated sludge unit. Water Res. 36, 2555–2560 (2002).
Sharrer, M. J., Tal, Y., Ferrier, D., Hankins, J. A. & Summerfelt, S. T. Membrane biological reactor treatment of a saline backwash flow from a recirculating aquaculture system. Aquac. Eng. 36, 159–176 (2007).
Jang, D., Hwang, Y., Shin, H. & Lee, W. Effects of salinity on the characteristics of biomass and membrane fouling in membrane bioreactors. Bioresour. Technol. 141, 50–56 (2013).
Qiu, G. & Ting, Y. P. Osmotic membrane bioreactor for wastewater treatment and the effect of salt accumulation on system performance and microbial community dynamics. Bioresour. Technol. 150, 287–297 (2013).
Tay, M. F. et al. Impact of salt accumulation in the bioreactor on the performance of nanofiltration membrane bioreactor (NF-MBR)+reverse osmosis (RO) process for water reclamation. Water Res. 170, 115352 (2020).
Wang, X., Chang, V. W. C. C. & Tang, C. Y. Osmotic membrane bioreactor (OMBR) technology for wastewater treatment and reclamation: advances, challenges, and prospects for the future. J. Membr. Sci. 504, 113–132 (2016).
Zhang, Q., Hu, J., Lee, D. J., Chang, Y. & Lee, Y. J. Sludge treatment: current research trends. Bioresour. Technol. 243, 1159–1172 (2017).
Wang, X., Yuan, B., Chen, Y., Li, X. & Ren, Y. Integration of micro-filtration into osmotic membrane bioreactors to prevent salinity build-up. Bioresour. Technol. 167, 116–123 (2014).
Holloway, R. W. et al. Long-term pilot scale investigation of novel hybrid ultrafiltration-osmotic membrane bioreactors. Desalination 363, 64–74 (2015).
Luo, W. et al. Phosphorus and water recovery by a novel osmotic membrane bioreactor-reverse osmosis system. Bioresour. Technol. 200, 297–304 (2016).
Holloway, R. W., Maltos, R., Vanneste, J. & Cath, T. Y. Mixed draw solutions for improved forward osmosis performance. J. Membr. Sci. 491, 121–131 (2015).
Holloway, R. W., Achilli, A. & Cath, T. Y. The osmotic membrane bioreactor: a critical review. Environ. Sci. Water Res. Technol. 1, 581–605 (2015).
Song, X. et al. Effects of sulphur on the performance of an anaerobic membrane bioreactor: Biological stability, trace organic contaminant removal, and membrane fouling. Bioresour. Technol. 250, 171–177 (2018).
Lin, H. et al. Membrane bioreactors for industrial wastewater treatment: a critical review. Crit. Rev. Environ. Sci. Technol. 42, 677–740 (2012).
Nguyen, H. T. et al. Exploring an innovative surfactant and phosphate-based draw solution for forward osmosis desalination. J. Membr. Sci. 489, 212–219 (2015).
Antoniou, P. et al. Effect of temperature and pH on the effective maximum specific growth rate of nitrifying bacteria. Water Res 24, 97–101 (1990).
Nawaz, M. S., Gadelha, G., Khan, S. J. & Hankins, N. Microbial toxicity effects of reverse transported draw solute in the forward osmosis membrane bioreactor (FO-MBR). J. Membr. Sci. 429, 323–329 (2013).
Ahmadizadeh, R., Shokrollahzadeh, S., Latifi, S. M., Samimi, A. & Pendashteh, A. Application of halophilic microorganisms in osmotic membrane bioreactor (OMBR) for reduction of volume and organic load of produced water. J. Water Process Eng. 37, 101422 (2020).
Nguyen, L. N. et al. Degradation of a broad spectrum of trace organic contaminants by anenzymatic membrane reactor: complementary role of membrane retention and enzymatic degradation. Int. Biodeterior. Biodegrad. 99, 115–122 (2015).
Asif, M. B. et al. Degradation of trace organic contaminants by a membrane distillation—enzymatic bioreactor. Appl. Sci. 7, 879 (2017).
Alharbi, M. B. et al. Degradation of diclofenac, trimethoprim, carbamazepine, and sulfamethoxazole by laccase from Trametes versicolor: transformation products and toxicity of treated effluent. Biocatal. Biotransform. 37, 399–408 (2019).
Nguyen, L. N. et al. A novel approach in crude enzyme laccase production and application in emerging contaminant bioremediation. Processes 8, 648 (2020).
Nguyen, L. N. et al. Enhancement of trace organic contaminant degradation by crude enzyme extract from Trametes versicolor culture: effect of mediator type and concentration. J. Taiwan Inst. Chem. Eng. 45, 1855–1862 (2014).
Nguyen, L. N. et al. Laccase-syringaldehyde-mediated degradation of trace organic contaminants in an enzymatic membrane reactor: Removal efficiency and effluent toxicity. Bioresour. Technol. 200, 477–484 (2016).
Asif, M. B. et al. Biocatalytic degradation of pharmaceuticals, personal care products, industrial chemicals, steroid hormones and pesticides in a membrane distillation-enzymatic bioreactor. Bioresour. Technol. 247, 528–536 (2018).
Ademakinwa, A. N. & Agboola, F. K. Biochemical characterization and kinetic studies on a purified yellow laccase from newly isolated Aureobasidium pullulans NAC8 obtained from soil containing decayed plant matter. J. Genet. Eng. Biotechnol. 14, 143–151 (2016).
Phan, H. V. et al. Bacterial community dynamics in an anoxic-aerobic membrane bioreactor—impact on nutrient and trace organic contaminant removal. Int. Biodeterior. Biodegrad. 109, 61–72 (2016).
García-Gómez, C. et al. Combined membrane bioreactor and electrochemical oxidation using Ti/PbO2 anode for the removal of carbamazepine. J. Taiwan Inst. Chem. Eng. 64, 211–219 (2016).
Predolin, L. M., Moya-Llamas, M. J., Vásquez-Rodríguez, E. D., Jaume, A. T. & Rico, D. P. Effect of current density on the efficiency of a membrane electro-bioreactor for removal of micropollutants and phosphorus, and reduction of fouling: a pilot plant case study. J. Environ. Chem. Eng. 9, 104874 (2021).
Song, H. L. et al. Degradation of sulfamethoxazole in low-C / N ratio wastewater by a novel membrane bioelectrochemical reactor. Bioresour. Technol. 305, 123029 (2020).
Yang, Y. et al. Degradation of sulfamethoxazole by UV, UV/H2O2 and UV/persulfate (PDS): formation of oxidation products and effect of bicarbonate. Water Res. 118, 196–207 (2017).
Ghaffour, N., Missimer, T. M. & Amy, G. L. Technical review and evaluation of the economics of water desalination: current and future challenges for better water supply sustainability. Desalination 309, 197–207 (2013).
Lo, C. H., McAdam, E. & Judd, S. The cost of a small membrane bioreactor. Water Sci. Technol. 72, 1739–1746 (2015).
DeCarolis, J. et al. Cost trends of MbR systems for municipal wastewater treatment. Proc. Water Environ. Fed. 2007, 3407–3418 (2012).
Adham, S., Hussain, A., Matar, J. M., Dores, R. & Janson, A. Application of membrane distillation for desalting brines from thermal desalination plants. Desalination 314, 101–108 (2013).
Khan, E. U. & Nordberg, Å. Thermal integration of membrane distillation in an anaerobic digestion biogas plant—a techno-economic assessment. Appl. Energy 239, 1163–1174 (2019).
Chia, W. Y., Chia, S. R., Khoo, K. S., Chew, K. W. & Show, P. L. Sustainable membrane technology for resource recovery from wastewater: Forward osmosis and pressure retarded osmosis. J. Water Process Eng. 39, 101758 (2021).
Le, N. L. & Nunes, S. P. Materials and membrane technologies for water and energy sustainability. Sustain. Mater. Technol. 7, 1–28 (2016).
Helfer, F., Lemckert, C. & Anissimov, Y. G. Osmotic power with pressure retarded osmosis: theory, performance and trends—a review. J. Membr. Sci. 453, 337–358 (2014).
Su, C. et al. Novel PTFE hollow fiber membrane fabricated by emulsion electrospinning and sintering for membrane distillation. J. Membr. Sci. 583, 200–208 (2019).
Burnwal, P. K., Pal, P., Midda, M. O. & Chaurasia, S. P. Synthesis of hybrid PVDF–PTFE membrane using nonhazardous solvent for ethanol–water separation through membrane distillation. J. Hazard., Toxic., Radioact. Waste 26, 1–11 (2022).
Chang, H. M. et al. Water reclamation and microbial community investigation: treatment of tetramethylammonium hydroxide wastewater through an anaerobic osmotic membrane bioreactor hybrid system. J. Hazard. Mater. 427, 128200 (2022).
Ashe, B. et al. Impacts of redox-mediator type on trace organic contaminants degradation by laccase: degradation efficiency, laccase stability and effluent toxicity. Int. Biodeterior. Biodegrad. 113, 169–176 (2016).
Choi, J. H., Fukushi, K. & Yamamoto, K. A submerged nanofiltration membrane bioreactor for domestic wastewater treatment: the performance of cellulose acetate nanofiltration membranes for long-term operation. Sep. Purif. Technol. 52, 470–477 (2007).
Xie, M., Nghiem, L. D., Price, W. E. & Elimelech, M. Comparison of the removal of hydrophobic trace organic contaminants by forward osmosis and reverse osmosis. Water Res 46, 2683–2692 (2012).
Judd, S. MBR Book: Principles and Applications of Membrane Bioreactors for Water and Wastewater Treatment, 2nd ed. Elsevier Science, Burlington (2010).
Lay, W. C. L. et al. Effect of pharmaceuticals on the performance of a novel osmotic membrane bioreactor (OMBR). Sep. Sci. Technol. 47, 543–554 (2012).
Zaviska, F., Drogui, P., Grasmick, A., Azais, A. & Heran, M. Nanofiltration membrane bioreactor for removing pharmaceutical compounds. J. Membr. Sci. 429, 121–129 (2013).
Nthunya, L. N. et al. Fouling, performance and cost analysis of membrane-based water desalination technologies: a critical review. J. Environ. Manag. 301, 113922 (2022).
Arcanjo, G. S. et al. Improving biological removal of pharmaceutical active compounds and estrogenic activity in a mesophilic anaerobic osmotic membrane bioreactor treating municipal sewage. Chemosphere 301, 1–12 (2022).
Asif, M. B. et al. Acid mine drainage and sewage impacted groundwater treatment by membrane distillation: organic micropollutant and metal removal and membrane fouling. J. Environ. Manag. 291, 112708 (2021).
Wijekoon, K. C. et al. Rejection and fate of trace organic compounds (TrOCs) during membrane distillation. J. Membr. Sci. 453, 636–642 (2014).
Arcanjo, G. S. et al. Effective removal of pharmaceutical compounds and estrogenic activity by a hybrid anaerobic osmotic membrane bioreactor-membrane distillation system treating municipal sewage. Chem. Eng. J. 416, 129151 (2021).
Xie, M., Nghiem, L. D., Price, W. E. & Elimelech, M. A forward osmosis-membrane distillation hybrid process for direct sewer mining: system performance and limitations. Environ. Sci. Technol. 47, 13486–13493 (2013).
Asif, M. B. et al. Elucidating the performance of an integrated laccase- and persulfate-assisted process for degradation of trace organic contaminants (TrOCs). Environ. Sci. Water Res. Technol. 6, 1069–1082 (2020).
Alturki, A. A. et al. Combining MBR and NF/RO membrane filtration for the removal of trace organics in indirect potable water reuse applications. J. Membr. Sci. 365, 206–215 (2010).
Aziz, M. & Ojumu, T. Exclusion of estrogenic and androgenic steroid hormones from municipal membrane bioreactor wastewater using UF/NF/RO membranes for water reuse application. Membranes 10, 37 (2020).
Mamo, J. et al. Fate of NDMA precursors through an MBR-NF pilot plant for urban wastewater reclamation and the effect of changing aeration conditions. Water Res. 102, 383–393 (2016).
Cartagena, P., El Kaddouri, M., Cases, V., Trapote, A. & Prats, D. Reduction of emerging micropollutants, organic matter, nutrients and salinity from real wastewater by combined MBR-NF/RO treatment. Sep. Purif. Technol. 110, 132–143 (2013).
Wang, Y. et al. Removal of pharmaceutical and personal care products (PPCPs) from municipal waste water with integrated membrane systems, MBR-RO/NF. Int. J. Environ. Res. Public Health 15, 269 (2018).
Chon, K., KyongShon, H. & Cho, J. Membrane bioreactor and nanofiltration hybrid system for reclamation of municipal wastewater: removal of nutrients, organic matter and micropollutants. Bioresour. Technol. 122, 181–188 (2012).
Kim, Y. et al. Assessing the removal of organic micro-pollutants from anaerobic membrane bioreactor effluent by fertilizer-drawn forward osmosis. J. Membr. Sci. 533, 84–95 (2017).
Arcanjo, G. S. et al. Draw solution solute selection for a hybrid forward osmosis-membrane distillation module: effects on trace organic compound rejection, water flux and polarization. Chem. Eng. J. 400, 125857 (2020).
Wang, X., Zhang, J., Chang, V. W. C., She, Q. & Tang, C. Y. Removal of cytostatic drugs from wastewater by an anaerobic osmotic membrane bioreactor. Chem. Eng. J. 339, 153–161 (2018).
Ricci, B. C. et al. A novel submerged anaerobic osmotic membrane bioreactor coupled to membrane distillation for water reclamation from municipal wastewater. Chem. Eng. J. 414, 128645 (2021).
Ayyavoo, J., Nguyen, T. P. N., Jun, B.-M., Kim, I.-C. & Kwon, Y.-N. Protection of polymeric membranes with antifouling surfacing via surface modifications. Colloids Surf. A Physicochem. Eng. Asp. 506, 190–201 (2016).
Mahlangu, O. T. & Mamba, B. B. Interdependence of contributing factors governing dead-end fouling of nanofiltration. Membr. Membr. 11, 47 (2021).
Kim, J., Kim, H. W., Tijing, L. D., Shon, H. K. & Hong, S. Elucidation of physicochemical scaling mechanisms in membrane distillation (MD): Implication to the control of inorganic fouling. Desalination 527, 115573 (2022).
Al-Juboori, R. A. & Yusaf, T. Biofouling in RO system: mechanisms, monitoring and controlling. Desalination 302, 1–23 (2012).
Kim, A. S., Chen, H. & Yuan, R. EPS biofouling in membrane filtration: an analytic modeling study. J. Colloid Interface Sci. 303, 243–249 (2006).
Herzberg, M. & Elimelech, M. Biofouling of reverse osmosis membranes: role of biofilm-enhanced osmotic pressure. J. Membr. Sci. 295, 11–20 (2007).
Li, Q. & Elimelech, M. Synergistic effects in combined fouling of a loose nanofiltration membrane by colloidal materials and natural organic matter. J. Membr. Sci. 278, 72–82 (2006).
Liu, Y. & Mi, B. Combined fouling of forward osmosis membranes: synergistic foulant interaction and direct observation of fouling layer formation. J. Membr. Sci. 407–408, 136–144 (2012).
Goh, S., Zhang, J., Liu, Y. & Fane, A. G. Fouling and wetting in membrane distillation (MD) and MD-bioreactor (MDBR) for wastewater reclamation. Desalination 323, 39–47 (2013).
Yao, M. et al. Volatile fatty acids and biogas recovery using thermophilic anaerobic membrane distillation bioreactor for wastewater reclamation. J. Environ. Manag. 231, 833–842 (2019).
Liu, C., Chen, L. & Zhu, L. Application of membrane distillation for the treatment of anaerobic membrane bioreactor effluent: an especial attention to the operating conditions. Chemosphere 208, 530–540 (2018).
Li, B. et al. Direct contact membrane distillation with softening Pre-treatment for effective reclaiming flue gas desulfurization wastewater. Sep. Purif. Technol. 277, 119637 (2021).
Lan, Y., Groenen-Serrano, K., Coetsier, C. & Causserand, C. Nanofiltration performances after membrane bioreactor for hospital wastewater treatment: fouling mechanisms and the quantitative link between stable fluxes and the water matrix. Water Res 146, 77–87 (2018).
Kappel, C. et al. Impacts of NF concentrate recirculation on membrane performance in an integrated MBR and NF membrane process for wastewater treatment. J. Membr. Sci. 453, 359–368 (2014).
Corzo, B. et al. Long-term evaluation of a forward osmosis-nanofiltration demonstration plant for wastewater reuse in agriculture. Chem. Eng. J. 338, 383–391 (2018).
Gündoğdu, B. et al. Effect of concentrate recirculation on the product water quality of integrated MBR–NF process for wastewater reclamation and industrial reuse. J. Water Process Eng. 29, 100485 (2019).
Zhang, X. & Sun, K. Study on fouling of nanofiltration membranes when treating the MBR effluent. Adv. Mater. Res. 442, 157–161 (2012).
Silva, N. C. M., Moravia, W. G., Amaral, M. C. S. & Figueiredo, K. C. S. Evaluation of fouling mechanisms in nanofiltration as a polishing step of yeast MBR-treated landfill leachate. Environ. Technol. 40, 3611–3621 (2019).
Wang, J., Li, K., Yu, D., Zhang, J. & Wei, Y. Fouling characteristics and cleaning strategies of NF membranes for the advanced treatment of antibiotic production wastewater. Environ. Sci. Pollut. Res. 24, 8967–8977 (2017).
Kimura, K., Okazaki, S., Ohashi, T. & Watanabe, Y. Importance of the co-presence of silica and organic matter in membrane fouling for RO filtering MBR effluent. J. Membr. Sci. 501, 60–67 (2016).
Kaya, Y. & Dayanir, S. Application of nanofiltration and reverse osmosis for treatment and reuse of laundry wastewater. J. Environ. Heal. Sci. Eng. 18, 699–709 (2020).
Cao, L., Zhang, Y., Ni, L. & Feng, X. A novel loosely structured nanofiltration membrane bioreactor for wastewater treatment: process performance and membrane fouling. J. Membr. Sci. 644, 120128 (2022).
Chon, K., Cho, J. & Shon, H. K. Fouling characteristics of a membrane bioreactor and nanofiltration hybrid system for municipal wastewater reclamation. Bioresour. Technol. 130, 239–247 (2013).
Sert, G. et al. Investigation of mini pilot-scale MBR-NF and MBR-RO integrated systems performance-preliminary field tests. J. Water Process Eng. 12, 72–77 (2016).
Manzoor, K., Khan, S. J., Khan, A., Abbasi, H. & Zaman, W. Q. Woven-fiber microfiltration coupled with anaerobic forward osmosis membrane bioreactor treating textile wastewater: use of fertilizer draw solutes for direct fertigation. Biochem. Eng. J. 181, 108385 (2022).
Sun, Y. et al. Membrane fouling of forward osmosis (FO) membrane for municipal wastewater treatment: a comparison between direct FO and OMBR. Water Res. 104, 330–339 (2016).
Linares, R. V. et al. Hybrid SBR–FO system for wastewater treatment and reuse: operation, fouling and cleaning. Desalination 393, 31–38 (2016).
Morrow, C. P. et al. Integrating an aerobic/anoxic osmotic membrane bioreactor with membrane distillation for potable reuse. Desalination 432, 46–54 (2018).
Zhang, J., Wang, D., Chen, Y., Gao, B. & Wang, Z. Scaling control of forward osmosis-membrane distillation (FO-MD) integrated process for pre-treated landfill leachate treatment. Desalination 520, 115342 (2021).
Qiu, G., Zhang, S., Srinivasa Raghavan, D. S., Das, S. & Ting, Y. P. The potential of hybrid forward osmosis membrane bioreactor (FOMBR) processes in achieving high throughput treatment of municipal wastewater with enhanced phosphorus recovery. Water Res. 105, 370–382 (2016).
Juntawang, C., Rongsayamanont, C. & Khan, E. Entrapped-cells-based anaerobic forward osmosis membrane bioreactor treating medium-strength domestic wastewater: Fouling characterization and performance evaluation. Chemosphere 225, 226–237 (2019).
Viet, N. D. & Jang, A. Fertilizer draw solution index in osmotic membrane bioreactor for simultaneous wastewater treatment and sustainable agriculture. Chemosphere 296, 134002 (2022).
Pathak, N. et al. Simultaneous nitrification-denitrification using baffled osmotic membrane bioreactor-microfiltration hybrid system at different oxic-anoxic conditions for wastewater treatment. J. Environ. Manag. 253, 109685 (2020).
Wang, X., Wang, H. & Xie, M. Secret underneath: fouling of membrane support layer in anaerobic osmotic membrane bioreactor (AnOMBR). J. Membr. Sci. 614, 118530 (2020).
Im, S. J., Rho, H., Jeong, S. & Jang, A. Organic fouling characterization of a CTA-based spiral-wound forward osmosis (SWFO) membrane used in wastewater reuse and seawater desalination. Chem. Eng. J. 336, 141–151 (2018).
Im, S. J., Jeong, G., Jeong, S., Cho, J. & Jang, A. Fouling and transport of organic matter in cellulose triacetate forward-osmosis membrane for wastewater reuse and seawater desalination. Chem. Eng. J. 384, 123341 (2020).
Lebron, A. R. Y. et al. Osmotic membrane bioreactor (OMBR) in refinery wastewater treatment: the impact of a draw solute with lower diffusivity in the process performance. Chem. Eng. J. 406, 127074 (2021).
Nawaz, M. S. et al. Performance and implications of forward osmosis-membrane distillation hybrid system for simultaneous treatment of different real produced water streams. Chem. Eng. J. 450, 138479 (2022).
Chun, Y. et al. Organic matter removal from a membrane bioreactor effluent for reverse osmosis fouling mitigation by microgranular adsorptive filtration system. Desalination 506, 115016 (2021).
Unal, B. O. Membrane autopsy study to characterize fouling type of RO membrane used in an industrial zone wastewater reuse plant. Desalination 529, 115648 (2022).
Ying, W., Siebdrath, N., Uhl, W., Gitis, V. & Herzebrg, M. New insights on early stages of RO membranes fouling during tertiary wastewater desalination. J. Membr. Sci. 466, 26–35 (2014).
Farias, E. L., Howe, K. J. & Thomson, B. M. Effect of membrane bioreactor solids retention time on reverse osmosis membrane fouling for wastewater reuse. Water Res. 49, 53–61 (2014).
Farias, E. L., Howe, K. J. & Thomson, B. M. Spatial and temporal evolution of organic foulant layers on reverse osmosis membranes in wastewater reuse applications. Water Res. 58, 102–110 (2014).
Malamis, S., Katsou, E., Takopoulos, K., Demetriou, P. & Loizidou, M. Assessment of metal removal, biomass activity and RO concentrate treatment in an MBR-RO system. J. Hazard. Mater. 209–210, 1–8 (2012).
Li, X., Liu, C., Yin, W., Chong, T. H. & Wang, R. Design and development of layer-by-layer based low-pressure antifouling nanofiltration membrane used for water reclamation. J. Membr. Sci. 584, 309–323 (2019).
Huang, Y., Jeffrey, P. & Pidou, M. A comparative evaluation of reverse osmosis membrane performance when combined with anaerobic or aerobic membrane bioreactors for indirect potable reuse applications. SSRN Electron. J. 50, 103295 (2022).
Srisukphun, T., Chiemchaisri, C., Chiemchaisri, W. & Thanuttamavong, M. Fouling and cleaning of reverse osmosis membrane applied to membrane bioreactor effluent treating textile wastewater. Environ. Eng. Res. 21, 45–51 (2016).
Inaba, T. et al. Microbiomes and chemical components of feed water and membrane-attached biofilm in reverse osmosis system to treat membrane bioreactor effluents. Sci. Rep. 8, 1–11 (2018).
Liu, H., Gu, J., Wang, S., Zhang, M. & Liu, Y. Performance, membrane fouling control and cost analysis of an integrated anaerobic fixed-film MBR and reverse osmosis process for municipal wastewater reclamation to NEWater-like product water. J. Membr. Sci. 593, 117442 (2020).
Xiao, Y. et al. Advanced treatment of semiconductor wastewater by combined MBR-RO technology. Desalination 336, 168–178 (2014).
Jamal-Uddin, A. T. & Zytner, R. G. Evaluation of fouling and RO performance for MBR treated fruit wastewater. Water Sci. Technol. 82, 2282–2295 (2020).
Kimura, K., Iwase, T., Kita, S. & Watanabe, Y. Influence of residual organic macromolecules produced in biological wastewater treatment processes on removal of pharmaceuticals by NF/RO membranes. Water Res. 43, 3751–3758 (2009).
Xu, R. et al. Influences of multi influent matrices on the retention of PPCPs by nanofiltration membranes. Sep. Purif. Technol. 212, 299–306 (2019).
Xu, P., Drewes, J. E., Kim, T. U., Bellona, C. & Amy, G. Effect of membrane fouling on transport of organic contaminants in NF/RO membrane applications. J. Membr. Sci. 279, 165–175 (2006).
Qu, F. et al. Tertiary treatment of secondary effluent using ultrafiltration for wastewater reuse: correlating membrane fouling with rejection of effluent organic matter and hydrophobic pharmaceuticals. Environ. Sci. Water Res. Technol. 5, 672–683 (2019).
Ng, H. Y. & Elimelech, M. Influence of colloidal fouling on rejection of trace organic contaminants by reverse osmosis. J. Membr. Sci. 244, 215–226 (2004).
Xie, M., Nghiem, L. D., Price, W. E. & Elimelech, M. Impact of organic and colloidal fouling on trace organic contaminant rejection by forward osmosis: role of initial permeate flux. Desalination 336, 146–152 (2014).
Kim, Y., Kim, L. H., Vrouwenvelder, J. S. & Ghaffour, N. Effect of organic micropollutants on biofouling in a forward osmosis process integrating seawater desalination and wastewater reclamation. J. Hazard. Mater. 401, 123386 (2021).
Zheng, L., Price, W. E. & Nghiem, L. D. Effects of fouling on separation performance by forward osmosis: the role of specific organic foulants. Environ. Sci. Pollut. Res. 26, 33758–33769 (2019).
Lin, Y. L. Effects of organic, biological and colloidal fouling on the removal of pharmaceuticals and personal care products by nanofiltration and reverse osmosis membranes. J. Membr. Sci. 542, 342–351 (2017).
Chon, K., Kim, S. J., Moon, J. & Cho, J. Combined coagulation-disk filtration process as a pretreatment of ultrafiltration and reverse osmosis membrane for wastewater reclamation: an autopsy study of a pilot plant. Water Res. 46, 1803–1816 (2012).
Zhang, X., Gu, J. & Liu, Y. Necessity of direct energy and ammonium recovery for carbon neutral municipal wastewater reclamation in an innovative anaerobic MBR-biochar adsorption-reverse osmosis process. Water Res. 211, 118058 (2022).
Borea, L., Naddeo, V. & Belgiorno, V. Application of electrochemical processes to membrane bioreactors for improving nutrient removal and fouling control. Environ. Sci. Pollut. Res. 24, 321–333 (2017).
Al Mahri, B. B. A., Balogun, H. A., Yusuf, A. & Giwa, A. Electro-osmotic thermal process model for performance enhancement of forward osmosis integrated with membrane distillation. Sep. Purif. Technol. 238, 116494 (2020).
Liu, L., Liu, J., Gao, B. & Yang, F. Minute electric field reduced membrane fouling and improved performance of membrane bioreactor. Sep. Purif. Technol. 86, 106–112 (2012).
Kim, Y., Li, S. & Ghaffour, N. Evaluation of different cleaning strategies for different types of forward osmosis membrane fouling and scaling. J. Membr. Sci. 596, 117731 (2020).
Aftab, B., Cho, J. & Hur, J. Intermittent osmotic relaxation: a strategy for organic fouling mitigation in a forward osmosis system treating landfill leachate. Desalination 482, 114406 (2020).
Stanford, B. D., Pisarenko, A. N., David Holbrook, R. & Snyder, S. A. Preozonation effects on the reduction of reverse osmosis membrane fouling in water reuse. Ozone Sci. Eng. 33, 379–388 (2011).
Singh, N., Petrinic, I., Hélix-Nielsen, C., Basu, S. & Balakrishnan, M. Influence of Forward Osmosis (FO) membrane properties on dewatering of molasses distillery wastewater. J. Water Process Eng. 32, 100921 (2019).
Zhou, Y., Huang, M., Deng, Q. & Cai, T. Combination and performance of forward osmosis and membrane distillation (FO-MD) for treatment of high salinity landfill leachate. Desalination 420, 99–105 (2017).
Ngo, M. T. T. et al. Fouling behavior and performance of a submerged flat-sheet nanofiltration membrane system for direct treatment of secondary wastewater effluent. J. Water Process Eng. 41, 101991 (2021).
Lebron, Y. A. R., Moreira, V. R., Moser, P. B., de Souza Santos, L. V. & Amaral, M. C. S. Screening cost effectiveness and salinity build up control in osmotic membrane bioreactors for refinery wastewater treatment: a draw solute with lower diffusivity and ultrafiltration implementation. Process Saf. Environ. Prot. 151, 195–207 (2021).
Zhang, Q., Wang, J. & Vecitis, C. D. Fouling reduction and recovery during forward osmosis of wastewater using an electroactive CNT composite. Membr. J. Membr. Sci. 620, 118803 (2021).
Wang, Y. & Xu, T. Anchoring hydrophilic polymer in substrate: an easy approach for improving the performance of TFC FO membrane. J. Membr. Sci. 476, 330–339 (2015).
Kasongo, G. et al. Surface grafting of polyvinyl alcohol (PVA) cross-linked with glutaraldehyde (GA) to improve resistance to fouling of aromatic polyamide thin film composite reverse osmosis membranes using municipal membrane bioreactor effluent. Water Pract. Technol. 14, 614–624 (2019).
Alkhatib, A., Ayari, M. A. & Hawari, A. H. Fouling mitigation strategies for different foulants in membrane distillation. Chem. Eng. Process. Process. Intensif. 167, 108517 (2021).
Acknowledgements
The authors would like to thank all anonymous reviewers and editors for their insightful comments and suggestions on revising and improving the quality of the review. This work was funded by the Institute for Nanotechnology and Water Sustainability, University of South Africa and the Strategic Water Infrastructure Laboratory, School of Civil Mining and Environmental Engineering, University of Wollongong, Australia.
Author information
Authors and Affiliations
Contributions
Conceptualization and writing of the first draft were done by O.T.M. T.I.N. acquired funding for the work. B.B.M. conducted reviews and editing, project administration, and funding acquisition. F.I.H. conceptualized the work, and conducted reviews, editing and approval of chapters. All authors have read and agreed to publish this version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahlangu, O.T., Nkambule, T.I., Mamba, B.B. et al. Strategies for mitigating challenges associated with trace organic compound removal by high-retention membrane bioreactors (HR-MBRs). npj Clean Water 7, 18 (2024). https://doi.org/10.1038/s41545-024-00313-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41545-024-00313-w