Abstract
Non-alcoholic steatohepatitis (NASH) is a progressive fibrotic form of non-alcoholic fatty liver disease. Liver fibrosis leads to liver cancer and cirrhosis, and drug therapy for NASH remains lacking. Ninjin’yoeito (NYT) has shown antifibrotic effects in a model of liver fibrosis without steatosis but has not been studied for NASH. Therefore, we evaluated the efficacy of NYT in mice fed a choline-deficient, L-amino acid-defined, high-fat diet (CDAHFD) as a NASH model. Compared with the normal diet group, mice fed CDAHFD showed decreased body weight and increased white adipose tissue, liver weight, and triglyceride content in the liver. Furthermore, a substantial increase in the hepatic concentration of hydroxyproline, expression of α-smooth muscle actin (α-SMA), and transforming growth factor-β was observed in CDAHFD-fed mice. Masson’s trichrome and Picro-Sirius red staining revealed a remarkable increase in collagen fiber compared with the normal diet group. Compared with mice that received CDAHFD alone, those supplemented with NYT exhibited reduced hepatic triglyceride and hydroxyproline levels and α-SMA expression. Additionally, compared with the group fed CDAHFD alone, the stained liver tissues of NYT-treated mice exhibited a reduction in Masson’s trichrome- and Picro-Sirius red-positive areas. Locomotor activity was significantly reduced in the CDAHFD-fed group compared with the normal diet group. In the NYT-treated group, the CDAHFD-induced decrease in locomotor activity was significantly suppressed. The findings indicate that NYT inhibited fatty and fibrotic changes in the livers of NASH mice and alleviated the decrease in locomotor activity. Therefore, NYT may serve as a novel therapeutic approach for NASH.
Graphical abstract

Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) cases are increasing worldwide, with a prevalence rate of 20–25% in the adult population [1, 2]. Non-alcoholic steatohepatitis (NASH), a progressive fibrotic form of NAFLD, comprises 25% of all NAFLD diagnoses [1, 2]. NASH is characterized by liver fibrosis with fatty liver as a pathological finding. Fibrosis refers to a condition in which the extracellular matrix, mainly composed of collagen, is excessively deposited in an organ. As liver fibrosis poses a risk of cirrhosis and cancer, the progression of liver fibrosis is associated with an increase in mortality [3,4,5]. Therefore, suppressing the progression of fibrosis is critical to effectively treat NASH. A multiple parallel hit hypothesis [6] has been proposed for the onset of NASH, which states that NASH is caused by the simultaneous progression of steatosis and fibrosis.
Currently, the main treatments for NASH are lifestyle improvements such as weight loss and aerobic exercise, as effective drug treatment methods targeting the liver have not yet been established [7, 8]. Therefore, the development of drugs against fibrosis in NASH is very important. In recent years, Kampo formulations that are effective against liver steatosis have attracted attention, and studies have reported their antifibrotic effects [9]. Ninjin’yoeito (NYT) is composed of 12 substances—Ginseng, Rehmannia Root, Japanese Angelica Root, Atractylodes Rhizome, Poria Sclerotium, Cinnamon Bark, Polygala Root, Peony Root, Citrus Unshiu Peel, Astragalus Root, Glycyrrhiza, and Schisandra Fruit (Table 1) [10]—and is used to treat decreased physical strength post-illness and post-surgery, fatigue, malaise, anorexia, night sweats, cold limbs, and anemia [11,12,13,14,15,16,17,18,19]. Previous studies have reported that NYT suppresses hepatic collagen accumulation in liver fibrosis model rats [20]. However, the efficacy of NYT in NASH mice, model mice with liver steatosis, is unknown. Therefore, in this study, we aimed to examine the protective effect of NYT using mice with NASH that were fed a choline-deficient, L-amino acid-defined, high-fat diet (CDAHFD). In addition, chronic liver diseases such as NASH/NAFLD are accompanied by behavioral changes such as fatigue and depression [21,22,23]. In fact, fatigue has been reported as a primary symptom in patients with NASH/NAFLD [24, 25]. Given that several reports have used reduced locomotor activity as a surrogate marker for fatiguing behavior [26,27,28], we also evaluated on spontaneous locomotor activity in NASH mouse model.
Materials and methods
NYT
NYT was manufactured by GMP Pharmaceutical Factory of Pharmaceutical Company, Kracie, Ltd. (lot no. E1510311A0, Qingdao, China). The analytical method and 3D-HPLC profile of the NYT extract have been reported previously [29].
Animals
Six-week-old C57BL/6J male mice were purchased from Japan SLC (Shizuoka, Japan) and maintained under the following conditions: temperature, 23 ± 2 °C; relative humidity, 55 ± 10%, and a 12:12 light–dark (L:D) cycle with the lights on from 8:00 to 20:00. During the preliminary breeding period, the mice were provided breeding feed, CE-2 (CLEA Japan, Tokyo, Japan).
NYT treatment in mice with NASH that were fed CDAHFD
Seven-week-old C57BL/6J male mice (weighing 19.3 ± 0.5 g) were divided into four groups (each group n = 8): normal diet group (normal group, CE-2), CDAHFD group (control group, A06071302; Research Diets, New Brunswick, NJ, USA), CDAHFD + 1000 mg/kg NYT group (NYT1000 group), and CDAHFD + 1500 mg/kg NYT group (NYT1500 group). Regarding NYT, a daily dose of 7.5 g (bulk extract 6.7 g) in humans (60 kg B.W.) is approximately 1500 mg/kg in mice (20 g B.W.) [30]. Based on this dosage, we divided the study into the low-dose 1000 mg/kg and equal-dose 1500 mg/kg groups. Given that CDAHFD reportedly induces NASH after 6 weeks of feeding [31], the duration of NYT administration was adjusted based on the time required to establish the NASH model. Subsequently, they were maintained for 6 weeks. An aqueous solution of NYT extract (0.1 ml per 10 g of mouse body weight) was orally administered once daily for 6 days a week to the NYT1000 and NYT1500 groups at 1000 and 1500 mg/kg of NYT, respectively. An equal quantity of distilled water was orally administered to the normal and control groups. The body weight of the mice was measured daily. On the last day of the experiment, the mice were subjected to laparotomy under anesthesia (isoflurane inhalation), and blood was collected from the abdominal inferior vena cava; the mice were subsequently euthanized. After blood collection, the liver, adipose tissue, and gastrocnemius muscle were harvested, and their wet weight was measured. Aspartate transaminase (AST), alanine transaminase (ALT), triglycerides, total cholesterol, and free fatty acid levels in the mice blood were measured using Transaminase CII Test Wako (FUJIFILM Wako Pure Chemical, Osaka, Japan), Triglyceride E-Test Kit (FUJIFILM Wako Pure Chemical), Cholesterol E-Test Wako Kit (FUJIFILM Wako Pure Chemical), LabAssay NEFA (FUJIFILM Wako Pure Chemical), and Synergy H1 Hybrid Multi-Mode Microplate Reader (BioTek Instruments, Tokyo, Japan).
Metabolic, histopathological, and gene expression analyses
Lipid extraction from the liver and gastrocnemius muscle was performed using a partial modification of the Folch method [32]; 5 and 2 ml of a chloroform and methanol mixture (2:1) was added to the liver and gastrocnemius muscle tissue sample, respectively, and homogenization was performed under ice-cold conditions. The mixture was then allowed to stand for 10 min and centrifuged at 3000 rpm for 10 min. The organic layer was separated, the organic solvent was removed using nitrogen gas, and 10 µl of ethanol was added. Then, triglyceride and total cholesterol levels were measured in the samples. Hydroxyproline was measured using Total Collagen Assay (QuickZyme Biosciences, Leiden, The Netherlands) according to the manufacturer’s recommended protocol and Synergy H1 Hybrid Multi-Mode Microplate Reader (BioTek Instruments).
Liver tissue fragments were fixed with 4% paraformaldehyde, embedded in paraffin, sliced into 4 µm sections, stained with hematoxylin–eosin (HE), Masson’s trichrome (Muto Pure Chemicals, Tokyo, Japan), and Picro-Sirius red staining (Polysciences, Warrington, PA, USA), and observed using an Axio Observer inverted microscope (Carl Zeiss Microscopy GmbH, Munich, Germany). For Oil red O staining (Junsei Chemical, Tokyo, Japan), liver tissues soaked in 4% paraformaldehyde were embedded using Tissue-Tek O.C.T. Compound (Sakura Finetek Japan, Torrance, CA, USA) and sliced into 10 µm sections and stained. Specimens were visualized using the Axio Observer Inverted microscope. Each stained section (three random microscopic fields per section; magnification: 200×) was quantified using ImageJ Fiji Software (National Institutes of Health, Bethesda, MD, USA).
Total RNA was extracted from liver samples stored in RNAlater Stabilization Solution (Thermo Fisher Scientific, Waltham, MA, USA) using TRIzol Reagent (Thermo Fisher Scientific). cDNA synthesis was performed using ReverTra Ace qPCR RT Master Mix with gDNA Remover (TOYOBO, Osaka, Japan). After the addition of THUNDERBIRD SYBR qPCR Mix (TOYOBO) and primers (Table 2), the gene expression level was calculated according to the 2–ΔΔCt method using StepOnePlus Real-Time PCR System (Thermo Fisher Scientific).
Locomotor activity analysis
Spontaneous locomotor activity of mice was measured 6 weeks after CDAHFD feeding using SUPERMEX PAT.P (Muromachi Kikai, Tokyo, Japan). The movement of mice was monitored using a passive infrared sensor and recorded using a digital counter. The mice were placed in plastic cages (30 × 20 × 13 cm; one mouse per cage). After acclimatization, spontaneous locomotor activity at night (12 h), which is the active phase of mice, was measured.
Statistical analysis
Measured values were expressed as mean ± standard deviation or median [25% tile, 75% tile]. Statistical analyses were performed using EZR [33], a statistical software that extends the functionality of R (version 3.6.1), and R Commander. Significance was determined using the Dunnett’s or Steel test; p < 0.05 was considered significant.
Results
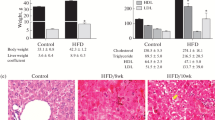
Effects of NYT on mice physiological characteristics
CDAHFD was given to the mice for 6 weeks to establish a NASH model [31]. After CDAHFD feeding, the control group showed significant weight loss and increased liver weight compared to the normal group, but no improvement was observed in the NYT1000 and NYT1500 groups (Table 3). Considering average daily food intake, there were no differences in the amount of food intake across the groups. The weight of white and brown adipose tissue substantially increased and tended to decrease (p = 0.06), respectively, in the control group compared with that of the normal group. The NYT1000 group showed a notable decrease and increase in white and brown adipose tissue weights, respectively, compared with that of the control group. Plasma biochemical analysis revealed changes in some metabolic markers due to CDAHFD feeding. However, no changes were induced by NYT treatment.
Effects of NYT on liver steatosis
To test whether NYT suppressed steatosis in the liver, steatosis-related factors were studied and histopathological analysis was conducted. HE and Oil Red O staining revealed increased lipid droplets in the control group compared with the normal group (Fig. 1a and b). Lipid droplets decreased in the NYT1000 and NYT1500 groups compared with that in the control group. In addition, triglyceride levels were drastically increased in the control group compared with that in the normal group and decreased in the NYT1500 group compared with that in the control group (Fig. 1c). Total cholesterol content was substantially reduced in the control group compared with that in the normal group (Fig. 1d). No such difference was observed in the NYT1000 and NYT1500 groups compared with that in the control group.
Effect of Ninjin’yoeito (NYT) on liver steatosis. a Hematoxylin–eosin (HE) and Oil red O staining of mouse livers to evaluate disease pathology (200×). b Percentage of Oil red O-positive area. c Hepatic triglycerides. d Hepatic total cholesterol. Data are presented as mean ± standard deviation (n = 6–8). *p < 0.05 and **p < 0.01 vs. the control group using Dunnett’s test
Effects of NYT on liver fibrosis
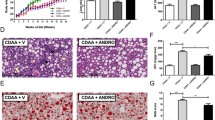
Next, histopathological analysis was performed, and fibrosis-related factors and gene expression were analyzed in liver fibrosis. Masson’s trichrome and Picro-Sirius red staining showed a clear increase in collagen fibers in the control group (Fig. 2a and b). Specifically, liver fibrosis was observed in mice with NASH. In addition, collagen fibers in mice with NASH were reduced by NYT treatment. Hydroxyproline was increased in the control group compared with that in the normal group but decreased in the NYT1000 and NYT1500 groups compared with that in the control group (Fig. 2c).
Effect of Ninjin’yoeito (NYT) on liver fibrosis. a Masson’s trichrome and Picro-Sirius red staining of the liver to evaluate disease pathology (200×). b The percentage of Masson’s trichrome-positive and Picro-Sirius red-positive areas. c Hepatic hydroxyproline. d Gene expression levels of α-smooth muscle actin (α-SMA) and transforming growth factor-β1 (TGF-β1) in the liver. Data are presented as mean ± standard deviation (n = 7–8). *p < 0.05 and **p < 0.01 vs. the control group using Dunnett’s test
The expression of α-smooth muscle actin (α-SMA), an activation marker of hepatic stellate cells, and transforming growth factor-β1 (TGF-β1), a promoter of hepatic fibrosis that binds to the receptor of hepatic stellate cells, was measured. The expression of α-SMA and TGF-β1 was drastically increased in the control group compared with that in the normal group (Fig. 2d). In addition, the expression of α-SMA was substantially decreased in the NYT1500 group compared with that in the control group.
Effects of NYT on spontaneous locomotor activity and skeletal muscle in mice with NASH
Measurement of spontaneous locomotor activity during the dark phase (12 h), which is the active phase in mice, revealed that compared with the normal group, the control group showed remarkably reduced locomotor activity (Fig. 3a). Specifically, a decrease in locomotor activity due to NASH was observed. However, locomotor activity increased in the NYT1000 and NYT1500 groups compared with that in the control group.
Effect of Ninjin’yoeito (NYT) on the locomotor activity and skeletal muscle weight of mice with non-alcoholic steatohepatitis (NASH). a Total locomotor activity during the dark phase (12 h) at 12 weeks. b Gastrocnemius muscle weight relative to body weight. c Triglycerides and d total cholesterol in the gastrocnemius muscle. Data are presented as a median [25% tile, 75% tile] and b-d mean ± standard deviation (n = 6–8). *p < 0.05 and **p < 0.01 vs. the control group using the Steel test
The weight and lipid content of the gastrocnemius muscle were measured after necropsy (Fig. 3b–d). No significant difference in the weight of gastrocnemius muscle was observed among the groups (Fig. 3b), and no locomotor disorders were observed. Furthermore, no significant differences in triglyceride and total cholesterol levels in the gastrocnemius muscle were observed among the groups (Fig. 3c and d).
Discussion
Various approaches can be employed to establish a NASH model. Although a high-fat diet is a widely recognized method and deemed more appropriate for evaluating drug effects in clinically relevant conditions compared with other methods, the development of fibrosis is a time-dependent process, and the precise timing of early fibrosis remains uncertain. Given that the main objective of the current study was to confirm the protective effect of NYT against the progression of liver fibrosis, we opted for the CDAHFD model, in which the initial stage of fibrosis can be observed at 6 weeks of feeding [31].
In this study, hepatic steatosis, hepatic fibrosis, and decreased locomotor activity during the active phase were confirmed in mice with NASH that were fed CDAHFD, which closely mimic the pathology of NASH in the human liver [31, 34]. Furthermore, the oral administration of NYT protected against the pathological characteristics of NASH in mice. However, it should be noted that CDAHFD-induced NASH is different from human NASH in that it disables intrahepatic triglyceride metabolism, decreasing triglyceride and cholesterol levels in the blood [31, 35]. Moreover, because pharmacotherapy for NASH is not well established and the mechanism of NYT in the treatment of NASH was largely unknown, a positive control was not established in this study.
The protective effect of NYT-induced hepatic triglyceride accumulation led to the amelioration of liver steatosis. Triglycerides are known to accumulate in the liver of NASH mice [34], and NYT suppressed their accumulation. Cholesterol content in the liver was decreased by CDAHFD feeding, as previously reported [35]. NYT had no effect on liver cholesterol. How NYT affects hepatic lipid incretion, de novo lipogenesis, β-oxidation, and very low-density lipoprotein-TG excretion remains unclear. Poria Cocos, Atractylodes Rhizome, Ginseng, and their constituent herbs of NYT increase the expression of peroxisome proliferator-activated receptor α (PPARα) [36,37,38]. In addition, ginsenoside Rb2, a component of ginseng, induces Sirtuin1 [39]. NYT has been reported to act on peroxisome proliferator-activated receptor gamma coactivator 1α(PGC-1α), which is involved in the expression of PPARα in skeletal muscle [40] and may suppress fat accumulation by promoting β-oxidation of fatty acids through increasing the expression of PPARα. Therefore, this drug may inhibit fat accumulation by promoting β-oxidation of fatty acids through enhancing the expression of PPARα.
Activated hepatic stellate cells produce collagen type I, which is the main cause of fibrosis [41,42,43]. Given that hepatic stellate cells are deeply involved in liver fibrosis in NASH [1, 44], the same may be true for the mouse model in this study. The levels of α-SMA, an activation marker of hepatic stellate cells [45], and TGF-β1, a promoter of hepatic fibrosis that binds to the receptor of hepatic stellate cells, were measured. As a reduction in α-SMA expression was observed, the antifibrotic action by NYT may be accompanied by the suppression of hepatic stellate cell activation. The activation of hepatic stellate cells involves various pathways such as the TGF-β/SMAD, Wnt, and Gas6/Axl signaling pathways [46, 47], and the development of hepatic fibrosis is very complex. Of these pathways, NYT does not affect TGF-β1 expression, suggesting that it may be involved in signaling pathways other than TGF-β1.
Skeletal muscle atrophy has been reported in NASH/NAFLD [48]. Therefore, in this study, muscular atrophy in the legs is assumed to be a cause of decreased locomotor activity during the active phase. However, no change in weight of the gastrocnemius muscle, an important lower limb muscle, was observed regardless of CDAHFD feeding or NYT treatment. In clinical practice, a relative decrease in skeletal muscle weight associated with fat accumulation in patients with NAFLD has been reported [49]; however, no change in lipid content was observed in the skeletal muscles of the mice in this study. Since triglyceride secretion from the liver to the blood vessels is suppressed by CDAHFD, fat accumulation may not have had an impact on the weight of the skeletal muscle. Therefore, from the results of skeletal muscle weight and fat content, it can be inferred that locomotor dysfunction was not involved in the decrease in locomotor activity. However, in some reports, decreased locomotor activity has been used as a surrogate marker for fatigue behavior [26,27,28]. Fatigue behavior with liver lesions has been reported in chronic liver diseases such as NASH [21,22,23]. Consequently, fatigue may be involved in the reduced locomotor activity of the mice in this study. Neuroinflammation in the brain, which is mediated by inflammation of the liver, is thought to be a cause of fatigue in NASH [23, 50,51,52]. NYT has been shown to be effective against fatigue and malaise [13, 15], improve liver lesions, and exert anti-inflammatory effects [20]. Therefore, the alleviation of locomotor activity reduction by NYT may involve a reduction of fatigue through the amelioration of liver lesions and inflammation. Future studies should elucidate the effectiveness of NYT on fatigue.
In this study, NYT was shown to improve hepatic steatosis, hepatic fibrosis, and spontaneous locomotor activity reduction during the active phase in NASH. In addition, considering that mice with NASH fed a CDAHFD are regarded to exhibit liver pathology closely resembling human NASH [31, 34], NYT holds promise as a potential therapeutic option for NASH treatment.
Data availability
All datasets generated during this study are included in the article.
References
Schwabe RF, Tabas I, Pajvani UB (2020) Mechanisms of fibrosis development in nonalcoholic steatohepatitis. Gastroenterology 158:1913–1928. https://doi.org/10.1053/j.gastro.2019.11.311
Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E (2018) Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 15:11–20. https://doi.org/10.1038/nrgastro.2017.109
Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, Sebastiani G, Ekstedt M, Hagstrom H, Nasr P, Stal P, Wong VWS, Kechagias S, Hultcrantz R, Loomba R (2017) Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology 65:1557–1565. https://doi.org/10.1002/hep.29085
Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F (2015) Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149:389-397.e10. https://doi.org/10.1053/j.gastro.2015.04.043
Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, Castellanos M, Aller-de la Fuente R, Metwally M, Eslam M, Gonzalez-Fabian L, Sanz MAQ, Conde-Martin AF, De Boer B, McLeod D, Chan AWH, Chalasani N, George J, Adams LA, Romero-Gomez M (2018) Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology 155:443-457.e17. https://doi.org/10.1053/j.gastro.2018.04.034
Tilg H, Moschen AR (2010) Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 52:1836–1846. https://doi.org/10.1002/hep.24001
Hallsworth K, Adams LA (2019) Lifestyle modification in NAFLD/NASH: facts and figures. JHEP Rep 1:468–479. https://doi.org/10.1016/j.jhepr.2019.10.008
Raza S, Rajak S, Upadhyay A, Tewari A, Anthony Sinha R (2021) Current treatment paradigms and emerging therapies for NAFLD/NASH. Front Biosci (Landmark Ed) 26:206–237. https://doi.org/10.2741/4892
Takahashi Y, Soejima Y, Kumagai A, Watanabe M, Uozaki H, Fukusato T (2014) Inhibitory effects of Japanese herbal medicines sho-saiko-to and juzen-taiho-to on nonalcoholic steatohepatitis in mice. PLoS ONE 9:e87279. https://doi.org/10.1371/journal.pone.0087279
Takayama S, Michihara S, Kimura Y, Morinaga A, Miyakawa K, Tsushima N, Otani K, Jinnai A, Aso Y, Okabayashi A, Arita R, Nogami T, Inui A (2023) Review of frequently used Kampo prescriptions: part 4, Ninjin’yoeito. Trad Kampo Med 10:224–252. https://doi.org/10.1002/tkm2.1387
Kuniaki H, Akihiko T, Tetsuya H, Hatsuko M, Tomoko K, Shin O, Sojiro K, Mayumi Y, Fumihiro Y, Shintaro S, Tsukasa O, Hironori S (2018) Improvement in frailty in a patient with severe chronic obstructive pulmonary disease after Ninjin’yoeito therapy: a case report. Front Nutr 5:71. https://doi.org/10.3389/fnut.2018.00071
Xu Y, Chen Y, Li P, Wang XS (2015) Ren Shen Yangrong Tang for fatigue in cancer survivors: a phase I/II open-label study. J Altern Complement Med 21:281–287. https://doi.org/10.1089/acm.2014.0211
Ito T, Konishi A, Tsubokura Y, Azuma Y, Hotta M, Yoshimura H, Nakanishi T, Fujita S, Nakaya A, Satake A, Ishii K, Nomura S (2018) Combined use of Ninjin’yoeito improves subjective fatigue caused by lenalidomide in patients with multiple myeloma: a retrospective study. Front Nutr 5:72. https://doi.org/10.3389/fnut.2018.00072
Ohsawa M, Tanaka Y, Tanaka Y, Ehara Y, Makita S, Onaka K (2017) A possibility of simultaneous treatment with the multicomponent drug, Ninjin’yoeito, for anorexia, apathy, and cognitive dysfunction in frail Alzheimer’s disease patients: an open-label pilot study. J Alzheimers Dis Rep 1:229–235. https://doi.org/10.3233/ADR-170026
Suzuki S, Aihara F, Shibahara M, Sakai K (2019) Safety and effectiveness of Ninjin’yoeito: a utilization study in elderly patients. Front Nutr 6:14. https://doi.org/10.3389/fnut.2019.00014
Iwase S, Yamaguchi T, Miyaji T, Terawaki K, Inui A, Uezono Y (2012) The clinical use of Kampo medicines (traditional Japanese herbal treatments) for controlling cancer patients’ symptoms in Japan: a national cross-sectional survey. BMC Complement Altern Med 12:222. https://doi.org/10.1186/1472-6882-12-222
Yoshikawa H, Ikeuchi T, Kai Y (1999) Clinical efficacy of Ninjin-Youei-To for recovery of reduced physical strength of the patients after prostate hypertrophy operation. Kampo Med (in Japanese) 49:617–622. https://doi.org/10.3937/KAMPOMED.49.617
Ando N (1999) Blood making effect of Ninjin-yoei-to (Ren-shen-yang-rong-tang) as monotherapy in obstetric and gynecologic patients with anemia. Kampo Med (in Japanese) 50:461–470. https://doi.org/10.3937/KAMPOMED.50.461
Motoo Y, Mouri H, Ohtsubo K, Yamaguchi Y, Watanabe H, Sawabu N (2005) Herbal medicine Ninjinyoeito ameliorates ribavirin-induced anemia in chronic hepatitis C: a randomized controlled trial. World J Gastroenterol 11:4013–4017. https://doi.org/10.3748/wjg.v11.i26.4013
Ochi T, Kawakita T, Nomoto K (2004) Effects of Hochu-ekki-to and Ninjin-youei-to, traditional Japanese medicines, on porcine serum-induced liver fibrosis in rats. Immunopharmacol Immunotoxicol 26:285–298. https://doi.org/10.1081/iph-120037726
Swain MG (2006) Fatigue in liver disease: pathophysiology and clinical management. Can J Gastroenterol 20:181–188. https://doi.org/10.1155/2006/624832
D’Mello C, Swain MG (2014) Liver-brain interactions in inflammatory liver diseases: implications for fatigue and mood disorders. Brain Behav Immun 35:9–20. https://doi.org/10.1016/j.bbi.2013.10.009
Swain MG, Jones DEJ (2019) Fatigue in chronic liver disease: new insights and therapeutic approaches. Liver Int 39:6–19. https://doi.org/10.1111/liv.13919
Newton JL, Jones DE, Henderson E, Kane L, Wilton K, Burt AD, Day CP (2008) Fatigue in non-alcoholic fatty liver disease (NAFLD) is significant and associates with inactivity and excessive daytime sleepiness but not with liver disease severity or insulin resistance. Gut 57:807–813. https://doi.org/10.1136/gut.2007.139303
Younossi ZM, Wong VW, Anstee QM, Romero-Gomez M, Trauner MH, Harrison SA, Lawitz EJ, Okanoue T, Camargo M, Kersey K, Myers RP, Goodman Z, Stepanova M (2020) Fatigue and pruritus in patients with advanced fibrosis due to nonalcoholic steatohepatitis: the impact on patient-reported outcomes. Hepatol Commun 4:1637–1650. https://doi.org/10.1002/hep4.1581
Burak KW, Le T, Swain MG (2002) Increased sensitivity to the locomotor-activating effects of corticotropin-releasing hormone in cholestatic rats. Gastroenterology 122:681–688. https://doi.org/10.1053/gast.2002.31878
Contarino A, Dellu F, Koob GF, Smith GW, Lee KF, Vale WW, Gold LH (2000) Dissociation of locomotor activation and suppression of food intake induced by CRF in CRFR1-deficient mice. Endocrinology 141:2698–2702. https://doi.org/10.1210/endo.141.7.7653
de Paiva VN, Lima SN, Fernandes MM, Soncini R, Andrade CA, Giusti-Paiva A (2010) Prostaglandins mediate depressive-like behaviour induced by endotoxin in mice. Behav Brain Res 215:146–151. https://doi.org/10.1016/j.bbr.2010.07.015
Murata K, Fujita N, Takahashi R, Inui A (2018) Ninjinyoeito improves behavioral abnormalities and hippocampal neurogenesis in the corticosterone model of depression. Front Pharmacol 9:1216. https://doi.org/10.3389/fphar.2018.01216
Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7:27–31. https://doi.org/10.4103/0976-0105.177703
Matsumoto M, Hada N, Sakamaki Y, Uno A, Shiga T, Tanaka C, Ito T, Katsume A, Sudoh M (2013) An improved mouse model that rapidly develops fibrosis in non-alcoholic steatohepatitis. Int J Exp Pathol 94:93–103. https://doi.org/10.1111/iep.12008
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509. https://doi.org/10.1016/S0021-9258(18)64849-5
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458. https://doi.org/10.1038/bmt.2012.244
Yanguas SC, Cogliati B, Willebrords J, Maes M, Colle I, van den Bossche B, Souza de Oliveira CPM, Andraus W, Alves VA, Leclercq I, Vinken M (2016) Experimental models of liver fibrosis. Arch Toxicol 90:1025–1048. https://doi.org/10.1007/s00204-015-1543-4
Yasuda D, Torii H, Shimizu R, Hiraoka Y, Kume N (2020) Reduced serum cholesterol and triglyceride levels in a choline-deficient L-amino acid-defined high-fat diet (CDAHFD)-induced mouse model of non-alcoholic steatohepatitis (NASH). Biol Pharm Bull 43:616–618. https://doi.org/10.1248/bpb.b19-00338
He J, Yang Y, Zhang F, Li Y, Li X, Pu X, He X, Zhang M, Yang X, Yu Q, Qi Y, Li X, Yu J (2022) Effects of Poria cocos extract on metabolic dysfunction-associated fatty liver disease via the FXR/PPARα-SREBPs pathway. Front Pharmacol 13:1007274. https://doi.org/10.3389/fphar.2022.1007274
Heo G, Kim Y, Kim EL, Park S, Rhee SH, Jung JH, Im E (2023) Atractylodin ameliorates colitis via PPARα agonism. Int J Mol Sci 24:802. https://doi.org/10.3390/ijms24010802
Xu Y, Yang C, Zhang S, Li J, Huang W (2018) Ginsenoside Rg1 protects against non-alcoholic fatty liver disease by ameliorating lipid peroxidation, endoplasmic reticulum stress, and inflammasome activation. Biol Pharm Bull 41:1638–1644. https://doi.org/10.1248/bpb.b18-00132
Huang Q, Wang T, Yang L, Wang HY (2017) Ginsenoside Rb2 alleviates hepatic lipid accumulation by restoring autophagy via induction of Sirt1 and activation of AMPK. Int J Mol Sci 18:1063. https://doi.org/10.3390/ijms18051063
Miyamoto A, Asai K, Kadotani H, Maruyama N, Kubo H, Okamoto A, Sato K, Yamada K, Yamada K, Ijiri N, Watanabe T, Kawaguchi T (2020) Ninjin’yoeito ameliorates skeletal muscle complications in COPD model mice by upregulating peroxisome proliferator-activated receptor γ coactivator-1α expression. Int J Chron Obstruct Pulmon Dis 15:3063–3077. https://doi.org/10.2147/COPD.S280401
Birukawa NK, Murase K, Sato Y, Kosaka A, Yoneda A, Nishita H, Fujita R, Nishimura M, Ninomiya T, Kajiwara K, Miyazaki M, Nakashima Y, Ota S, Murakami Y, Tanaka Y, Minomi K, Tamura Y, Niitsu Y (2014) Activated hepatic stellate cells are dependent on self-collagen, cleaved by membrane type 1 matrix metalloproteinase for their growth. J Biol Chem 289:20209–20221. https://doi.org/10.1074/jbc.M113.544494
Hohenester S, Kanitz V, Kremer AE, Paulusma CC, Wimmer R, Kuehn H, Denk G, Horst D, Oude Elferink R, Beuers U (2020) Glycochenodeoxycholate promotes liver fibrosis in mice with hepatocellular cholestasis. Cells 9:281. https://doi.org/10.3390/cells9020281
Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, Pradere JP, Schwabe RF (2013) Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun 4:2823. https://doi.org/10.1038/ncomms3823
Koyama Y, Brenner DA (2017) Liver inflammation and fibrosis. J Clin Invest 127:55–64. https://doi.org/10.1172/JCI88881
Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, Pellicoro A, Raschperger E, Betsholtz C, Ruminski PG, Griggs DW, Prinsen MJ, Maher JJ, Iredale JP, Lacy-Hulbert A, Adams RH, Sheppard D (2013) Selective αv integrin depletion identifies a core, targetable molecular pathway that regulates fibrosis across solid organs. Nat Med 19:1617–1624. https://doi.org/10.1038/nm.3282
Zhang CY, Yuan WG, He P, Lei JH, Wang CX (2016) Liver fibrosis and hepatic stellate cells: etiology, pathological hallmarks and therapeutic targets. World J Gastroenterol 22:10512–10522. https://doi.org/10.3748/wjg.v22.i48.10512
Xu F, Liu C, Zhou D, Zhang L (2016) TGF-β/SMAD pathway and its regulation in hepatic fibrosis. J Histochem Cytochem 64:157–167. https://doi.org/10.1369/0022155415627681
Pasmans K, Adriaens ME, Olinga P, Langen R, Rensen SS, Schaap FG, Olde Damink SWM, Caiment F, van Loon LJC, Blaak EE, Meex RCR (2021) Hepatic steatosis contributes to the development of muscle atrophy via inter-organ crosstalk. Front Endocrinol (Lausanne) 12:733625. https://doi.org/10.3389/fendo.2021.733625
Linge J, Ekstedt M, Dahlqvist Leinhard O (2021) Adverse muscle composition is linked to poor functional performance and metabolic comorbidities in NAFLD. JHEP Rep 3:100197. https://doi.org/10.1016/j.jhepr.2020.100197
Gerber LH, Weinstein AA, Mehta R, Younossi ZM (2019) Importance of fatigue and its measurement in chronic liver disease. World J Gastroenterol 25:3669–3683. https://doi.org/10.3748/wjg.v25.i28.3669
D’Mello C, Swain MG (2011) Liver-brain inflammation axis. Am J Physiol Gastrointest Liver Physiol 301:G749–G761. https://doi.org/10.1152/ajpgi.00184.2011
Mondal A, Bose D, Saha P, Sarkar S, Seth R, Kimono D, Albadrani M, Nagarkatti M, Nagarkatti P, Chatterjee S (2020) Lipocalin 2 induces neuroinflammation and blood-brain barrier dysfunction through liver-brain axis in murine model of nonalcoholic steatohepatitis. J Neuroinflammation 17:201. https://doi.org/10.1186/s12974-020-01876-4
Acknowledgements
We would like to thank our colleagues at Pharmaceutical Company, Kracie, Ltd. for providing test assistance in this study. We would also like to thank Editage (www.editage.com) for English language editing.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
KT, MK, and YA performed the experiments and analyzed the data. NF, SC, SM, L-KH, and RT initiated and supervised the study. KT, MK, YA, SM, and L-KH designed the experiments. KT, MK, YA, SC, and L-KH wrote the manuscript. All the authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
All the authors are employees of Pharmaceutical Company, Kracie, Ltd.
Ethics approval
All animal experiments were conducted in accordance with the Experimental Animal Care Committee of Kracie, Ltd. guidelines and Standards for Breeding and Storage of Experimental Animals (Prime Minister’s Office Notification No. 6 of March 1980).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takano, K., Kaneda, M., Aoki, Y. et al. The protective effects of Ninjin’yoeito against liver steatosis/fibrosis in a non-alcoholic steatohepatitis model mouse. J Nat Med (2024). https://doi.org/10.1007/s11418-024-01786-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11418-024-01786-2