Abstract
Understanding the dynamic changes in grape characteristics during ripening is crucial for enhancing grape quality, which, in turn, influences overall viticulture practices. This study aimed to investigate the temporal and cultivar-specific variations in grape characteristics, focusing on BAs, phenolic compounds, antioxidant enzymes, and organic acids. Our results revealed that Bozcaada Çavuşu*Kuntra and Kuntra grapes exhibited the most significant growth in size, weight, and maturity index at the final developmental stage (EL-38), while Vasilâki showed a nuanced maturation pattern with significant increases in berry width but not weight. Across all varieties, the highest maturity index and Total Soluble Solids percentages were observed at EL-38. The investigation of BAs revealed a significant decrease in Put levels from 2.62 at EL-32 to 2.04 mg L−1 at EL-38, contrasting with an increasing trend in Agm concentrations from 0.25 at EL-29 to 0.37 mg L−1 at EL-38. Bozcaada Çavuşu*Kuntra stood out with the highest Cad concentration at 2.96 mg L−1. The analysis of phenolic compounds highlighted a distinct temporal pattern for gallic acid, increasing from 1.60 at EL-29 to 3.77 g L−1 at EL-32 and maintaining stability at 3.74 g L−1 at EL-38. Bozcaada Çavuşu*Vasilâki consistently exhibited higher concentrations of phenolic compounds, such as epicatechin (5.69 g L−1) and tyrosol (7.00 g L−1). Antioxidant enzymes like catalase and superoxide dismutase in grape cultivars showed significant temporal variations, indicating a dynamic oxidative stress response during berry ripening. Catalase activity decreased from 14.04 to 11.20 EU/gr berry between EL-29 and EL-38. The multifaceted regulation of superoxide dismutase by sampling time and cultivar highlighted the complexity of antioxidant dynamics in grapevine physiology. In conclusion, this comprehensive study sheds light on the intricate interplay of temporal and cultivar-specific factors influencing grape characteristics, providing valuable insights for optimizing viticulture practices and enhancing overall grape quality.
Similar content being viewed by others
Introduction
The reproductive development of grapevines exerts a direct influence on yield and significantly impacts the quality of berries and wine, encompassing traits, such as berry and cluster size, cluster compactness, and the ratio of berry skin to pulp (Najmaddin et al. 2011). However, the flowers of rare Vitis vinifera L. cv varieties like Bozcaada Çavuşu are not capable of being fertilized by their own pollen (Dardeniz et al. 2011). The expression of female dominance in certain grape cultivar introduces a fascinating dimension to the intricate world of grape reproductive biology (Jovanović-Cvetković et al. 2016). The downward curvature of its stamens signifies a clear emphasis on the female reproductive organs, notably the pistil, in the grapevine’s overall strategy for reproduction. This trait is not merely a botanical peculiarity; rather, it provides valuable insights into the evolutionary history and ecological context of the cultivar. In this regards, the physiological adaptations associated with female dominance in Bozcaada Çavuşu likely play a role in optimizing pollination and fertilization processes. Grape varieties manifesting self-incompatibility are commonly distinguished by a phenomenon wherein their incapacity for self-pollination necessitates the presence of other grape cultivars harboring compatible pollen to facilitate successful fertilization and fruit set (Tello et al. 2018). This characteristic indicates the importance of male grape cultivars as compatible pollinators in achieving optimal reproductive outcomes. The intricate fertilization biology of grape cultivars has, indeed, been the focus of numerous studies, particularly in exploring pollination and fertilization in wild grapevine relatives and locally significant varieties facing challenges in the processes of fertilization and berry setting (Caporali et al. 2003; Abreu et al. 2006; Gallardo et al. 2009). Although the morphological development of grapevine inflorescences is well documented, there is limited information concerning varietal dynamics related to compatibility during cross-pollination and cross-breeding, as well as the potential outcomes of different cross-breeds. Despite the prevalent biological process of pollination in cultivated grapevines, uncertainties persist regarding the implications of cross-fertilization on grape productivity and quality. Moreover, there is a scarcity of research exploring the intricate relationships among floral morphology, reproductive strategies, and nutritional characteristics in grape varieties, with a specific emphasis on the unique traits exhibited by different grape cultivars. A comprehensive understanding of the nuanced aspects of pollination, such as the distinctive downward curvature of stamens observed in these cultivars, is essential for successful cultivation, necessitating the identification of compatible pollinizer counterparts. This knowledge holds paramount importance for grape producers aiming to enhance fruit setting volume, attain high-quality yields, and contribute to a more comprehensive comprehension of grapevine reproductive biology and nutritional composition.
Previous studies reveal diverse approaches to pollination in grapevine varieties (V. vinifera ssp.), combining characteristics of both insect- and wind-pollinated plants (Munoz-Rodríguez et al. 2011). The methods through which pollination occurs whether by insects, wind, or self-pollination vary among researchers. Some argue that hermaphroditic grapevine varieties are primarily self-pollinating (Rombough 2002), while others assert simultaneous self- and cross-pollination, with self-pollination holding greater significance (Gurasashvili and Vashakidze 2004). On the contrary, Vashakidze et al. (1982) suggest the simultaneous occurrence of both types of pollination, with prevalence determined by genotype. These varying perspectives underscore the complex fertilization biology of grapes, necessitating further research for reliable information in hybridization studies and to optimize yield and quality. In the realm of grape development, while we possess a comprehensive understanding of the inflorescence’s morphological growth, crossing compatibility, and potential outcomes, there is a notable lack of information concerning the interplay among grape varieties regarding physiological femaleness (female dominance) and their available pollinator varieties. Despite these reports, literature investigations surprisingly offer limited insights into the impact of physiological femaleness and their available pollinator varieties on biogenic amines, phenolic compounds, antioxidant enzymes, and organic acids in berries. This understanding is essential for grape producers aiming to maximize fruit setting volume, attain yields of high quality, and contribute to a more comprehensive comprehension of grapevine reproductive biology and nutritional composition. The primary objectives of this study were to (I) reveal biogenic amines, phenolic compounds, antioxidant enzymes, and organic acids of the cross-pollination with Vasilâki and Kuntra in berry set of Bozcaada Çavuşu, the popular grape cultivar that is well accepted as physiological femaleness in Türkiye and (ii) to compare the levels of biogenic amines, phenolic compounds, antioxidant enzymes, and organic acids in well-known commercial grape varieties, including Vasilâki and Kuntra, with those in Bozcaada Çavuşu.
Materials and Methods
Plant Material
The study focused on 30-year-old grapevine cultivars, namely Bozcaada Çavuşu, Kuntra (Karasakız), and Vasilâki, all grafted onto 5 BB rootstock, situated in Bozcaada, Turkey (coordinates: 26° 05′ 40″ East Longitude and 39° 49′ 11″ North Latitude). The investigation included two trials: one involving the pollination of Bozcaada Çavuşu by Kuntra and the other by Vasilâki, with a paternity (pollinator) ratio of 2/4 for the varieties ‛Kuntra’ and ‛Vasilâki’ to ‛Bozcaada Çavuşu’ in the year 2022. The research encompassed two distinct vineyard settings, each meticulously designed to facilitate open pollination conditions for the grape varieties under investigation. To achieve this, the vineyard layout followed a specific trial design known as the 2/4 pattern. In this arrangement, two Kuntra grape varieties were planted alongside four Bozcaada Çavuşu grape varieties. This pattern was consistently applied to the other vineyard conditions as well, where two Vasilâki grape varieties were positioned next to four Bozcaada Çavuşu grape varieties, adhering to the same trial configuration. The Bozcaada Çavuşu grape variety is recognized for its large, amber yellow berries with a distinctive flavor. These grapes are noted for their juicy content and thin skin, boasting a unique flavor inherent to the variety. The grape clusters were described as being particularly large, winged conical in shape, and relatively sparse in density. For pollination purposes, the Bozcaada Çavuşu was crossed with Kuntra and Vasilâki pollinator varieties in specific ratios, following the method outlined by Dardeniz et al. (2011). The Kuntra variety, with reddish purple, round, and large berries, ripens mid-season and is typically pruned short. The clusters are winged conical, large, and densely structured. Vasilâki, also pruned short and maturing mid-season, yields yellow, round, medium-large berries, and its clusters are cylindrical, ranging from small to medium size, with a dense structure. The planting arrangement employed a 1.4 × 1.4 m design with a goble trellis and the spur-pruned vines displayed 12–15 shoots per plant. This spacing allowed for optimal sunlight exposure and air circulation, essential for vine health and disease prevention. To ensure the well-being of the vines, a range of cultural practices was implemented. This included regular pruning to manage growth and enhance fruit production, meticulous canopy management to improve light and air around grape clusters, and soil management techniques. Integrated pest management and precise nutrient management were also integral parts of the vineyard’s practices. These measures were tailored to the vineyard’s specific conditions and regularly adjusted to maintain vine health and productivity. Sample collections were randomly conducted from the upper, middle, and lower sections of the clusters, with three collection instances. In this investigation, the stages EL-29, EL-32, and EL-38 corresponded to the phases where berries begin to veraison, berries brighten in color, and berries are ripe for harvest, respectively (Fig. 1). The sampling protocol adhered to the guidelines outlined by Coombe (1995). Irrigation was not employed in the vineyard at any point during the year; viticulture was conducted under dry-farming conditions. Climate data pertinent to the vineyard locale is provided in Supplementary Material 1. Collected clusters were promptly stored at 4 °C in the laboratory and subsequently preserved at − 80 °C for further analysis.
Chemicals and Cluster Characteristics, and Must Composition
Standard solutions encompassing spermine, agmatine, spermidine, serotonin, histamine, tryptamine, dopamine, norepinephrine, cadaverine, trimethylamine, putrescine, and tyramine were procured from Sigma-Aldrich Chemie, Steinheim, Germany. The berries were manually pressed using a hand-press, and the resulting grape musts were promptly subjected to analysis. Subsequently, the juice underwent centrifugation at 4000 revolutions per minute (rpm) for a duration of 7 min, facilitated by the Hettich Zentrifugen Universal 320 centrifuge from Germany. The resulting clarified juice was then utilized for the assessment of titratable acidity (TA), maturation index (MI-Brix), and total soluble solids (TSS). Total soluble solids were quantified using a digital refractometer (BRX-242 Erma, Tokyo, Japan). The maturation index and TA were determined through the utilization of an autotitrator. Specifically, must samples be analyzed for TA employing 0.1-M sodium hydroxide (NaOH) with the G20S autotitrator from Mettler Toledo, Switzerland, with results expressed as g/L. Berry dimensions, encompassing width and length, were meticulously measured using a precision caliper. Berry weight, on the other hand, was accurately determined utilizing a high-precision scale.
Isolation of Amines from Grape Varieties
For each grape variety, 5 g of berries underwent homogenization using an Ultra-turax homogenizer with 0.5 mL of 70% perchloric acid. The resulting homogenate (X g) was centrifuged at 10,000 rpm for 10 min, and the supernatant was collected, filtered through a 0.22 mm membrane, and then diluted with 10% perchloric acid to match the initial homogenate weight. Subsequently, the sample was filtered through 0.45 μm and injected into the HPLC.
Identification of Amines from Grape Varieties by HPLC
The separation and quantification of biogenic amines followed the method outlined by Incesu et al. (2022), with adaptations. Samples were injected onto a reverse-phase column (Bondapak C18, 300 × 3.9 mm, 10 mm; Waters, Milford, MA, USA) connected to a Waters Alliance Liquid Chromatograph with a Waters 474 fluorescence detector (Milford, MA, USA). Post-column derivatization, employing 2-mercaptoethanol and o-phthalaldehyde, was implemented to enhance detection. Peaks were identified using authentic standards, and quantitation relied on calibration curves within the range of 1 to 30 mg/L for spermine and 0.1 to 10 mg/L for other amines. The biogenic amine content of samples was expressed in mg L−1 fresh weight.
Identification of Phenolic Compounds from Grape Cultivars by HPLC
Berry samples were sourced from the grape varieties, collected at different developmental stages as outlined in “Plant Material” section, and underwent analysis for phenolic compounds. Sample preparation involved triturating whole berry samples obtained from clusters using a conventional beater until a homogeneous berry sample was achieved for analysis. The analyzed phenolic compounds included gallic acid, vanillic acid, trans-caffeic acid, trans-p-coumaric acid, ferulic acid, kaftaric acid, catechin, epicatechin, quercetin, routine, myricetin, and tyrosol. A modified version of the method by Barrado et al. (2009) was employed for phenolic compound extraction, with three replications. The triturated berry samples were mixed with distilled water at a 1:1 ratio and then centrifuged at 15.000 rpm for 15 min. The upper part of the samples was filtered using 0.45-μm MF-Millipore filters, and the filtered samples were injected into an HPLC device for phenolic compound analysis. Chromatography assays were conducted using an Agilent 1100 HPLC device equipped with a diode array detector (Agilent, Santa Clara, CA, USA) and a 4-μm octadecyl-silica column (4.6 × 250 mm, Hichrom, Reading, UK). The mobile phase consisted of two components: A—methanol:water:acetic acid (10:28:2) and B—methanol:water:acetic acid (90:8:2). Phenolic compounds were detected at 254 nm and 280 nm, with an injection volume of 20 μL and a flow rate set at 1 mL/min.
Identification of Antioxidants from Grape Cultivars by HPLC
Berry samples from grape varieties underwent a preparatory process in accordance with the protocol outlined by Keskin et al. (2022). Enzymatic activities were spectrophotometrically detected at a temperature of 25 °C using a Shimadzu 1208 UV spectrophotometer (Kyoto, Japan). To assess glutathione (GSH) activity in the berries, the method by Minucci et al. (2009) was applied, allowing for the quantification of GSH activity in the raisin samples. Additionally, the activities of two other enzymes, namely glutathione reductase (GR, EC 1.8.1.7) and glutathione S-transferase (GST, EC 2.5.1.18), were determined following the methodology outlined by Chikezie et al. (2009), with the initiation of the enzymatic reactions indicated by the addition of the enzyme solution. Furthermore, the activities of superoxide dismutase (SOD, EC 1.15.1.1), catalase (CAT, EC 1.11.1.6), and peroxidase (POD, EC 1.11.1.7) in the raisin samples were assessed based on the methods outlined by Abedi and Pakniyat (2010) and Angelini et al. (1990). These enzymatic activities were evaluated spectrophotometrically at 25 °C.
Identification of Organic Acids from Grape Cultivars by HPLC
The extraction of organic acids followed the method developed by Keskin et al. (2021). The extraction process commenced by preparing a mixture that combined 5 mL of grape must with 20 mL of a 0.009-M NH2SO4 solution. This mixture underwent thorough homogenization, 1 h of agitation on a shaker, and subsequent centrifugation at 15.000 rpm for 15 min. The resulting supernatants were subjected to filtration, initially through filter paper to remove larger particles, followed by two additional filtrations using a 0.45-μm membrane filter to eliminate finer particulate matter. The filtered solutions underwent further purification through passage through a SEP-PAK C18 cartridge. For the subsequent analysis of the extracted organic acids, high-performance liquid chromatography (HPLC) was employed. An Aminex column (HPX-87 H, 300 mm × 7.8 mm) served as the chromatographic medium for the separation and quantification of organic acids in the samples. Throughout this study, chemicals with a high degree of analytical purity were utilized. Standards, specifically tartaric, malic, citric, succinic, and fumaric acids, were sourced from Sigma-Aldrich in St. Louis, USA.
Statistical Analysis
All descriptive analyses were executed utilizing the agricolae package in R Studio (Mendiburu 2023). The influence of sampling time, cultivar, and their interactions on biogenic amines, phenolic compounds, antioxidant enzymes, organic acids, and berry features was investigated through ANOVA in R Studio (R Core 2013). A model encompassing all main effects and interaction effects underwent testing for normality assumptions. Linear models (lm function) were applied to assess the main effects (sampling time and cultivar) on biogenic amines, phenolic compounds, antioxidant enzymes, organic acids, and berry features. Post hoc analysis using Tukey HSD was carried out with the agricolae package in R Studio (Mendiburu 2023). Principal Component Analyses (PCAs) for biogenic amines, phenolic compounds, antioxidant enzymes, and organic acids datasets were conducted using ggbiplot2 within R Studio (Vu 2011). PCA, being a valuable analytical technique, aids in reducing multidimensional data into a more interpretable format, facilitating the identification of underlying patterns and trends within complex datasets. The heatmap was generated using the pheatmap package in R Studio (Kolde R 2019).
Results
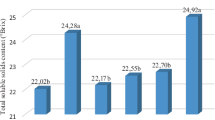
Figure 2 illustrates the growth metrics of grape berries at three developmental stages.These measurements were taken for different grape varieties and sampling times, specifically Bozcaada Cavusu*Kuntra and Bozcaada Cavusu* Vasilâki, Kuntra, and Vasilâki. In the category of Bozcaada Cavusu*Kuntra, the greatest berry length was observed at EL-38 and it was significantly higher than the other sampling times. The same trend was noted for Kuntra and Vasilâki, where EL-38 presented the longest berries. For berry weight, the highest values were again found at EL-38 across all varieties, with Bozcaada Cavusu*Kuntra and Kuntra showing statistically significant differences from the other sampling times, while Vasilâki did not show a significant difference between EL-32 and EL-38. Berry width followed a similar pattern in Bozcaada Cavusu*Kuntra and Kuntra, with the largest widths at EL-38. For Vasilaki, the widest berries were also observed at EL-38, significantly wider than at EL-29, but not significantly different from EL-32. The maturity index showed a discernible pattern as well; the highest index was found at EL-38 for Bozcaada Cavusu with Kuntra and a similar trend was observed for Kuntra and Vasilâki. However, Vasilâki did not exhibit a significant difference between EL-32 and EL-38. The Total Soluble Solids (TSS oBrix), visible in the Vasilâki category, displayed the highest percentage at EL-38, markedly different from EL-29 and EL-32, respectively. In examining the titratable acidity (TA) levels across grape varieties, it was observed that the variety designated as EL-29 consistently exhibited the highest TA values. This trend was marked by a gradual decrease in TA as the examination progressed through to varieties EL-32 and EL-38. Notably, EL-38 demonstrated the lowest TA levels among the studied varieties. Overall, the data indicated that the measurements for berry length, weight, width, and maturity index were generally highest at the final sampling time (EL-38). On the other hand, in the EL-29 stage, the berry weight for Bozcaada Çavuşu*Kuntra was consistent across sampling times, while Kuntra also maintained a steady berry weight. Vasilâki exhibited a slight increase in berry weight, and Bozcaada Çavuşu*Vasilâki remained consistent with a minor rise by the end. Berry width during EL-29 was stable for both Bozcaada Çavuşu*Kuntra and Kuntra. Similarly, Vasilâki and Bozcaada Çavuşu*Vasilâki maintained consistent berry widths. The maturity index for Bozcaada Çavuşu*Kuntra was initially higher but decreased later on, whereas Kuntra, Vasilâki, and Bozcaada Çavuşu*Vasilâki showed stable maturity indices throughout EL-29. For TSS (oBrix), Bozcaada Çavuşu*Kuntra began with a higher percentage, but it stabilized, and the other cultivars, including Kuntra, Vasilâki, and Bozcaada Çavuşu*Vasilâki, maintained consistent TSS (oBrix) levels throughout this stage. Moving into EL-32, Bozcaada Çavuşu*Kuntra displayed a slight increase in berry weight, while Kuntra’s berry weight stayed consistent, as did Vasilâki and Bozcaada Çavuşu*Vasilâki. Berry width for all cultivars remained stable, with Vasilâki showing a slight increase toward the end of this stage. The maturity index for Bozcaada Çavuşu*Kuntra remained relatively unchanged from EL-29, Kuntra continued to be consistent, and Vasilâki began to show an increase by the end of EL-32, with Bozcaada Çavuşu*Vasilâki maintaining stability. TSS (oBrix) levels for all cultivars did not show significant changes during EL-32, staying relatively steady. In the final observed stage, EL-38, the berry weight for Kuntra displayed a noticeable increase toward the end of the stage, with Vasilâki also showing a significant rise in berry weight. Bozcaada Çavuşu*Kuntra and Bozcaada Çavuşu*Vasilâki exhibited minor increases. The berry width for all cultivars remained consistent with previous stages. In terms of the maturity index, there was a slight increase observed in Kuntra and a more pronounced rise in Vasilâki by the end of EL-38, while Bozcaada Çavuşu*Kuntra and Bozcaada Çavuşu*Vasilâki’s maturity indices remained stable. The TSS (oBrix) for all cultivars continued to be stable, with no notable increases or decreases, suggesting that the soluble solids content had reached a plateau by this stage. Overall, the EL-38 stage marked a period of continued growth and maturity for the cultivars, with some showing more growth than others, yet all achieving a level of stability in TSS (oBrix) (Fig. 3).
Analysis of berry developmental metrics (berry weigh, berry width, berry length, total soluble solid, titratable acidity, and maturity index) across different sampling times for Kuntra, Bozcaada Çavuşu* Kuntra, Vasilâki, and Bozcaada Çavuşu*Vasilâki grape varieties. For a given factor (different letters within a column represent significant differences (Tukey test, *Significant at p value < 0.05). Data are stated as averages of the data and their standard deviations
Berry weight, berry width, berry length, total soluble solid, titratable acidity, and maturity index of grapes (Kuntra, Bozcaada Çavuşu* Kuntra, Vasilâki, and Bozcaada Çavuşu*Vasilâki) harvested in each EL-29, EL-32, and EL-38 phenological stages. For a given factor (different letters within a column represent significant differences (Tukey test, *Significant at p value < 0.05). Data are stated as averages of the data and their standard deviations
In the investigation of biogenic amines (BAs) in grapes harvested at different phenological stages (EL-29, EL-32, and EL-38) and across various cultivars (Kuntra, Bozcaada Çavuşu*Kuntra, Vasilâki, and Bozcaada Çavuşu*Vasilâki), several noteworthy trends emerged. Analyzing the temporal dynamics (S), Put levels exhibited a significant decrease from EL-32 (2.62 ± 0.58 mg/L) to EL-38 (2.04 ± 0.21 mg/L). Cad concentrations, on the other hand, peaked in EL-32 (3.53 ± 0.66 mg/L) and decreased in EL-38 (1.52 ± 0.14 mg/L). Agm concentrations showed an increasing trend from EL-29 (0.25 ± 0.05 mg/L) to EL-38 (0.37 ± 0.05a mg/L). Considering the impact of different cultivars (C), variations in cadaverine (Cad) levels were significant, with Vasilaki exhibiting the highest concentration (3.20 ± 1.31 mg/L). Other BAs such as Put and Agm did not show significant differences among cultivars (Table 1). Upon scrutinizing the numerical data presented in Table 2, a comprehensive analysis of phenolic compounds in grapes harvested at different phenological stages (EL-29, EL-32, and EL-38) and across various cultivars (Kuntra, Bozcaada Çavuşu*Kuntra, Vasilâki, and Bozcaada Çavuşu*Vasilâki) revealed nuanced insights into the variations over time and among different grape types. Gallic acid, a key phenolic compound, demonstrated a distinct temporal pattern. Its concentration increased progressively from 1.60 ± 0.61 in EL-29 to 3.77 ± 1.64 g L−1 in EL-32, maintaining a relatively stable level at 3.74 ± 0.81 g L−1 in EL-38. Analyzing other phenolic compounds, such as vanillic acid, trans-caffeic acid, and trans p-coumaric acid, revealed similar temporal patterns, and this indicated overall rise in their concentrations as grapes transitioned through different phenological stages. For instance, vanillic acid increased from 3.54 ± 2.63 in EL-29 to 5.26 ± 1.04 g L−1 in EL-38. Comparing different grape varieties provided additional layers of complexity to the analysis. Bozcaada Çavuşu*Vasilâki consistently exhibited higher concentrations of various phenolic compounds, such as epicatechin (5.69 ± 1.78 g L−1) and ferulic acid (7.51 ± 1.71 g L−1), underscoring the unique phenolic profiles associated with specific grape varieties. The statistical significance tests conducted reaffirmed the robustness of these observed differences. The low p-values indicated highly significant variations for most phenolic compounds and their interactions across different sampling times and cultivars.
The examination of antioxidant enzymes in grape varieties, as detailed in Table 3, provided valuable insights into the oxidative stress response during different phenological stages (EL-29, EL-32, and EL-38) and across various grape varieties (Kuntra, Bozcaada Çavuşu* Kuntra, Vasilâki, and Bozcaada Çavuşu*Vasilâki). Antioxidant enzymes played a crucial role in mitigating oxidative damage and maintaining cellular homeostasis, making their dynamics an essential aspect of grapevine physiology. Catalase activity exhibited a significant temporal variation (S) with a decrease from 14.04 ± 2.02 EU/gr berry in EL-29 to 11.20 ± 1.66 EU/gr berry in EL-38. When comparing different grape cultivars, Bozcaada Çavuşu*Kuntra consistently displayed higher levels of peroxidase (24.09 ± 2.41 EU/gr berry) compared to other cultivars. Moreover, the overall statistical significance tests revealed that CAT, POX, SOD, GR, G6PD, and GST were significantly affected by sampling time. In the our investigation of organic acids in grape cultivars, the levels of various compounds were examined across different phenological stages (EL-29, EL-32, and EL-38) and grape cultivars (Kuntra, Bozcaada Çavuşu*Kuntra, Vasilâki, and Bozcaada Çavuşu*Vasilâki). Upon analyzing the numerical values, significant differences were observed in several organic acids concerning the sampling time (S) factor. For instance, oxalic acid exhibited a notable increase from EL-29 (18.06 ± 3.18 g/L) to EL-32 (21.28 ± 3.48 g/L), followed by a decrease in EL-38 (17.58 ± 4.28 g/L). A similar trend was observed for butyric acid, which increased from EL-29 (21.01 ± 3.00 g/L) to EL-32 (26.45 ± 3.46 g/L) and then decreased in EL-38 (25.41 ± 7.27 g/L). When comparing organic acids across different grape cultivars (C), tartaric acid showed significant variability. For Kuntra, the tartaric acid level was 20.19 ± 3.39 g/L, whereas Bozcaada Çavuşu*Kuntra exhibited a lower value of 16.51 ± 2.92 g/L. Vasilâki and Bozcaada Çavuşu*Vasilâki showed intermediate values, and this emphasized the cultivar-specific differences in organic acid accumulation.
The presented PCA scatter plots provided a comprehensive visualization of the sample distributions and relationships within the dataset, specifically focusing on biogenic amines (BAs) and phenolic compounds. In Fig. 4, the scatter plot depicted the distribution of samples in the biogenic amine dataset across the first two principal components (PC1 and PC2), collectively explaining 48.9% and 23.8% of the variance, respectively. Each sample, labeled with an identifier, such as EL-29_BK.1, was color coded based on the sampling time (EL-29, EL-32, EL-38). The clustering of points, especially the tight grouping of samples from EL-38, suggested similarities among samples. This clustering indicated comparable profiles of biogenic amines within the same phenological stage. Moving to the biplot for BAs, the loadings of variables on PC1 and PC2 were illustrated, demonstrating the contribution of each biogenic amine (e.g., Ser, Dop) to the principal components. The color-coded arrows, based on the squared cosine (cos2), conveyed the quality of representation of each variable. The direction and length of the arrows provided insights into how biogenic amines correlated with the principal components and with each other. Dop, Tma, Nor, Agm, and Spd were close to each other in the third quadrant, whereas the other BAs was close to each other (except for Try) and was located in the second quadrant. Similarly, in Fig. 5, the scatter plot for phenolic compounds showcased the distribution of samples based on PC1 and PC2, explaining 71.4% and 10.4% of the variance, respectively. The dominance of PC1 in explaining variance suggested a more strongly patterned or less diffuse distribution of phenolic compound data. Ellipses, color coded by sampling time, likely represented confidence regions for different time points. Finally, the biplot for phenolic compounds (Fig. 5) revealed the loadings of each compound on the principal components. The cos2 values indicated the quality of representation of each compound. The plot elucidated relationships between phenolic compounds, showcasing which compounds had similar profiles based on their proximity to each other and how they contributed to the described variation by the principal components. In the examination of antioxidant enzymes through PCA (Fig. 6), the scatter plot vividly illustrated the distribution of samples based on their scores on the first two principal components. PC1, which explained 50.6% of the variance, and PC2, explaining 26.2%, unveiled some separation influenced by sampling time, particularly notable between EL-29 and the other groups. The color-coded samples and ellipses denoting confidence regions enhanced the perception of distinct profiles of antioxidant enzymes at different sampling times. Phenolic compounds were close to each other in the second and third quadrant. Moving to the biplot for antioxidant enzymes (Fig. 6), the direction and length of the arrows conveyed the correlation and influence of each enzyme on the principal components. The color-coded arrows, representing cos2 values, highlighted enzymes with a substantial impact on the sample distribution. Longer arrows signified a more significant contribution to the variance described by the PCA. Antioxidant enzymes were close to each other in the second and third quadrant, except for APX. Transitioning to the PCA of organic acids (Fig. 7), the scatter plot revealed the sample distribution based on PC1 (31.2%) and PC2 (23.5%). The spread of samples and the overlap of confidence ellipses suggested a greater similarity between groups compared to antioxidant enzymes. This implied a more consistent organic acid profile across different sampling times. In the biplot for organic acids (Fig. 7), the contribution of each acid to the principal components was depicted. Similar to enzymes, arrow direction and length indicated the correlation and influence, while cos2 values signified the representation quality on the plot. Longer arrows and more saturated colors denoted acids with a greater impact on the variance explained by the PCA. Organic acids were close to each other in the second and third quadrant, except for maleic.
In the results that were analyzed, strong positive correlations were indicated by large blue circles, such as those found between Spn and Spd as well as between His and Spd. Conversely, strong negative correlations were depicted as large red circles, with the relationships between Ser and Tyr and between Dop and Nor being particularly noteworthy. Smaller circles represented weaker correlations, with their blue and red hues designating positive and negative directions, respectively. These weaker correlations were dispersed across the matrix, and due to the lack of clarity in the labels, it was challenging to identify the specific variable pairs they represented (Fig. 8A). On the other hand, the heatmap for phenolic compounds depicted a comprehensive analysis of the correlation between different phenolic compounds. There were strong positive correlations throughout, as evidenced by the prevalence of dark blue circles. For example, gallic acid showed a strong positive correlation with nearly all other compounds listed, particularly vanillic acid, trans-caffeic acid, and trans-p-coumaric acid. Similarly, catechin and epicatechin displayed a high degree of positive association with one another. The uniformity of the dark blue circles across the heatmap indicated that most of these compounds tend to increase or decrease together within the samples analyzed. The analysis did not present any significant negative correlations, as there were no red circles indicating such a relationship. The lack of lighter-colored circles also suggested that weak correlations were uncommon among these compounds. This pattern suggested a tendency for these phenolic compounds to be involved in related pathways or to share similar sources (Fig. 8B). In our results, the correlations between various enzymes involved in oxidative stress response were depicted. Strong positive correlations were represented by large blue circles, as seen between SOD and both GST and G6PD. Meanwhile, APX exhibited a significant negative correlation with G6PD, as indicated by a large red circle. The heatmap also revealed a mix of moderate correlations, with varying sizes of circles suggesting the different strengths of association among the enzymes. Smaller circles, both blue and red, indicated weaker positive and negative correlations, respectively, across the range of enzymes. Interestingly, APX showed a unique pattern with small circles of both colors. The patterns captured in this heatmap provided insights into the intricate relationships that existed among the enzymes and offered a snapshot of the complex interplay within the cell’s response to oxidative stress (Fig. 8C). In addition, a strong positive correlation was observed between oxalic and tartaric acids, as well as between malonic and butyric acids, depicted by the large blue circles. Conversely, citric acid was found to have a pronounced negative correlation with succinic acid, a pattern also seen between fumaric and succinic acids, as indicated by the large red circles. Medium-sized circles on the heatmap suggested moderate correlations, notably a positive association between malic and lactic acids and a negative relationship between maleic and succinic acids. Smaller circles throughout the heatmap signaled weaker correlations, with propionic acid showing faint associations with several other acids. In certain cases, such as the one between oxalic and succinic acids, a lack of colored circles or the presence of very small ones denoted an absence of significant correlation (Fig. 8D).
On the other hand, in the analysis of the heatmap with hierarchical clustering, the color-coded representation provided insights into the patterns and similarities within the dataset. The heatmap employed a color gradient, ranging from green (indicating low values) to white (medium values) to blue (indicating high values), suggesting that the depicted values were standardized scores or measurements. The top and left dendrograms in the image illustrated the outcomes of hierarchical clustering. The top dendrogram, representing columns (experimental conditions or samples), exhibited branches of varying lengths, reflecting the degree of similarity between clusters. Shorter branches indicated higher similarity. Similarly, the side dendrogram clustered rows, likely representing different measured variables, with branch lengths indicating the degree of similarity between row elements. The labels on the columns, such as EL_36_V and EL_37_K, served as identifiers for different samples or conditions. Meanwhile, the labels on the rows, such as G6PD and Routine, represented the variables or measurements associated with each sample. While the exact numerical values corresponding to the colors were not visible in the provided image, a color key or legend would typically accompany the heat map to elucidate this information. Three primary clusters for sampling times emerged as a result of the grouping process, with the parameters being categorized into seven main groups. A robust correlation was observed between Spn and Agm, both belonging to the same group. Additionally, G6PD, GPD6, SOD, GR, Spd, Dop, and Tma were collectively grouped and demonstrated a strong association among them (Fig. 9).
Discussion
In the study of grapevine phenology, the observed trends in Fig. 2 are consistent with what is documented in the literature regarding the growth and maturation of grape berries (Keskin et al. 2022). It is well established that the final stages of berry development, typically corresponding to the veraison to harvest period, are critical for significant increases in berry size and weight due to cell expansion and accumulation of sugars, which is reflected in the EL-38 measurements across all varieties. The similar patterns of berry weight and width increases in Bozcaada Çavuşu*Kuntra and Kuntra suggest a genetic or environmental consistency that influences these specific cultivars in a similar manner, an observation that aligns with previous studies indicating a strong genetic control over berry size traits (CooMbe and McCarthy 2000). The maturity index’s rise at EL-38 is also in agreement with the literature, where berry maturity is known to increase as the berries approach full ripeness, with changes in tannin composition and a drop in acid levels, contributing to the overall maturity of the berries. For Vasilâki, the lack of significant difference between EL-32 and EL-38 for berry weight and maturity index might suggest a plateau in development, which could be attributed to either a reaching of full physiological maturity or environmental factors that limit further growth, a phenomenon that has been noted in other climatic studies (Coombe 1992; Guillaumie et al. 2011). The Total Soluble Solids (TSS oBrix) increase is critical for winemaking, as it correlates with sugar content and potential alcohol levels in wine. The marked difference in TSS oBrix at EL-38 for Vasilâki is indicative of the berries accumulating sugars in preparation for harvest, a key factor in determining harvest dates for optimal wine quality, as supported by numerous viticultural studies (Coombe 1992; Guillaumie et al. 2011). The findings from Fig. 3 reinforce the concept that the timing of these developmental stages is crucial for grape quality and yield, which are paramount for viticulturists and winemakers alike. The consistency of these results with the existing body of literature indicates the importance of monitoring these growth parameters at specific phenological stages to inform practices in the vineyard and anticipate the quality of the harvest (Peña-Neira et al. 2023). Furthermore, the data indicate a more pronounced increase in growth metrics during the EL-38 stage, aligning with the concept of a growth surge before ripening, which has been previously described. This stage, often marked by veraison, is a key transitional period where berries begin to soften and a rapid accumulation of sugars occurs, alongside a decrease in acid concentration. The increase in berry dimensions and the TSS oBrix in the EL-38 stage may also be reflective of the shift from vegetative to reproductive growth, as the plant diverts resources toward the berry. The stability of TSS (oBrix) in earlier stages suggests a slower rate of sugar accumulation which could be attributed to the berries’ focus on cell division and enlargement rather than sugar storage. This stage-specific growth pattern emphasizes the need for careful monitoring during the EL-29 and EL-32 stages to ensure that the vines are on the correct trajectory for optimal berry development. Temporal variations in acidity levels, peaking at EL-29 and declining at EL-38, corroborate the common pattern of acidity dynamics during berry development noted by Conde et al. (2007a, b). TA was, on the other hand, observed that the variety designated as EL-29 consistently exhibited the highest TA values. This trend was marked by a gradual decrease in TA as the examination progressed through to varieties EL-32 and EL-38. Notably, EL-38 demonstrated the lowest TA levels among the studied varieties, indicating a significant variation in acidity profiles within these grapes. This pattern highlights the diversity in acidity characteristics inherent to different grape cultivars, underscoring the importance of variety-specific analysis in viticultural studies. Transitioning from grape berry development to viticultural practices, the influence of grape pollinizer individuals on berry development, as highlighted by studies such as those by Gupton (2000) and Williams (1957), is evident in our study. The advanced state of maturity in Bozcaada Çavuşu*Vasilâki and Bozcaada Çavuşu*Kuntra compared to other cultivars is consistent with previous research (Dardeniz et al. 2011).
In our investigation of BAs in grapes harvested at different phenological stages (EL-29, EL-32, and EL-38) and across various cultivars (Kuntra, Bozcaada Çavuşu*Kuntra, Vasilâki, and Bozcaada Çavuşu*Vasilâki), we observed distinctive trends in the temporal dynamics of specific BAs. Notably, Put levels exhibited a significant decrease from EL-32 to EL-38, indicating a fluctuation in this particular amine during late phenological stages. Conversely, Cad concentrations peaked in EL-32 and decreased in EL-38, suggesting a dynamic response during berry ripening. Agm concentrations showed a consistent increasing trend from EL-29 to EL-38, highlighting its accumulation across the three periods and these findings are consistent with previous results (Incesu et al. 2022). The authors reported that berries harvested at EL-38 and EL-37 stages exhibited elevated levels of BAs compared to berries collected at other phenological stages for both grape varieties. Specifically, Put and His content reached their peak at EL-37, whereas lower concentrations of Spn, Nor, and Spd were observed at EL-38 across different grape cultivars. When considering the impact of different cultivars, significant variations in Cad levels were observed, with Vasilaki exhibiting the highest concentration. This cultivar-specific difference in Cad levels suggests a unique metabolic response to late phenological stages. However, other BAs, such as Put and Agm, did not show significant differences among cultivars, indicating a degree of consistency across the varieties studied. Furthermore, some researchers, in alignment with our results, have documented the existence of primary amines, including histamine, tyramine, putrescine, cadaverine, tryptamine, agmatine, phenylethylamine, methylamine, isoamylamine, and ethylamine, in wine products derived from various grape sources (Restuccia et al. 2018). The interaction effects revealed nuanced relationships between sampling time and cultivar for all variables except Try concentrations. This intricate interplay emphasizes that the influence of sampling time on BAs varies depending on the specific cultivar, reinforcing the complexity of the factors governing BAs dynamics in grapes (Ates et al. 2022). Comparing our findings with the provided literature, both sources underscore the intricate nature of BAs in grape varieties. This was to be expected, considering the documented variation in BAs content in grapes among different varieties (Yue et al. 2021). The observed decrease in Put levels aligns with the general understanding of BAs variations during grape ripening, as discussed in the literature. The increase and subsequent decrease in Cad concentrations during EL-32 and EL-38, respectively, echo the dynamic patterns noted in the effects of different applications on grape and wine chemical composition across various grape varieties. The differences in Cad levels among cultivars, particularly the elevated concentration in Bozcaada Çavuşu*Kuntra, resonate with the reported variations in BAs content across different grape varieties discussed in the literature. However, the consistent levels of other BAs such as Put and Agm across cultivars suggest a certain level of uniformity in the response of these amines to sampling times (Table 1). On the other hand, the presented findings on the phenolic compounds in grapes, particularly gallic acid and other compounds, reveal valuable insights into the dynamic changes across different phenological stages and grape cultivars. The study identified a distinct temporal pattern in gallic acid concentration, with an increase from 1.60 in EL-29 to 3.77 g L−1 in EL-32, maintaining stability at 3.74 g L−1 in EL-38 (Table 2). Similar patterns were observed for other phenolic compounds, like vanillic acid, trans-caffeic acid, and trans p-coumaric acid. Bozcaada Çavuşu*Vasilâki consistently exhibited higher concentrations of various phenolic compounds, such as epicatechin and tyrosol, emphasizing unique phenolic profiles associated with specific grape varieties. Pantelić et al. (2016) reported similar cultivar-specific variations in phenolic content among different grape varieties. The statistical significance tests conducted in the study reaffirmed the robustness of observed differences in phenolic compound concentrations across different sampling times and cultivars. Similar findings were reported by Đorđević et al. (2017) regarding the statistical significance of variations in phenolic compounds in grapes under different environmental conditions. In addidion, Cheng et al. (2015) found that temporal patterns in polyphenolic compounds can be influenced by seasonal weather variations and climatic conditions.
The investigation of antioxidant enzymes in grape cultivars, as delineated in Table 3, yields valuable insights into the oxidative stress response across distinct phenological stages (EL-29, EL-32, and EL-38) and diverse grape cultivars (Kuntra, Bozcaada Çavuşu* Kuntra, Vasilâki, and Bozcaada Çavuşu*Vasilâki). Notably, catalase activity exhibits a pronounced temporal variation (S), manifesting as a decrease from 14.04 EU/gr berry in EL-29 to 11.20 EU/gr berry in EL-38 (Table 3). This temporal modulation of catalase aligns with the established role of this pivotal antioxidant enzyme in dynamically responding to oxidative stress concomitant with the varying developmental stages of grape berries. Supporting this observation, pertinent studies such as those conducted by Pompella et al. (2003) underscore the significance of catalase in detoxifying hydrogen peroxide, a reactive oxygen species intricately associated with oxidative stress in biological systems. This finding corroborates the prevailing understanding that SOD, as a crucial antioxidant enzyme, and is subject to intricate regulation influenced by a confluence of intrinsic and extrinsic factors, including developmental stages and genetic variations among cultivars. Previous scholarly works, indeed, exemplified by Mittler (2002), have shed light on the intricate regulatory mechanisms governing SOD, emphasizing its sensitivity to diverse environmental and developmental determinants. In the comparative analysis of different grape varieties, Bozcaada Çavuşu*Kuntra consistently exhibits elevated levels of peroxidase (22.23 ± 2.41 EU/gr berry) in comparison to other varieties. This observation aligns harmoniously with extant literature positing that peroxidase activity manifests variances among grape cultivars, indicative of genetic disparities and potential adaptive responses to oxidative stress. Research by Hasanuzzaman et al. (2017) provides additional context by delving into the genetic variability inherent in peroxidase activity across plant species, thereby emphasizing the pivotal role of peroxidase in detoxifying reactive oxygen species. The comprehensive statistical analyses indicate the temporal regulation of antioxidant enzyme activities, revealing that peroxidase, CAT, SOD, GR, G6PD, and GST are significantly influenced by sampling time. This aligns seamlessly with the overarching understanding that antioxidant enzyme activities in grapes exhibit temporal regulation, likely responsive to dynamic environmental conditions and developmental stages. Indeed, Mhamdi et al. (2010) have expounded upon the temporal variability characterizing antioxidant enzyme activities, elucidating their responsiveness to environmental stimuli. This shows the multifaceted relationship between genetic determinants and environmental influences in shaping the dynamics of antioxidant enzymes across disparate grape cultivars and phenological stages. The scholarly contributions of Noctor et al. (2016) provide additional support to this notion, emphasizing the complex interactions that influence antioxidant enzyme activities and underscoring the imperative to consider both genetic and environmental factors in deciphering these intricate regulatory mechanisms. On the other hand, the findings from our study highlight the intricate dynamics of organic acids, particularly oxalic acid, butyric acid, tartaric acid, and malic acid, in grape berries. The significant differences observed in these acids concerning both sampling time and grape cultivar indicate the importance of considering temporal and varietal factors in grape chemistry studies. The study emphasizes the cultivar-specific variations in tartaric acid levels, emphasizing its crucial role in grape potential quality. The results on tartaric acid biosynthesis aligns with existing knowledge about the unique metabolic pathways involved. The study’s identification of oxalic acid and L-threonate as intermediates in tartaric acid formation and the limitation of tartaric acid biosynthesis from post-anthesis until EL-38 corroborate with earlier research (de Bolt et al. 2006). Besides, the observed temporal fluctuations in oxalic acid and butyric acid levels, with an increase from EL-29 to EL-32 followed by a decrease in EL-38, indicate a dynamic metabolic response during distinct stages of grape development (Table 4). This aligns with previous studies emphasizing the influence of sampling time on organic acid profiles in grape berries (Conde et al. 2007a, b). The temporal trends suggest that metabolic processes governing the accumulation of these acids may be stage specific, reflecting the intricate interplay between grape physiology and environmental factors. The significant variability in tartaric acid levels among different grape cultivars, such as Kuntra, Bozcaada Çavuşu*Kuntra, Vasilâki, and Bozcaada Çavuşu*Vasilâki, indicates the impact of genetic factors on organic acid composition (Bell and Henschke 2005). The findings emphasize the need for viticulturists and grape breeders to consider the genetic diversity of grape varieties when aiming to optimize grape quality for winemaking. This holistic perspective is consistent with the multifaceted nature of grape metabolism and highlights the necessity of considering both aspects concurrently in studies related to organic acid dynamics in grapes. The significant interactions for various acids, including oxalic acid, tartaric acid, and butyric acid, emphasize the interconnectedness of temporal and varietal factors (Conde et al. 2007a, b). In a practical context, these findings offer valuable insights for grape cultivation and winemaking. Viticulturists can leverage this knowledge to optimize harvest timing based on the desired organic acid composition, thus influencing the sensory attributes of resulting berries (Williams and Matthews 1990). In our results, the PCA scatter plots presented a detailed overview of the dataset, focusing on BAs, antioxidant enzymes, phenolic compounds, and organic acids. In the BAs dataset (Fig. 4), samples clustered notably by sampling time, with EL-38 samples tightly grouped. The biplot illustrated the contribution of each BA to principal components, revealing potential correlations. The scatter plot for phenolic compounds (Fig. 5) indicated a strongly patterned distribution, and the biplot showcased relationships between compounds. The PCA of antioxidant enzymes (Fig. 6) revealed separation influenced by sampling time, with the biplot highlighting enzymes impacting PCA variance. In the PCA analysis of organic acids (Fig. 7), the samples displayed a consistent profile across various time points. The corresponding biplot unveiled correlations among different acids. In our comprehensive analysis across multiple datasets, we observed a variety of correlation strengths that inform our understanding of biochemical relationships. In the enzymatic heatmap (Fig. 8C), strong positive correlations, particularly between SOD with GST and G6PD, suggest a coordinated response in oxidative stress mechanisms. Contrastingly, APX’s strong negative correlation with G6PD and its unique pattern of weaker correlations with other enzymes may indicate a distinct regulatory role in redox homeostasis. Similarly, in the analysis of organic acids (Fig. 8D), the strong positive correlation between oxalic and tartaric acids and between malonic and butyric acids implies a shared biosynthetic pathway or synchronicity in metabolic processes. The significant negative correlations noted between citric and succinic acids and between fumaric and succinic acids could reflect competitive or compensatory pathways in cellular metabolism. The phenolic compound heatmap (Fig. 8B) uniquely displayed a network of strong positive correlations, such as those between gallic, vanillic, trans-caffeic, and trans-p-coumaric acids, which might indicate similar roles in plant defense mechanisms or antioxidant activity. These findings align with established biochemical pathways and previous studies that emphasize the complexity of metabolic interactions (Bell and Henschke 2005; Mhamdi et al. 2010; Cheng et al. 2015; Yue et al. 2021). These correlations, strong and weak, underscore the interdependent nature of biological systems and the importance of understanding these interactions to fully grasp the intricacies of cellular and physiological processes. The heatmap with hierarchical clustering (Fig. 9) underscored notable patterns and associations, such as the correlation between Spn and Agm. These visualizations collectively enhance our comprehension of the dataset, unveiling clear clusters and correlations that contribute to the intricate dynamics of grape composition throughout diverse phenological stages.
Conclusion
In this study, a nuanced analysis of BAs, antioxidant enzymes, phenolic compounds, and organic acids provided a comprehensive understanding of the intricate temporal and variety-specific trends that governed berry ripening. Grape berries exhibited significant increases in size, weight, and maturity index, particularly in the final developmental stage (EL-38), and the TSS oBrix demonstrated variety-specific maturation patterns, with notable differences observed across different grape varieties. The consistently distinctive phenolic profiles observed in Bozcaada Çavuşu*Vasilâki, surpassing those of other varieties, underscored its uniqueness and potential for producing grapes with exceptional qualities. The dynamic variations in antioxidant enzymes and organic acids across different phenological stages and these grape cultivars offered valuable insights into the complex oxidative stress response and metabolic processes influencing berry development. Looking forward, future research endeavors should focus on harnessing the female dominance Bozcaada variety, leveraging its unique characteristics for further advancements in grape quality. This knowledge will undoubtedly contribute to the refinement of viticultural practices, ultimately optimizing grape quality and bolstering the overall success of grape cultivation.
Data Availability
The data from this study will be made available upon request.
Abbreviations
- BAs:
-
Biogenic amines
- Put:
-
Putrescine
- Cad:
-
Cadaverine
- Agm:
-
Agmatine
- Spn:
-
Spermine
- Spd:
-
Spermidine
- His:
-
Histamine
- Ser:
-
Serotonin
- Tyr:
-
Tyramine
- Tma:
-
Trimethylamine
- Dop:
-
Dopamine
- GSH:
-
Glutathione
- SOD:
-
Superoxide dismutase
- POD:
-
Peroxidase
- CAT:
-
Catalase
- GR:
-
Glutathione reductase
- GST:
-
Glutathione S-transferase
- APX:
-
Ascorbate peroxidase
References
Abedi T, Pakniyat H (2010) Antioxidant enzymes changes in response to drought stress in ten cultivars of oilseed rape (Brassica napus L.). Czech J Genet Plant Breed 46(1):27–34
Abreu I, Costa I, Oliveria M, Cunha M, De Castro R (2006) Ultrastructure and germation of Vitisvinifera L. cv Loureiropollen. Protoplasma 228:131–135
Angelini R, Manes F, Federico R (1990) Spatial and functional correlation between diamine-oxidase and peroxidase activities and their dependence upon de-etiolation and wounding in chick-pea stems. Planta 182:89–96
Ates F, Kaya O, Keskin N, Turan M (2022) Biogenic amines in raisins of one vintage year: Influence of two chemical pre-treatments (dipping in oak ash solution or potassium carbonate solution). Mitt Klosterneubg 72:51–59
Barrado E, Rodriguez JA, Castrillejo Y (2009) Determination of primary amino acids in wines by high performance liquid magneto-chromatography. Talanta 78(3):672–675
Bell SJ, Henschke PA (2005) Implications of nitrogen nutrition for grapes, fermentation and wine. Aust J Grape Wine Res 11(3):242–295
Caporali E, Spada A, Marziani G, Failla O, Scienza A (2003) The arrest of development of abortive reproductive organs in the unisexual flower of Vitisvinifera ssp. silvestris. Sex Plant Reprod 15:291–300
Cheng G, Liu Y, Yue TX, Zhang ZW (2015) Comparison between aroma compounds in wines from four Vitis vinifera grape varieties grown in different shoot positions. Food Sci Technol 35:237–246
Chikezie PC, Chikezie CM, Uwakwe AA, Monago CC (2009) Comparative study of glutathione S-transferase activity of three human erythrocyte genotypes infected with Plasmodium falciparum. J Appl Sci Environ Manag 13(3)
Conde C, Silva P, Fontes N, Dias ACP, Tavares RM, Sousa MJ, Gerós H (2007a) Biochemical changes throughout grape berry development and fruit and wine quality
Conde C, Silva P, Fontes N, Dias AC, Tavares RM, Sousa MJ, Gerós H (2007b) Biochemical changes throughout grape berry development and fruit and wine quality. Food 1(1):1–22
R Core Team. R (2013) A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Aus-tria, 2013. Available online: https://www.R-project.org/ (accessed on 8 August 2023).
Coombe BG (1992) Research on development and ripening of the grape berry. Am J Enol Vitic 43(1):101–110
Coombe BG (1995) Adoption of a system for identifying grapevine growth stages. Aust J Grape Wine Res 1:100–110
CooMbe BG, McCarthy MG (2000) Dynamics of grape berry growth and physiology of ripening. Aust J Grape Wine Res 6(2):131–135
Dardeniz A, Şeker M, Yancar A, Gökbayrak Z, Bahar E, Kahraman KA (2011) Çanakkale’de Bozcaada Çavuşu Üzüm Çeşidi Yetiştiriciliği ve Karşılaşılan Sorunlar. Uluslararası Katılımlı I. Ali Numan Kıraç Tarım Kongresi ve Fuarı. 27–30 Nisan. Eskişehir, Türkiye
de Bolt S, Cook DR, Ford CM (2006) L-Tartaric acid synthesis from vitamin C in higher plants. Proc Natl Acad Sci USA 103:5608–5613
Đorđević NO, Novaković MM, Pejin B, Mutić J, Vajs V, Pajović SB, Tešević V (2017) Supplementary data for article: Đorđevic, NO; Novaković, MM; Pejin, B.; Mutić, J.; Vajs, V.; Pajovic, SB; Tešević, V. Comparative analytical study of the selected wine varieties grown in montenegro. Nat Prod Res 31(15):1825–1830. https://doi.org/10.1080/14786419.2017
Gallardo A, Ocete R, Angeles Lopez M, Lara M, Rivera D (2009) Assessment of pollen dimorphisim in populations of Vitisvinifera L. subsp. sylvestris (Gmelin) Hegi in Spain. Vitis 48:59–62
Guillaumie S, Fouquet R, Kappel C, Camps C, Terrier N, Moncomble D, Dunlevy JD, Davies C, Boss PK, Delrot, S (2011) Transcriptional analysis of late ripening stages of grapevine berry. BMC Plant Biol 11 (1): 165. https://doi.org/10.1186/1471-2229-11-165.
Gupton C (2000) Grapes. In: The encyclopedia of fruit and nuts. CABI, pp 287–312
Gurasashvili V, Vashakidze L (2004) Type of pollination and frequeny of fruit-set of Georgian grapevine variety Gorula. J Probl Agric Sci 26:72–75
Hasanuzzaman M, Hossain MA, da Silva JAT, Fujita M (2017) Plant response and tolerance to abiotic oxidative stress: antioxidant defense is a key factor. In: Abiotic stress-mediated sensing and signaling in plants. Springer, New York, pp. 235–258
Incesu M, Karakus S, Seyed Hajizadeh H, Ates F, Turan M, Skalicky M, Kaya O (2022) Changes in biogenic amines of two table grapes (cv. Bronx Seedless and Italia) during berry development and ripening. Plants 11:2845
Jovanović-Cvetković T, Mićić N, Đurić G, Cvetković M (2016) Pollen morphology and germination of indigenous grapevine cultivars Žilavka and Blatina (Vitisvinifera L.). AgroLife Sci J 5:105–109
Keskin N, Kunter B, Çelik H, Kaya Ö, Keskin S (2021) ANOM approach for the statistical evaluation of organic acid contents of clones of the grape variety ‘Kalecik Karası. Mitt Klosterneuburg 71(2):126–138
Keskin N, Kaya O, Ates F, Turan M, Gutiérrez-Gamboa G (2022) Drying grapes after the application of different dipping solutions: effects on hormones, minerals, vitamins, and antioxidant enzymes in Gök Üzüm (Vitis vinifera L.) raisins. Plants 11(4):529
Kolde R (2019) Pheatmap: pretty heatmaps. R package version, 1(2): 726.
Mendiburu F (2023) agricolae: statistical procedures for agricultural research. R package version 1.4-0. https://CRAN.R-project.org/package=agricolae
Mhamdi A, Queval G, Chaouch S, Vanderauwera S, Van Breusegem F, Noctor G (2010) Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. J Exp Bot 61(15):4197–4220
Minucci A, Giardina B, Zuppi C, Capoluongo E (2009) Glucose-6-phosphate dehydrogenase laboratory assay: how, when, and why? IUBMB Life 61:27–34
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9):405–410
Munoz-Rodríguez AF, Tormo R, Silva MI (2011) Pollination dynamics in Vitis vinifera L. Am J Enol Vitic 62:113–117
Najmaddin C, Khatijah H, Maideen H (2011) Comparative study on the anatomy and palynology of the three variety of Vitisviniferavarity (family Vitaceae). Afr J Biotechnol 10:16866–16874
Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez-Garcia B, Queval G (2016) Glutathione in plants: an integrated overview. Plant Cell Environ 39(2):454–488
Pantelić MM, Zagorac DČD, Davidović SM, Todić SR, Bešlić ZS, Gašić UM, Natić MM (2016) Identification and quantification of phenolic compounds in berry skin, pulp, and seeds in 13 grapevine varieties grown in Serbia. Food Chem 211:243–252
Peña-Neira A, Cortiella MGI, Ubeda C, Pastenes C, Villalobos L, Contador L, Gómez C (2023) Phenolic, polysaccharides composition, and texture properties during ripening and storage time of new table grape cultivars in chile. Plants 12(13):2488
Pompella C, Visvikis A, Paolicchi A, Tata VD, Casini AF, Lodovici M (2003) The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol 66(8):1499–1503
Restuccia D, Loizzo MR, Spizzirri UG (2018) Accumulation of biogenic amines in wine: role of alcoholic and malolactic fermentation. Fermentation 4:6
Rombough L (2002) The grape grower. Chelsea Green, Vermont
Tello J, Ibáñez J, Muñoz-Organero G (2018) Microsatellite markers in grapevine reveal DNA stability during processing and after six months of simulated shipping conditions. Sci Rep 8(1):7771
Vashakidze L, Gogiava E, Bejuashviliu N (1982) The biological peculiarities of some Georgian grapevine varieties by breeding. In: Conference on ‘Productivity of Subtropical Crops’. Maxaradze-Anaseuli, Georgia
Vu VQ (2011) _ggbiplot: a ggplot2 based biplot_. R package version 0.55, http://github.com/vqv/ggbiplot
Williams MW (1957) Grapes. In: The New York Times Natural Foods Cookbook. Random House, pp 497–506
Williams LE, Matthews MA (1990) Grapevine. In: Irrigation of agricultural crops. American Society of Agronomy, Crop Science Society of America, Soil Science Society of America, pp 637–673
Yue X, Zhao Y, Ma X, Jiao X, Fang Y, Zhang Z, Ju Y (2021) Effects of leaf removal on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in Cabernet Sauvignon (Vitis vinifera L.) grapes. J Sci Food Agric 101:3214–3224
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
F.A., H.D., T.Y., A.D., and O.K played a multifaceted role in this study, contributing to funding acquisition, conceptualization, investigation, project administration, data curation, resources, supervision, validation, and visualization. O.K wrote the manuscript, participated in data curation, formal analysis, methodology, and software, and contributed to the review and editing of the manuscript. M.T conducted field and laboratory work, focusing on analyzing samples in the laboratory, and also contributed to field and laboratory work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Ethcial Approval
Not applicable.
Informed Consent
Not applicable.
Additional information
Handling Editor: Fabrizio Costa.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ates, F., Delavar, H., Dardeniz, A. et al. Dynamics of Berry Characteristics, Biochemical Composition, and Physiological Responses Across Ripening Stages: Investigating the Impact of Pollinizer Varieties on Physiological Femaleness in Bozcaada Çavuşu (Vitis vinifera L. cv). J Plant Growth Regul (2024). https://doi.org/10.1007/s00344-024-11263-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00344-024-11263-5