Abstract
Background
The real-world application of STRIDE-II treatment targets to identify whether disease control is optimal in Crohn’s disease (CD) and ulcerative colitis (UC) is not well known.
Aims
This study aimed to estimate proportions of patients with suboptimally controlled CD and UC in real-world Canadian healthcare settings and the impact on quality of life (QoL).
Methods
The noninterventional, multicenter, observational IBD-PODCAST Canada study comprised a single study visit involving routine assessments, patient- and clinician-completed questionnaires, and a retrospective chart review. Primary outcomes were proportions of patients with STRIDE-II-based red flags indicative of suboptimal disease control and mean ± standard deviation Short Inflammatory Bowel Disease Questionnaire (SIBDQ) scores. Secondary outcomes included proportions of patients and clinicians subjectively reporting suboptimal control.
Results
Among 163 enrolled patients from 10 sites, 45/87 patients with CD (51.7%; 95% CI: 40.8%, 62.6%) and 33/76 patients with UC (43.3%; 95% CI: 32.1%, 55.3%) had suboptimal disease control based on STRIDE-II criteria. Suboptimal control was subjectively reported at lower proportions (patients: CD, 15.0%; UC, 18.6%; clinicians: CD, 19.5%; UC, 25.0%). Numerically lower SIBDQ scores were observed with suboptimal control (CD, 43.0 ± 10.8; UC, 42.5 ± 12.0) than with optimal control (CD, 58.2 ± 7.2; UC, 57.8 ± 6.6).
Conclusions
Approximately 50% (CD) and 40% (UC) of patients from real-world Canadian practices had suboptimal disease control based on STRIDE-II criteria. Suboptimal control was underestimated by patients and clinicians and accompanied by reduced QoL, suggesting further efforts to implement STRIDE-II treat-to-target strategies are needed.
Similar content being viewed by others
Introduction
Crohn’s disease (CD) and ulcerative colitis (UC) are chronic, incurable, and idiopathic types of inflammatory bowel disease (IBD) with impactful gastrointestinal and constitutional symptoms and may result in irreversible tissue damage, reduced quality of life (QoL), and permanent disability if untreated [1]. Medical therapies in CD and UC generally target the underlying inflammatory process to attain the treatment goals of reducing symptoms, preventing complications, and restoring QoL. Despite the expanding availability of therapeutic options for patients with IBD, the treatment goals are inconsistently attained, which may increase the risk of future complications and result in unnecessary patient suffering. Therefore, frequent monitoring of patients with IBD is important to ensure that treatment goals are being reached and therapies are being adjusted as necessary. As symptoms of CD and UC do not necessarily correlate with the presence or severity of inflammation, patients with CD and UC require objective testing for inflammation at regular intervals to reduce the risk of irreversible long-term complications and/or detrimental effects on QoL, functional ability, and economic burden [1,2,3].
In 2021, the International Organization for the Study of IBD committee published a set of short, medium-, and long-term targets to assist clinicians in attaining the treatment goals for IBD, which are known as the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE)-II recommendations [4, 5]. This goal-oriented approach involves the regular time-anchored assessment of symptom burden, inflammatory activity, and health-related QoL (HRQoL), the determination of whether prespecified targets are met, and the consideration of changes to the therapeutic regimen when targets are not achieved [6]. The STRIDE-II recommendations have confirmed the long-term treatment target of endoscopic healing with clinical remission [5]. Treatment targets that were added include the intermediate targets of C-reactive protein (CRP) normalization and fecal calprotectin (FC) reduction and the long-term targets of an absence of disability and a restoration of QoL.
The incorporation of STRIDE-II guidelines and the frequency of treatment target achievement in real-world practice settings are unclear. Variations in the adoption and application of STRIDE-II guidelines in different practice settings may lead to avoidable adverse outcomes. Therefore, the evaluation of how evidence-based treatment targets are attained in diverse practice settings is important. We undertook the Proportion Of inadequate Disease Control And Strategy of Treatment in IBD (IBD-PODCAST) study to systematically assess the frequency of STRIDE-II–based treatment target achievement and suboptimal disease control across Canadian IBD practices, with the primary goal to determine the proportion of suboptimal disease control in patients with CD and UC. We further sought to assess the impact of suboptimal disease control on HRQoL, healthcare resource utilization (HCRU), and other demographic and disease-related factors among Canadians with IBD in ambulatory practice settings.
Methods
Study Design
This phase 4, noninterventional, multicenter observational study (Fig. 1) included both cross-sectional and retrospective assessments of adult outpatients with CD or UC from real-world Canadian IBD-focused practices.
The IBD-PODCAST study design. †Aligns with routine clinical appointments. CRP C-reactive protein, EIM extraintestinal manifestation, FACIT-F Functional Assessment of Chronic Illness Therapy—Fatigue, FC fecal calprotectin, HBI Harvey-Bradshaw Index, HCRU healthcare resource utilization, IBD inflammatory bowel disease, P‑SCCAI Patient Simple Clinical Colitis Activity Index, SIBDQ Short Inflammatory Bowel Disease Questionnaire, STRIDE Selecting Therapeutic Targets in Inflammatory Bowel Disease, TIM targeted immunomodulator, WPAI Work Productivity and Activity Impairment
Through chart review, we identified patients who were at least 19 years of age and had received a physician-confirmed CD or UC diagnosis at least one year before enrollment. Other inclusion criteria were the ability and willingness to provide informed consent and to read, understand, and complete the patient study materials. Patients with less than 12 months of available documentation in their medical records were excluded from the study, as were patients receiving any unapproved investigational therapy, patients with an IBD diagnosis not classified as CD or UC, and patients with a history of proctocolectomy. Other surgeries were not excluded, and patients were not excluded if starting a new IBD therapy. Eligible patients were enrolled and provided informed consent between May 10, 2022, and October 21, 2022, which involved completion of questionnaires and agreement to have their clinic charts reviewed. The questionnaires used and the data collected from the charts are shown in Fig. 1.
Definition of Suboptimal Control
Enrolled patients were considered to have suboptimal disease control if they had “red flags,” defined as not having met prespecified STRIDE-II–based treatment targets within the accepted time horizon from the start of their current medical therapy. These “red flags” indicating suboptimal control are listed in Table 1. Patients were asked whether they believed their IBD was optimally controlled to assess the relationship between the patient’s perception of disease control and the objective definition based on STRIDE-II treatment targets. Through chart review, we also enumerated HCRU, history of medical treatment, and the time from the initiation of their current treatment regimen until the time of the first indication of suboptimal disease control.
Outcome Measures
All outcome measures were evaluated separately for CD and UC. There were two primary outcomes assessed: the proportion of patients with red flags indicative of suboptimal disease control based on STRIDE-II recommendations; and differences in Short Inflammatory Bowel Disease Questionnaire (SIBDQ) scores (higher scores indicate better QoL) [7] between patients with optimal and suboptimal disease control.
Secondary outcome measures included the proportions of patients who subjectively reported suboptimal disease control, HRQoL outcomes, symptom burden, HCRU outcomes, the types of monitoring performed during the 12 months prior to enrollment (i.e., symptoms, laboratory evaluations, imaging, and endoscopic assessments), and the classes of IBD-targeting medical therapies received. Patients were also characterized according to comorbidities and extraintestinal manifestations (EIMs). Exploratory outcome measures of QoL and daily life burden included IBD Disk scores [8] and stool urgency as determined by the Patient Simple Clinical Colitis Activity Index [9]. Symptomatic status (i.e., presence or absence of clinical disease activity) and inflammatory status (i.e., presence or absence of objective indicators of inflammation) were also evaluated as an exploratory outcome measure.
Study Procedures
All patient data were documented in the investigators’ patient files, which served as source documents. During the study visit, the investigators or site staff entered the data in web-based electronic case report forms (eCRFs), and patients entered their data directly in electronic patient-reported outcome forms. No additional protocol-mandated diagnostic or monitoring procedures were conducted during patient visits beyond those typically performed in accordance with the therapeutic strategy. Efforts to address the potential for bias during this study included predefined eligibility of patients and sites, specific site instructions (i.e., on the protocol, eCRF functionality, and source document maintenance), continuous study oversight, and a comprehensive data validation program for quality control.
Statistical Methods
All analyses were descriptive and evaluated separately for patients with CD and UC who had available data and met the inclusion criteria. Mean ± standard deviation (SD) data were reported for continuous variables. Numbers and percentages of the total study population, excluding any missing data, were reported for categorical variables. No imputation for missing values occurred. Corresponding two-sided 95% confidence intervals (CIs) were reported where applicable. Comparative statistical analyses were not performed due to the descriptive nature of this study. Annualized summary HCRU measures were calculated over the course of the 12 months before the study visit. Total annual healthcare costs were calculated using sourced Canadian reference costs [10,11,12,13,14,15,16]. All analyses were performed using SAS Version 9.4 (SAS Institute Inc., Cary, NC).
Study Size Determination
The sample size was based on feasibility and precision rather than statistical power due to the descriptive nature of this study. A total of 150 patients were planned for inclusion (75 CD; 75 UC). For the second primary endpoint, assumed proportions of patients with suboptimal and optimal disease control were 20% (15/75) and 80% (60/75), respectively, and the assumed SD was 15 for the mean SIBDQ score. With these assumptions, a sample size of 15 patients with suboptimal disease control would have an expected precision of ± 7.6 for the two-sided 95% CI of the mean SIBDQ score; and a sample size of 60 patients with optimal disease control would have an expected precision of ± 3.8 for the two-sided 95% CI of the mean SIBDQ score. Since a larger sample size results in smaller standard error and consequently a narrower CI, higher proportions of patients with suboptimal control up to 50% would lead to smaller CIs, with larger CIs for those with optimal control.
Results
Patients
A total of 163 patients (87 CD; 76 UC) were screened and subsequently enrolled at 10 participating Canadian private practice sites. No hospital patients were recruited. Mean demographics and clinical disease characteristics for suboptimal and optimal groups in the CD and UC study populations are shown in Table 2. Overall, patients with CD (44.8% female) were 32.9 years of age at diagnosis and patients with UC (55.3% female) were 34.2 years, with median disease durations of 10.0 and 10.5 years, respectively. The overall mean body mass index was high for CD (27.7 kg/m2) and UC (26.0 kg/m2). There were fewer current smokers in this study (CD, 9.3%; UC, 5.3%) than in historical cohorts (CD, 29.7–39.6%; UC, 15.3–20%) [17,18,19,20]. Mean disease activity scores were a Harvey-Bradshaw Index score of 2.2 (CD) and a partial Mayo Clinic score of 1.0 (UC). The majority of patients were employed (CD, 70.0%; UC, 65.7%). The mean ± SD CRP levels for patients with CD and UC were 0.4 ± 0.47 and 0.4 ± 0.60 mg/dL, respectively, and the mean FC levels for patients with CD and UC were 132.3 ± 131.1 and 621.9 ± 709.3 μg/g, respectively.
Suboptimal Disease Control
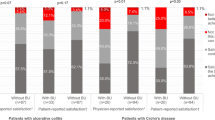
At the study visit, the proportions of patients with STRIDE-II–based red flags indicative of suboptimal disease control were 51.7% (45/87; 95% CI: 40.8%, 62.6%) for CD and 43.4% (33/76; 95% CI: 32.1%, 55.3%) for UC (Fig. 2). The majority of the patients with suboptimal disease control were identified according to the long-term red flags listed in Table 1 (CD, 93.3% [42/45]; UC, 90.9% [30/33]). The individual red flag that contributed most to the identification of suboptimal disease control was impaired QoL (SIBDQ < 50) for CD (73.3%; 33/45) and UC (78.8%; 26/33) (Supplemental Table 1). Other red flags that commonly identified patients with suboptimal CD control were the failure to achieve clinical remission (37.8%; 17/45) and the presence of disease- or treatment-associated clinically significant EIMs (31.1%; 14/45). In UC, failure to achieve clinically meaningful improvement (27.3%; 9/33) and the overuse of systemic corticosteroids (30.3%; 10/33) were red flags that commonly identified suboptimal control.
The proportions of patients and clinicians with subjectively reported suboptimal disease control (Supplemental Table 2) were lower than the STRIDE-II-determined suboptimal control rate. Only 15.0% (12/87) of patients with CD and 18.6% (13/76) of patients with UC self-identified as having suboptimal disease control. According to clinician reports, 19.5% (17/87) of patients with CD and 25.0% (19/76) of patients with UC had suboptimal disease control.
Patient- and clinician-reported suboptimal disease control each comprised only a small proportion of the total number of patients with STRIDE-II-based red flag-indicated suboptimal disease control (Fig. 3). Most patients with suboptimal disease control were identified solely according to STRIDE-II-based red flags (CD, 53.3% [24/45]; UC, 46.2% [18/39]). Only 15.6% (7/45) of patients with CD and 20.5% (8/39) of patients with UC had STRIDE-II-based red flags and self- and clinician-perceived suboptimal disease control. Furthermore, despite most subjective reports suggesting optimal disease control, 17.6% (22/125) and 18.6% (22/127) of patients with self-reported and clinician-reported control, respectively, still had underlying severe impairments in QoL as measured by the SIBDQ.
Proportions of outpatients with suboptimal disease control according to patient and clinician reports and STRIDE-II-based red flags. †Missing patient, clinician, or red flag assessments for 3 patients; percentages are based on patients with non-missing assessments. CD Crohn’s disease, STRIDE Selecting Therapeutic Targets in Inflammatory Bowel Disease, UC ulcerative colitis
QoL and Daily Life Burden
Consistent with the definition of the impaired QoL red flag, mean SIBDQ scores were numerically lower in patients with red flag-indicated suboptimal disease control (CD, 43.0 ± 10.8; UC, 42.5 ± 12.0) than in those with optimal control (CD, 58.2 ± 7.2; UC, 57.8 ± 6.6) (Fig. 4). These SIBDQ scores represent considerable between-group differences of approximately 15 for both CD and UC. Findings were similar across all subscores of the SIBDQ, with the bowel and emotional subscores primarily driving the total scores.
Mean Short Inflammatory Bowel Disease Questionnaire scores among patients with and without optimal disease control. †Missing SIBDQ scores for 3 patients with suboptimal disease control and 4 patients with optimal disease control; mean ± SD SIBDQ scores are based on patients with non-missing data. ‡Missing SIBDQ scores for 5 patients with optimal disease control; mean ± SD SIBDQ scores are based on patients with non-missing data. CD Crohn’s disease, SD standard deviation, SIBDQ Short Inflammatory Bowel Disease Questionnaire, UC ulcerative colitis
A greater burden on patients’ ability to fully participate in activities of daily living was reflected in all categories of the IBD Disk when disease was suboptimally controlled (according to STRIDE-II-based red flags) than when optimally controlled, with the most pronounced differences observed in the energy, sleep, and work and education categories for both CD and UC as well as the joint pain category in CD (Supplemental Fig. 1). Other measures of daily life burden demonstrated impairments consistent with the results of the IBD Disk: the group with suboptimal control had higher Work Productivity and Activity Impairment: General Health scores (Supplemental Fig. 2), lower Functional Assessment of Chronic Illness Therapy-Fatigue scores (Supplemental Fig. 3), and higher proportions of patients with abdominal pain (45.9% [17/37] versus 5.9% [2/34] in patients with suboptimal and optimal CD control, respectively) and stool urgency (Supplemental Table 3). Stool urgency appeared to be particularly burdensome on QoL for patients with suboptimal disease control, with considerable overlap between these two outcomes. Among those with both suboptimal control and an SIBDQ score of less than 50, 90.3% to 95.5% (CD) and 84.6% to 94.1% (UC) had an inability to hold stool for 15 min and/or a need to adjust activities to ensure a nearby toilet (Supplemental Table 3).
HCRU
Among all HCRU events evaluated, only outpatient visits were more common with suboptimal disease control than with optimal control for both CD and UC. Over the course of the 12 months before the study visit, outpatient visits occurred at a higher rate per person-year in patients with suboptimal disease control (CD, 3.0 [95% CI: 2.5, 3.5]; UC, 3.3 [95% CI: 2.8, 4.0]) than in those with optimal control (CD, 2.4 [95% CI: 2.0, 2.9]; UC, 2.1 [95% CI: 1.7, 2.6]) (Fig. 5A). Similarly, the proportion of patients with at least one outpatient visit was higher among patients with suboptimally controlled disease (CD, 86.7% [39/45]; UC, 75.8% [25/33]) than in those with optimal control (CD, 81.0% [34/42]; UC, 74.4% [32/43]). Most outpatient visits were with gastroenterologists (CD, 81.6% [71/87]; UC, 72.4% [55/76]), with less than 5% of visits with general practitioners and less than 20% with IBD nurses (Fig. 5B). The remaining HCRU events evaluated (Fig. 6A) and total annual healthcare costs (Fig. 6B) were higher in patients with suboptimal disease control for UC but not for CD.
Monitoring
During the 12 months before the study visit, at least one imaging or endoscopic assessment had been performed in 43.5% (37/85) of patients with CD and 36.8% (28/76) of patients with UC (Supplemental Fig. 4A). Of these assessments, endoscopy was the most used in CD (83.8%; 31/37) and UC (96.4%; 27/28). Magnetic resonance imaging or magnetic resonance enterography assessments were performed in 13.5% (5/37) of patients with CD and 7.1% (2/28) of patients with UC, and ultrasound was performed in 13.5% (5/37) of CD, but none (0/28) of UC patients. Imaging or endoscopic assessments had been performed more frequently in the past 12 months in patients with suboptimally controlled UC at the study visit than in patients with UC who did not have any STRIDE-II–based red flags (Supplemental Fig. 4B).
Overall, patients’ symptoms were monitored closely, with symptomatic status unknown for only 15.3% (25/163) of patients (Supplemental Table 4). In contrast, most patients’ inflammatory status was unknown at the time of the study visit (68.1%; 111/163), suggesting that objective indicators of inflammation were not being monitored.
Treatment Patterns
In the 12 months before the study visit, more patients with suboptimally controlled disease (CD, 8.9% [4/45]; UC, 27.3% [9/33]) than optimally controlled disease (CD, 0% [0/42]; UC, 4.7% [2/43]) had received more than one corticosteroid course (Supplemental Table 5). Compared with patients having optimal disease control, patients with suboptimally controlled disease were more likely to be TIM experienced (82.2% [37/45] versus 73.8% [31/42] in CD; 63.6% [21/33] versus 55.8% [24/43] in UC). Approximately 47.2% (17/36; CD) and 45.5% (6/11; UC) of the eligible TIM-experienced patients with suboptimal disease control were not yet receiving escalated TIM dosing at the study visit and had the potential for optimization of their TIM therapy (Supplemental Fig. 5). At the study visit, few patients received treatment adjustments, with only 6.9% (6/87) patients with CD and 7.9% (6/76) patients with UC initiating a new treatment (Supplemental Table 6).
Comorbidities and EIMs
The subgroup with suboptimal disease control had higher proportions of patients with EIMs (Supplemental Fig. 6). Rheumatologic EIMs were most common, with 20.5% (16/78) of suboptimally controlled patients and 2.4% (2/85) of optimally controlled patients presenting with at least one of these EIMs. The most common comorbidities were mental disorders (9.2%; 15/163), arterial hypertension (4.9%; 8/163), type 2 diabetes mellitus (3.7%; 6/163), and anemia (3.7%; 6/163) (Supplemental Table 7).
Discussion
In the IBD-PODCAST Canada study, we determined that approximately half of patients with IBD (51.7% CD and 43.4% UC) had red flags that indicated suboptimal disease control, predominantly driven by suboptimal QoL as determined by the SIBDQ. However, our data clearly identified a discordance between physician and patient perceptions of suboptimal disease control and objectively determined suboptimal control based on STRIDE-II criteria. Importantly, we also demonstrated that patients with suboptimal disease control based on STRIDE-II criteria were more likely to report impairments in QoL, with those with UC having higher rates of HCRU. The lack of increased HCRU in suboptimally controlled CD patients may be explained by the observation that patients with optimal CD control were more likely to have reported surgical intervention in the 12 months preceding their study visit, and such intervention may have been more likely to optimize disease control.
Collectively, these data support the presence of challenges with the current implementation of STRIDE-II treatment targets in real-world practice within Canada. The high percentages of patients with suboptimal disease control observed in this study are comparable to those reported in the limited literature available from other geographic regions [21, 22]. However, further evaluations, including those reporting IBD-PODCAST data from other countries, are underway to provide additional information specifically relevant to the achievement of STRIDE-II treatment targets. The discordance among the red flag-based, patient, and clinician assessments of suboptimal disease control in the current study is consistent with previous reports of subjectively high patient satisfaction in IBD management and the differing goals of treatment between patients and physicians [23, 24]. The discordance may be a result of the wide intra-individual variation in QoL features that drive patients’ subjective reports of disease control, which may not be accurately captured by the SIBDQ and other objective QoL assessments. Opportunities remain to optimize IBD control in Canadian clinical practice through further awareness of under-recognized STRIDE-II-based red flags such as impaired QoL.
Patients’ under-recognition of objective red flags may be related to their under-communicated disease features experienced in daily life, which could also contribute to clinician unawareness of these features. In a large 2019 North American and European survey, most patients (CD, 53%; UC, 52%) but fewer physicians (CD, 41%; UC, 38%) believed that better disease control could be achieved, regardless of their reported satisfaction with treatment goals [23]. This finding supports that patients perceived as having optimal disease control may still be living their daily lives with certain levels of disease burden that they are unable or unwilling to communicate. This “accepted” burden may comprise stigmatizing aspects affecting QoL, such as stool urgency, which are under-recognized by physicians potentially due to patients’ reticence to discuss their psychosocial concerns [23, 25, 26]. Consistent with this, results from a Japanese internet survey demonstrated that up to 30% of respondents with UC were embarrassed to discuss such disease features with a clinician [26]. However, recognition and subsequent management of these features could substantially improve patients’ disease experience. Stool urgency is strongly associated with compromised QoL and disease activity [27,28,29], consistent with findings from the current study where most patients with both suboptimally controlled IBD and poor QoL (SIBDQ < 50) had urgency-related concerns. Therefore, stool urgency could be a useful surrogate to prompt further QoL evaluations in clinical practice for the identification and treatment of more patients with suboptimally controlled IBD.
These study results suggest a low rate of adherence to STRIDE-II assessments within the recommended time frame. The implementation of STRIDE-II recommendations for disease monitoring may be challenging in many IBD care settings due to requirements for the design and implementation of a systematic approach to patient evaluations and for access to allied health professionals who can order the assessments and monitor whether targets are reached. In the Canadian healthcare environment, access to these resources is limited outside of highly specialized centers. In addition, limited access to endoscopy and other diagnostic testing, including FC assays and diagnostic imaging, further challenges the ability to assess for objective and reliable measures of inflammatory activity. Access to IBD nursing, mental health professionals, and social workers is beneficial for the achievement of QoL and daily functioning targets but is not covered by most governmental insurance plans and has limited coverage under private insurance. Nurses, in particular, should play an integral role in the evaluation of patients with IBD. This role is reflected in guidelines such as the Nurses European Crohn’s and Colitis Organisation consensus statements, which encourage leveraging the expertise of IBD nurses in the use of assessment tools to ensure awareness of their patients’ physical, social, and emotional concerns and the associated impact on QoL [30]. All of the aforementioned challenges may complicate the ability of healthcare professionals and patients to adhere to STRIDE-II recommendations and may lead to long-term decrements in the wellbeing of Canadians with IBD.
Increasing the frequency of monitoring for the STRIDE-II treatment targets represents an opportunity to further implement relevant guidelines in real-world Canadian settings and address the well-recognized disconnect between clinical symptoms and underlying inflammation [31,32,33]. The STRIDE-II guidance recommends that changing treatment should be considered if monitored targets have not been achieved [5]. However, this consideration may be impractical due the inability of some patients to achieve certain treatment targets. For example, patients with advanced disease and long-term complications without a clear inflammatory burden may never achieve adequate symptom control but should not necessarily be switched between several different therapies or receive increasing doses. Nevertheless, the frequency of treatment adjustments, which was also low in the current study, would likely increase with more monitoring according to the STRIDE-II recommendations.
A key strength of this study is its real-world setting. As the study visit aligned with routine clinical appointments, the results are generalizable to patients seeking routine care for IBD and their clinicians’ monitoring and treatment behaviors in real-world Canadian practice. In addition, the inclusion criteria were minimal to allow for the enrollment of patients from various Canadian regions with a broad range of comorbidities, medical and treatment histories, and socioeconomic factors. Limitations of this observational study also exist. First, the minimal application of specific criteria could not ensure that patients with certain symptoms or behaviors were represented in this cohort. The study population may not be representative of patients with IBD who do not seek care as frequently (e.g., those with milder disease). Second, the quality, accuracy, and completeness of study data that were collected retrospectively from existing patient records may have important inter-site differences potentially affecting the interpretation of findings. Third, selection bias could be introduced due to lack of data collected on ‘refusals’ of patients to participate. Finally, in patients with CD, HCRU and associated costs were generally lower when CD was suboptimally controlled, a counterintuitive finding that may have been influenced by the surgeries performed during the study. Among all patients with IBD (CD or UC), only two surgeries occurred, both in patients with optimally controlled CD. These surgeries, a non-elective ileocecal resection and an elective bowel resection, may have driven some of the other health events and the higher healthcare costs for these patients. The surgeries may also have interfered with the systematic approach of the study design that required access to objective patient evaluations to capture and monitor for red flags indicative of suboptimal control (e.g., imaging or biomarker assessments). Further studies of the economic burden of suboptimal IBD control in Canada are needed.
In conclusion, more than 50% of patients with CD and more than 40% of patients with UC from real-world clinical settings in Canada did not achieve optimal disease control as defined using STRIDE-II guidance, primarily due to unattained long-term treatment targets. Patient and clinician perceptions underestimated the actual proportions of patients with suboptimal control. Greater impairments in QoL, daily life burden, and higher utilization of outpatient resources were observed in patients with suboptimally controlled disease than in those with optimally controlled disease. The findings of this study suggest the need for a better understanding of the enablers and barriers to the implementation of STRIDE-II treat-to-target strategies in Canadian practice for optimal disease control and better QoL for patients with IBD.
Data availability
Data relevant to this study will be made available to other researchers upon reasonable request.
References
Lamb CA, Kennedy NA, Raine T et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1–s106.
GBD Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2020;5:17–30.
Wang R, Li Z, Liu S, Zhang D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: a systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open. 2023;13:e065186.
Peyrin-Biroulet L, Sandborn W, Sands BE et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–1338.
Turner D, Ricciuto A, Lewis A et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160:1570–1583.
Bouguen G, Levesque BG, Feagan BG et al. Treat to target: a proposed new paradigm for the management of Crohn’s disease. Clin Gastroenterol Hepatol. 2015;13:1042–1050.
Irvine EJ, Zhou Q, Thompson AK. The short inflammatory bowel disease questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s relapse prevention trial. Am J Gastroenterol. 1996;91:1571–1578.
Ghosh S, Louis E, Beaugerie L et al. Development of the IBD disk: a visual self-administered tool for assessing disability in inflammatory bowel diseases. Inflamm Bowel Dis. 2017;23:333–340.
Bennebroek Evertsz F, Nieuwkerk PT, Stokkers PC, Ponsioen CY, Bockting CL, Sanderman R, Sprangers MA. The patient simple clinical colitis activity index (P-SCCAI) can detect ulcerative colitis (UC) disease activity in remission: a comparison of the P-SCCAI with clinician-based SCCAI and biological markers. J Crohns Colitis. 2013;7:890–900.
Detection of infliximab and anti-infliximab antibodies with ELISA or high-performance liquid chromatography, 2014. https://www.cadth.ca/sites/default/files/pdf/lab-tests/09_Detection_of_Infliximab_and_Anti-Infliximab_Antibodies_e.pdf. Accessed 17 August, 2023.
Job Bank, 2023. https://www.jobbank.gc.ca/home. Accessed 17 August, 2023.
Employee benefits and benefits packages: what Ontario employers should know, 2023. https://learn.marsdd.com/article/employee-benefits-and-benefits-packages-what-ontario-employers-should-know/. Accessed 17 August, 2023.
Ontario Case Costing Initiative, 2017. https://data.ontario.ca/en/dataset/ontario-case-costing-initiative-occi. Accessed 17 August, 2023.
OHIP Schedule of Benefits and Fees, 2023. https://www.health.gov.on.ca/en/pro/programs/ohip/sob/. Accessed 17 August, 2023.
Average usual hours and wages by selected characteristics, monthly, unadjusted for seasonality, 2023. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1410032002. Accessed 17 August, 2023.
Zhang W, Wong CH, Chavannes M, Mohammadi T, Rosenfeld G. Cost-effectiveness of faecal calprotectin used in primary care in the diagnosis of inflammatory bowel disease. BMJ Open. 2019;9:e027043.
Biedermann L, Fournier N, Misselwitz B et al. High rates of smoking especially in female Crohn’s disease patients and low use of supportive measures to achieve smoking cessation–data from the Swiss IBD cohort study. J Crohns Colitis. 2015;9:819–829.
Chhaya V, Saxena S, Cecil E, Subramanian V, Curcin V, Majeed A, Pollok RC. Emerging trends and risk factors for perianal surgery in Crohn’s disease: a 20-year national population-based cohort study. Eur J Gastroenterol Hepatol. 2016;28:890–895.
Hammer T, Nielsen KR, Munkholm P, Burisch J, Lynge E. The Faroese IBD study: incidence of inflammatory bowel diseases across 54 years of population-based data. J Crohns Colitis. 2016;10:934–942.
Zhai H, Huang W, Liu A et al. Current smoking improves ulcerative colitis patients’ disease behaviour in the northwest of China. Prz Gastroenterol. 2017;12:286–290.
Bokemeyer B, Picker N, Wilke T, Rosin L, Patel H. Inadequate response, treatment patterns, health care utilization, and associated costs in patients with ulcerative colitis: retrospective cohort study based on German claims data. Inflamm Bowel Dis. 2022;28:1647–1657.
Gibble TH, Naegeli AN, Grabner M, Isenberg K, Shan M, Teng CC, Curtis JR. Identification of inadequate responders to advanced therapy among commercially-insured adult patients with Crohn’s disease and ulcerative colitis in the United States. BMC Gastroenterol. 2023;23:63.
Rubin DT, Sninsky C, Siegmund B et al. International perspectives on management of inflammatory bowel disease: opinion differences and similarities between patients and physicians from the IBD GAPPS survey. Inflamm Bowel Dis. 2021;27:1942–1953.
Gonczi L, Kurti Z, Verdon C et al. Perceived quality of care is associated with disease activity, quality of life, work productivity, and gender, but not disease phenotype: a prospective study in a high-volume IBD centre. J Crohns Colitis. 2019;13:1138–1147.
Dibley L, Norton C, Whitehead E. The experience of stigma in inflammatory bowel disease: an interpretive (hermeneutic) phenomenological study. J Adv Nurs. 2018;74:838–851.
Hibi T, Ishibashi T, Ikenoue Y, Yoshihara R, Nihei A, Kobayashi T. Ulcerative colitis: disease burden, impact on daily life, and reluctance to consult medical professionals: results from a Japanese internet survey. Inflamm Intest Dis. 2020;5:27–35.
Dubinsky MC, Panaccione R, Lewis JD et al. Impact of bowel urgency on quality of life and clinical outcomes in patients with ulcerative colitis. Crohns Colitis 360. 2022;4:otac016.
Kulyk A, Shafer LA, Graff LA, Stone J, Witges K, Targownik LE, Bernstein CN. Urgency for bowel movements is a highly discriminatory symptom of active disease in persons with IBD (the Manitoba Living with IBD study). Aliment Pharmacol Ther. 2022;56:1570–1580.
Sninsky JA, Barnes EL, Zhang X, Long MD. Urgency and its association with quality of life and clinical outcomes in patients with ulcerative colitis. Am J Gastroenterol. 2022;117:769–776.
Kemp K, Dibley L, Chauhan U et al. Second N-ECCO consensus statements on the European nursing roles in caring for patients with Crohn’s disease or ulcerative colitis. J Crohns Colitis. 2018;12:760–776.
Maaser C, Sturm A, Vavricka SR et al. ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144–164.
Peyrin-Biroulet L, Reinisch W, Colombel JF et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut. 2014;63:88–95.
Torres J, Bonovas S, Doherty G et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2020;14:4–22.
Acknowledgments
AbbVie and the authors thank Kenneth Atkinson, MD, David Ford, MD, Daniel Green, MD, Rajveer Hundal, MD, and Mark Silverberg, MD, for serving as investigators in this study. AbbVie and the authors also thank the study sites, study coordinators, and patients who participated in the trial. AbbVie initiated and supported the study design, data interpretation, and writing, together with an expert panel of dedicated IBD experts. The authors thank Séverine Vermeire, MD, PhD, for taking part in the study design. Fortrea, funded by AbbVie, was in charge of data management, biostatistics, and clinical study report medical writing. AbbVie reviewed and approved the publication. All authors had access to all relevant data, participated in data interpretation, writing, and review of this manuscript, and approved the final version of the article, including the authorship list. No honoraria or payments were made for authorship. Use of Inflammatory Bowel Disease Questionnaire, authored by Dr. Jan Irvine et. al, was made under license from McMaster University, Hamilton, Canada. The FACIT and all related works are owned and copyrighted by, and the intellectual property of David Cella, PhD. Permission for use of the FACIT-F is obtained by contacting Dr. Cella at information@facit.org.
Funding
This study, including the initial data analyses, was funded in full by AbbVie Corporation. The preparation of this paper was funded in part by AbbVie. Writing support was provided by Stefanie McFarlane and Linda J. Cornfield, PhD, CMPP™ of Alimentiv Inc. and was funded by AbbVie.
Author information
Authors and Affiliations
Contributions
Conception and design: JS, CL, CHC, LT. Senior author: LT. Data analysis/interpretation: all authors. Manuscript drafting: CHC. Manuscript editing for important intellectual content: all authors. All authors had access to the data and have approved the final version of this manuscript for submission. Guarantor of the article: JS.
Corresponding author
Ethics declarations
Conflict of interest
JS has received consultancy and/or speaking honoraria fees from AbbVie, BioJamp, Bristol Myers Squibb, Celltrion, Ferring, Fresenius Kabi, Janssen, Pfizer, and Takeda; and educational grants from AbbVie and BioJamp. SS has received speaker and consulting fees from AbbVie, Takeda, Ferring, Kabi Fresenius, BMS, Pfizer, and Janssen. SMS has received speaker fees, consultancy fees and/or Advisory board fees from AbbVie, Bristol Myers Squibb, Takeda, Gilead, BIO-Jamp, and Janssen. JI has received speaker fees, consulting fees, and advisory board fees from AbbVie, Takeda, and BioJamp, and advisory board fees from Eli Lilly. THS is an employee of AbbVie Corporation. CL is an employee of AbbVie Corporation. CHC is an employee of AbbVie Corporation. LT has received grants or contracts from AbbVie Canada, Janssen Canada, and Pfizer, Canada; received consulting fees from AbbVie, Amgen, Bristol Myers Squibb, Fresenius Kabi, Janssen, Lily, Pfizer, Sanofi, Takeda, and Viatris; has received payment or honoraria from AbbVie, Amgen, Bristol Myers Squibb, Fresenius Kabi, Janssen, Pfizer, Sanofi, Takeda, and Viatris; has participated on a Data Safety Monitoring Board or Advisory Board for Bristol Myers Squibb, GoodCap Pharmaceuticals, Janssen, Pfizer, and Takeda; and has received services from AbbVie, Bristol Myers Squibb, and Pfizer.
Ethical approval
Ethics Committee approval was obtained from Advarra as necessary per local laws and regulations. The study was conducted in accordance with ethical principles that have their origin in the current Declaration of Helsinki and followed the principles of International Conference Harmonization Good Clinical Practice and Good Epidemiology Practice and applicable regulatory requirements.
Informed consent
All patients provided written informed consent before study inclusion.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Siffledeen, J., Singh, S., Shulman, S.M. et al. Effect of Suboptimal Disease Control on Patient Quality of Life: Real-World Data from the Observational IBD-PODCAST Canada Trial. Dig Dis Sci (2024). https://doi.org/10.1007/s10620-024-08313-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10620-024-08313-z