Abstract
South American Leaf Blight (SALB) is the primary limitation to the establishment of new rubber (Hevea brasiliensis) crops in Latin America. This study aimed to assess the temporal dynamics of resistance to SALB in 99 elite Colombian genotypes and the IAN 873 cultivar (control) of H. brasiliensis in small-scale clone trials conducted under conditions with a high disease prevalence in the northwestern Colombian Amazon. Resistance monitoring was carried out on a monthly basis and analyzed over five climatic periods. Three variables were recorded: attack severity (AT, ranging from 0 to 4), reaction type (TR, ranging from 1 to 6) and stromal density (ST, ranging from 0 to 4). The maximum scores for TR and ST were used to classify the level of resistance of the genotypes. Highly significant differences in the mean values of AT, TR and ST among the genotypes, across the periods, and in the interaction between genotype and period were found. Over time, an increase in symptoms (AT) and signs (TR and ST) of SALB was observed. During the rainy periods, SALB intensity was highest, resulting in a leaf area affected ranging from 16 to 30%. Eight genotypes showed partial resistance (PR) (TR < 5 and ST < 2), while 16 genotypes demonstrated complete resistance (CR) (TR < 3 and ST = 0), and 76 genotypes were highly susceptible (HS) (TR > 5 or ST > 2). The temporal analysis identified 23 Colombian superior genotypes with varying degrees of SALB resistance, which can be a valuable breeding resource for improving SALB management in the Colombian Amazon region.
Similar content being viewed by others
Introduction
One of the main diseases that develop in rubber crops (Hevea brasiliensis) is South American Leaf Blight (SALB), caused by the fungus Pseudocercospora ulei (Hora Júnior et al., 2014). SALB is considered a highly damaging disease because the pathogen responsible for it spreads rapidly, is difficult to control and the damage it causes to plants is very significant (Gasparotto & Pereira, 2012). The disease is characterized by the premature shedding of leaves, which disrupts the photosynthetic process, reducing crop growth and yield (Sterling & Rodríguez, 2011); moreover, it can lead to the death of mature trees after consecutive episodes of severe defoliation in the same year (Gasparotto & Pereira, 2012; Lieberei, 2007).
The species H. brasiliensis is endemic to South America and is the main source of natural rubber in the world (Silva et al., 2012), representing up to 90% of the rubber marketed in global markets (Wu et al., 2017). Its latex is used in the manufacturing of tires, paints, plastics, sporting goods, pharmaceutical products and other items (De Santana et al., 2018). According to the International Rubber Study Group—IRSG (2022), in 2021, global production of natural rubber reached 13.8 million tons, with production concentrated in Asia (88.4%), Africa (6.8%) and Latin America (4.7%).
Colombia has approximately 69,000 hectares planted with rubber tree, with a national production of 7500 tons per year, mainly distributed in the departments of Meta, Santander, Antioquia, Vichada and Caquetá (MADR, 2019). Caquetá has an estimated production of 1,456 tons, ranking as the third department with the highest productivity in Colombia after Meta and Santander (MADR, 2021).
Low yields in Latin America, especially in very humid regions like the Amazon, are primarily due to the high pressure of SALB on susceptible cultivars (Furtado et al., 2020; Sterling & Rodriguez, 2018). In Caquetá, for example, on 6,700 affected hectares, yields decreased from 1.3 to 0.9 tons per hectare per year between 2007 and 2020. This issue involves more than 1,200 families who depend on the crop for their livelihood (ASOHECA, 2020). This situation has created the need to replace current plantations with new genotypes that are disease-tolerant and have better latex yields (Sterling et al., 2021).
In the Colombian Amazon and particularly in the department of Caquetá, various studies have been conducted to expand the genetic base of H. brasiliensis (Sterling et al., 2012), analyzing, among other aspects, the effect of environmental variations on the adaptive responses of new promising rubber clones with potential use in the region (Sterling et al., 2019a, 2019b, 2019c). Likewise, scientific efforts have focused on researching strategies for disease control since chemical control measures and fungal management are neither ecologically nor economically viable (Jaimes et al., 2015).
One of the most promising strategies is genetic improvement, using promising germplasm sources with varying levels of SALB resistance, especially under high fungal inoculum pressure conditions (Cardoso et al., 2014; Sterling et al., 2019a, 2019b, 2019c). However, the degree of resistance and the temporal stability of the reaction to SALB in the local superior rubber tree germplasm with potential use in the Amazon region are unknown. This knowledge is essential for implementing more effective SALB management in non-escape conditions. Therefore, these studies are crucial for identifying new planting materials with desirable phytosanitary traits in the new rubber promotion programs in the region (Gasparotto & Pereira, 2012; Sterling & Rodriguez, 2018).
We hypothesize that rubber tree elite genotypes identified from local germplasm may express both partial and complete resistance to SALB that remains stable over time, even with an increased inoculum pressure of P. ulei under SALB non-escape conditions. To test this hypothesis, this study aimed to assess the temporal dynamics of resistance to SALB in 99 elite Colombian genotypes and the IAN 873 cultivar (control) of H. brasiliensis in small-scale clone trials conducted under conditions with a high disease prevalence in the northwestern Colombian Amazon. The primary purpose was identify early new sources of SALB resistance through the monitoring of local elite germplasm, serving as a valuable breeding resource for enhancing SALB management under disease non-escape conditions in the Colombian Amazon region.
Materials and methods
Study area
The study was conducted at the Colchagua farm in the La Chocho rural settlement area in the municipality of Belén de los Andaquíes, department of Caquetá (1°27′01.22" North and 75°48′31.79" West, at an elevation of 287 m above sea level), in the Colombian Amazon. The study area is morphologically situated on a plateau, with terrain characterized by hilltops with slopes ranging from 7–30% and low-lying areas with slopes ranging from 0–3%, featuring soils with low to moderate fertility (IGAC, 2010). The area is identified as a no-escape zone to SALB (Castañeda, 1997).
Climate
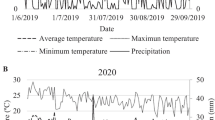
The department of Caquetá has a tropical humid climate, with an average temperature (T) of 25 °C, annual precipitation of 3235 mm, and relative humidity (RH) of 80% (Murad & Pearse, 2018). The climate in Belén de los Andaquíes follows a monomodal pattern, with two distinct climatic periods: a dry period from November to February and a rainy period from March to June, with a transition to the dry period from July to October (IGAC, 2010). The annual precipitation was 3340 mm, with a temperature of 25.2 °C, relative humidity of 85.7%, solar brightness of 1452.3 h year−1, and evaporation of 1205.5 mm. Microclimatic data for the study site were recorded using a Portable Microclimate Station (Decagon Devices Inc, USA). Thus, monthly means of precipitation, T and RH in the cycle of assessments between November 2009 and May 2011 were calculated for five period: period 1 (P1): dry 1 (November 2009 to February 2010); period 2 (P2): rainy 1 (March to May 2010); period 3 (P3): transition to dry 2 (June to October 2010); period 4 (P4): dry 2 (November 2010 to February 2011) and period 5 (P5): rainy 2 (March to May 2011) (Fig. 1).

Source: Intituto de Hidrología Meteorolgía y Estudios Ambientales—IDEAM (2011)
Climatic conditions for the five evaluated periods (from November 2009 to May 2011) in La Chocho rural settlement area, Belén de los Andaquíes municipality (Caquetá, Colombia).
Soils
The soils in Caquetá are classified as Inceptisols and Oxisols (USDA classification), characterized by fine texture and drainage limitations. They are acidic, with a pH ranging from 4.5 to 5.8, low base saturation, high aluminum saturation, and limited contents of carbon, phosphorus, potassium, and magnesium (IGAC, 2014). The study site had clayey-textured soil with organic carbon (1.01%), total nitrogen (0.15%), cation exchange capacity (16.6%), pH between 4.4 and 4.5, exchangeable acidity (6.1 meq 100 g−1), calcium (0.18 meq 100 g−1), magnesium (0.19 meq 100 g−1), potassium (0.14 meq 100 g−1), sodium (0.05 meq 100 g−1), phosphorus (0.76 mg kg−1), minor elements such as manganese (2.3 mg kg−1), iron (34.6 mg kg−1), zinc (0.16), copper (0.29 mg kg−1), and boron (0.10 mg kg−1).
Plant material
Ninety-nine Colombian H. brasiliensis genotypes from the ECC-100 (Élite Caquetá Colombia) series, resulting from a breeding program led by SINCHI Amazonian Institute of Scientific Research in Caquetá, Colombia, were evaluated, labeled ECC 1 to ECC 99. These genotypes were obtained from the asexual propagation (cloning) of plus trees aged between 28 to 35 years old (i.e., trees characterized by high production, good agronomic performance, and disease tolerance). These plus trees originated from natural cross-pollination with unknown parentals in producer farms (Sterling & Rodríguez, 2011; Sterling et al., 2011, 2019a, 2019b, 2019c). The IAN 873 clone was chosen as a control due to its good adaptation to the environment, robust agronomic performance, health and productivity in farms across various regions of Colombia (CCC, 2015).
Experimental design and trial management
The experiment, known as small-scale clonal trial (SSCT) (Clément-Damange et al., 1995), followed a randomized complete block design with 100 treatments (genotypes). The experiment was established in an area of 10 ha, which was divided into four blocks (replications) of 2.5 ha each. In each block, the 100 treatments (99 elite genotypes and the control clone IAN 873) were placed in plots consisting of 12 plants, organized in two rows of six plants and planted at a distance of 7.0 m × 3.0 m, resulting in 1200 plants per block.
No chemical controls were employed in the SSCT. Weed eradication was carried out around each rubber plant, covering a 70 cm radius to eliminate all competing weeds for light and nutrients during the initial growth stages. Mechanical weeding was conducted every six months. Cariniana pyriformis species was used as a windbreak barrier around the SSCT. These barriers helps reduce the speed of surface runoff and retains the soil transported by it (Sterling & Rodríguez, 2011). Following the methodology described by Sterling and Rodríguez (2011), fertilization was applied every six months, with each plant receiving 1 kg of organic compost-type fertilizer supplemented with a mixture of CaO and MgO (200 g plant−1), NPK (200 g plant−1) and minor elements (50 g plant−1).
Evaluation of SALB resistance
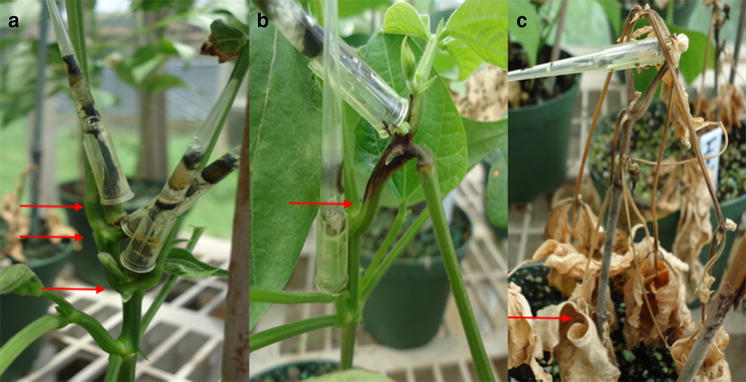
The clonal resistance of H. brasiliensis to SALB was evaluated on a monthly basis and the data were analyzed by climatic period. Observations were made on 7 randomly selected plants from each plot, starting three months after planting—from November 2009 to May 2011. Resistance to P. ulei was assessed on young leaves (stage C) and mature leaves (stage D), based on the leaf stages according to Hallé et al. (1978). For the three variables (AT, TR, and ST), the leaves with the most severe symptoms and/or signs of the disease were evaluated on each plant.
The attack severity (AT) was assessed using the adapted scale from Chee and Holliday (1986), with five classes based on the percentage of leaf surface with disease symptoms: 0: < 1% (no attack or very minor attack); 1: 1—5% (mild attack); 2: 6—15% (moderate attack); 3: 16—30% (severe attack); and 4: > 30% (very severe attack). The intensity of conidial sporulation on lesions formed on the surface of leaves in stage C was visually evaluated using the "type of reaction" (TR) variable with a scale from 1 to 6, as proposed by Junqueira et al. (1988), adapted by Mattos et al. (2003), defining the following levels: 1: Necrotic lesions without spores; 2: Non-necrotic lesions without spores; 3: Very weak sporulation on the lower side of the lesion; 4: Strong sporulation partially covering the lower side of the lesion; 5: Very strong sporulation covering the entire lower side of the lesion; 6: Very strong sporulation covering the entire lower side of the lesion and strong on the upper side. The density of stromata (ST) was evaluated on the upper side of mature leaves (stage D), according to the scale proposed by Mattos et al. (2005), which defines the following levels: 0: absence of stromata; 1: fewer than 5 stromata per leaflet; 2: between 5 and 10 stromata per leaflet; 3: between 11 and 30 stromata per leaflet; and 4: more than 30 stromata per leaflet.
The SALB resistance was evaluated in the leaflets with the highest attack severity per plant, and the average for each parameter was estimated for each genotype (Sterling et al., 2019a, 2019b, 2019c). Furthermore, the maximum scores for each variable were obtained and used to classify genotypes according to the resistance scale for SALB, modified from Le Guen et al. (2002) by Sterling et al. (2021): CR: Completely resistant = TR < 3, ST = 0; PR: Partially resistant = TR ≤ 5 and ST ≤ 2 and HS: Highly susceptible = TR > 5 or ST > 2.
Statistical analysis
A frequency distribution analysis was used to quantify the number of genotypes for each class of each SALB resistance variable. Subsequently, a linear mixed-effects (LME) model was fitted, with genotype and climatic period as fixed effects, and block and plot as random effects. Normality and homoscedasticity were assessed through model residuals analysis. Data normalization was achieved using the transformation: (Variable + 0.5)1/2, similar to previous studies (Sterling et al., 2019a, 2019b, 2019c, 2020, 2021). The residual variance was modeled to address heteroscedasticity in the fixed effects of the model, and residual correlation for successive observations on the same plant was analyzed using a compound symmetry model. Akaike (AIC) and Bayesian (BIC) criteria were used to select the residual variance and correlation structure. The nlme library (Pinheiro et al., 2018) in R 4.0.3 (R Core Team, 2020) was utilized for model fitting using the interface in InfoStat V. 2020 (Di Rienzo et al., 2020). When the fixed effects were significant (p < 0.05), Fisher's LSD multiple comparisons test was performed. In addition, a hierarchical clustering on principal components (HCPC) was employed to identify groups of similar genotypes with respect to SALB resistance variables. Finally, significant differences between clusters were assessed using an analysis of variance. The FactoMineR (Husson et al., 2020) and factoextra (Kassambara & Mundt, 2020) R packages were used for performing HCPC and visualizing the results, respectively.
Results
Climate
The data showed that precipitation and relative humidity had higher mean values during the rainy periods compared to dry periods (Fig. 1). Regarding the average temperature (°C), an inverse trend was observed with the relative humidity and precipitation data, as an increase in these variables led to a decrease in temperature, ranging from 25.3 °C in period 3 to 26.6 °C in period 1 (Fig. 1). Furthermore, in this study, the transition to the dry period between periods 2 and 4 was evident, characteristic of the monomodal regime of the northwestern Colombian Amazon.
SALB resistance
The identification of new sources of resistance to SALB based on the early field selection of resistant elite genotypes, revealed a broad range of responses in the tested germplasm (Fig. 2). The attack severity (AT) was very severe in 56 of the tested genotypes, while only 15 genotypes, including the control clone IAN 873, showed no attack or very minor attack (Fig. 2A). Furthermore, 46 genotypes did not exhibit any type of reaction (TR) to P. ulei (Fig. 2B). With respect to the stromal density (ST), most genotypes had more than 30 stromata per leaflet, and 17 genotypes showed no stromata (included the IAN 873 clone) (Fig. 2C).
The LME models revealed highly significant differences in the mean values of AT, TR and ST among the genotypes, across the periods, and in the interaction between genotype and period (Table 1). Overall, an increase in symptoms and signs of SALB was observed over time (Online Resource 1). In the period 1, the ECC 49, ECC 74, ECC 77, ECC 83 and ECC 88 genotypes showed mild attack severity (maximum AT score of 1), and the ECC 35 and ECC 32 genotypes had the highest score (2). In the period 2, there was an increasing in the severity, where five genotypes had maximum AT scores of 4. In the period 3, the number of genotypes strongly affected was increased to 13, with maximum scores of 4. In the period 4, 33 genotypes showed the highest levels of attack (maximum AT scores of 4), and in the period 5, 54 genotypes had maximum score of 4.
Regarding TR in young leaves (Online Resource 1), in the period 1, 6% of the genotypes had maximum scores of 3. In the period 2, 6% of the genotypes also had a maximum score of 3, while genotypes ECC 18 and ECC 43 had maximum scores of 4 and 6, respectively. In the period 3, 40% of the genotypes had maximums scores ranging from 1 to 4, and 4% scored 6. In the period 4, 70% of the genotypes had maximum scores between 1 and 4, and 9% scored 6. In the period 5, 78% of the genotypes had maximum scores between 1 and 4, and 12% scored 6. With respect to ST in mature leaves (Online Resource 1), 2%, 6%, 20%, 52%, and 72% of the genotypes had the highest stromata production (maximum scores > 2) in the periods 1, 2, 3, 4, and 5, respectively.
A total of 76 highly susceptible genotypes (HS) were identified (TR > 5 or ST > 2). Eight genotypes showed partial resistance (PR) (TR ≤ 5 and ST ≤ 2), specifically ECC 7, EC 23, ECC 31, ECC 52, ECC 60, ECC 79, ECC 82, and ECC 86, while 16 genotypes demonstrated complete resistance (CR) (TR < 3 and ST = 0). These genotypes included ECC 1, ECC 5, ECC 13, ECC 14, ECC 27, ECC 36, ECC 53, ECC 55, ECC 58, ECC 64, ECC 66, ECC 68, ECC 83, ECC 97, ECC 99, and IAN 873 (control) (Online Resource 1).
The hierarchical clustering on principal components (HCPC) was performed to analyzed the relationship between the genotypes groups and the means values of the three SALB variables measured in the five climatic periods (Fig. 3). Thus, the two principal component analysis (PCA) axes explained 64.9% of the total variability (Fig. 3A), while the hierarchical clustering analysis (HCA) separated the genotypes into three groups or clusters (p < 0.01; 43% of explained variance) (Fig. 3B). The group 1 contained the highest number genotypes (78), followed by the groups 2 (18) and 3 (4). In general, groups 1 and 2 comprised genotypes with the lowest average SALB scores across the five evaluated periods, whereas group 3 consisted of genotypes with higher average scores, particularly in terms of TR and ST, mainly in periods 3, 4, and 5 (Table 2).
Panel A displays a Principal Components Analysis (PCA) with a "Biplot" conducted for the three evaluated variables (AT, TR and ST), each in combination with the five climatic periods. Panel B shows hierarchical clustering for the classification of the 100 Hevea brasiliensis genotypes. Panel C presents a hierarchical clustering (with ward method and Euclidean distance) on principal components (HCPC) in a 3D view, illustrating the distribution of genotypes groups on the PCA ordination plane
To enhance the precision and efficiency of clone selection, genotypes within each group were ranked in descending order based on their maximum scores for the evaluated variables (AT, TR, and ST) (Table 3). In this context, ECC 1, ECC 5, ECC 13, ECC 14, ECC 27, ECC 36, ECC 53, ECC 55, ECC 64, ECC 68, ECC 97, ECC 99, and IAN 873 genotypes were identified as the best performers, exhibiting complete resistance (CR) to SALB. Closely following were ECC 82, ECC 7, ECC 23, ECC 31, and ECCC 83, which demonstrated partial resistance (PR). The most resistant genotypes belonged to group 1, whereas groups 2 and 3 contained genotypes highly susceptible to SALB.
Discussion
The climatic conditions that characterize the southern region of Caquetá (northwestern Colombian Amazonia) during both rainy periods (HR > 85% and precipitation > 342 mm month−1) and dry periods (HR > 84% and precipitation > 148.5 mm month−1), confirm its classification as a SALB no-escape zone (Correa‑Pinilla et al., 2022; Jaimes et al., 2016; Sterling & Rodriguez, 2018). These climatic conditions favor the presence and dissemination of P. ulei (Bevenuto et al., 2017; Gasparotto et al., 2012; Goncalves et al., 2017), thereby increasing the risk of infection for the most susceptible genotypes (Guyot & Le Guen, 2018).
During the rainy periods (periods 2 and 5), there was an increase in the attack severity (AT) in the most susceptible genotypes, resulting in leaf area affected ranging from 16 to 30%. These climatic conditions favor the epidemiology of P. ulei by facilitating the development of the asexual phase of the fungus, which adheres to the leaf surface and directly penetrates to rapidly colonize the internal leaf tissues (Guyot & Le Guen, 2018; Hora Júnior et al., 2014). This could explain the higher susceptibility observed (HS) (TR > 5 or ST > 2) in 76 out of the 100 genotypes evaluated. These findings contrast with those reported by (Gasparotto et al., 1991; Rivera, 2003), who found that SALB severity is higher during periods with lower rainfall.
Overall, the AT increased over time. Consequently, during the period 1 (dry 1), there were lower AT scores (maximum AT score of 2). In contrast, during period 5 (rainy 2), 76 genotypes exhibited high susceptibility, similar to that reported by (Rivano et al., 2013a, 2013b), who found that SALB does not significantly affect the early stages of rubber cultivation. In this regard, it was observed that in period 5 (rainy 2), with an average temperature of 25.5 ºC, the highest average scores were recorded for all SALB variables (AT, TR, and ST). This correspond with the findings of (Sterling et al., 2019a, 2019b, 2019c) and (Guyot et al., 2014), who confirmed that the greatest SALB attack occurs at average temperatures between 21 and 28 °C.
During dry periods (periods 1 and 4) a reduction in AT was observed, coinciding with a decrease in relative humidity and precipitation during these periods. In period 1 (dry 1), mild attack (maximum score of 1) were recorded, primarily in the ECC 49, ECC 74, ECC 77, ECC 83, and ECC 88 genotypes. Although there was a slight increase in AT during period 4 (dry 2), this increase was not significant (p > 0.05). This suggests a strong correlation between the decrease in P. ulei infection and the reduction in these climatic parameters, consistent with findings from previous studies (Jaimes et al., 2016; Rivano et al., 2015; Sterling et al., 2021). In this regard, Sterling et al., (2019a, 2019b, 2019c), found that rubber crops in the Caquetá department exhibited enhanced performance and lower levels of SALB severity during the dry period.
On the other hand, an increase in sporulation intensity (TR) in young leaves and stromal density (ST) in mature leaves was also evident with the rise in AT, especially in period 5 (rainy 2). During this period, the highest number of genotypes (76) with high susceptibility to SALB was reported. This aligns with findings from previous studies where SALB symptom expression has shown a strong correlation with disease signs, mainly influenced by increased rainfall and relative humidity (Jaimes et al., 2016; Rivano et al., 2010; Sterling & Melgarejo, 2014; Sterling et al., 2021).
In this study, 16 completely resistant genotypes (CR) (TR < 3 and ST = 0) were identified in the field (ECC 1, ECC 5, ECC 13, ECC 14, ECC 27, ECC 36, ECC 53, ECC 55, ECC 58, ECC 64, ECC 66, ECC 68, ECC 83, ECC 97, ECC 99, and IAN 873). These genotypes did not exhibit lesions with stromata during the five evaluated periods, which represents a key genetic barrier in the host–pathogen interaction, as it disrupts the sexual stage of P. ulei, where the highest physiological variability of this pathogen occurs (Sterling et al., 2010; Gasparotto et al., 2012; Hora Júnior et al., 2014).
The clone IAN 873 used as the control in this research, has been commercially planted in the Caquetá department since the 1960s and was chosen among the top ten H. brasiliensis clones for its phytosanitary performance in the Amazon region (Sterling & Rodríguez, 2012). Similarly, it is one of the clones reported to have better agronomic performance and higher profitability in Guararapes plantations, State of São Paulo, Brazil (Silva et al., 2010). In this regard, according to Gasparotto et al. (2012) and Guyot and Le Guen (2018), low levels of susceptibility are related to the resistance of the plant material used and the type of climatic period it faces, which aligns with the data obtained for clone IAN 873, where the development of the pathogen's sexual phase was not observed. This result aligns with that was described by (Rivano, 1997), who evaluated the IAN 873 clone and found that it had less than 1% of leaf surface affected, demonstrating no attack or resistance to P. ulei. In contrast, the most recent studies in the Colombian Amazon have reported high susceptibility in IAN 873 clone in large-scale clonal trials in a SALB no-escape zone, thus confirming the loss of resistance to P. ulei in this cultivar (Sterling et al., 2019a, 2019b, 2019c, 2020, 2021).
Rivano et al., (2013a, 2013b), clearly highlight the need to focus on the selection of new genotypes that exhibit long-lasting resistance to SALB. This entails not only evaluating resistance under current conditions but also anticipating and preventing possible P. ulei adaptations that could overcome rubber tree resistance in the future. Furthermore, it is crucial to accurately identify the epidemiological conditions that favor SALB development, as emphasized in previous (Furtado et al., 2008; Souza et al., 2013). It is important to emphasize that long-lasting resistance benefits not only farmers by reducing crop losses but also contributes to the sustainability of the rubber industry in the regions where it is cultivated.
In the Colombian Amazon, the evaluation of high-performance genotypes during the growth and tapping phases have been performed, and there has been a priority in searching for new sources of genetic resistance to P. ulei through the selection and evaluation of new genotypes of American origin with potential for the region (Sterling et al., 2019a, 2019b, 2019c, 2020, 2021). In this research, obtaining genotypes completely resistant to P. ulei under local conditions highlights the need for these materials to be considered for future evaluation in large-scale designs and controlled conditions, with the goal of their commercial release, leading to an expansion of the genetic base of Hevea spp. in the region and the country.
A detailed understanding of environmental factors such as humidity, temperature, and precipitation that contribute to increased genotype susceptibility to P. ulei is essential for developing effective management strategies. Likewise, a comprehensive approach that includes plant genetics, pathogen dynamics, and environmental conditions is required to achieve long-lasting resistance in rubber plantations (Guyot & Le Guen, 2018; Rivano et al., 2013a, 2013b).
The lack of efficiency and practical control mechanisms for SALB limits the large-scale production of natural rubber in South America (Guyot & Guen, 2018). The selection and production of clones that combine low susceptibility to SALB with good agronomic performance and latex productivity levels superior to or equivalent to traditionally cultivated rubber clones reveal an alternative for rubber producers in non-escape areas from SALB. This alternative should be evaluated over several years on a large scale and under different environmental conditions ( Rivano et al., 2010, 2015, 2016).
In conclusion, this study has revealed early new sources of SALB resistance that remain stable over time and under varying climatic conditions, despite the observed increase in SALB intensity throughout all five monitoring periods. These sources can be categorized as follows: (i) eight genotypes (ECC 7, ECC 23, ECC 31, ECC 52, ECC 60, ECC 79, ECC 82 and ECC 86) exhibiting partial resistance (PR) (TR ≤ 5 and ST ≤ 2) to SALB, and (ii) 16 genotypes (ECC 1, ECC 5, ECC 13, ECC 14, ECC 27, ECC 36, ECC 53, ECC 55, ECC 58, ECC 64, ECC 66, ECC 68, ECC 83, ECC 97, ECC 99 and IAN 873) showing complete resistance (CR) (TR < 3 and ST = 0). This study, conducted with various elite Colombian genotypes in a SALB non-escape zone, represents a methodological approach in rubber tree genetic breeding, indicating promising prospects for genotypes with good phytosanitary performance in the Colombian Amazon. Nevertheless, it is essential to carry out several years of large-scale evaluation under diverse edaphoclimatic conditions in the region before providing definite recommendations to local producers.
Data availability
All data that were produced and analyzed in this research are included in this manuscript.
Code availability
No applicable.
References
Asociación de Reforestadores y Cultivadores de Caucho del Caquetá, ASOHECA. (2020). Estadísticas del sector cauchero en Caquetá, Colombia. 30p.
Bevenuto, J. A., De Souza, J. R., & Furtado, E. L. (2017). Microcyclus ulei races in Brazil. Summa Phytopathologica, 43(4), 326–336. https://doi.org/10.1590/0100-5405/172339
Cardoso, S. E. A., Freitas, T. A., Da Silva, C. D., Gouvea, L. R. L., Goncalves, P. D. S., Mattos, C. R. R., & Garcia, D. (2014). Comparison of growth, yield and related traits of resistant Hevea genotypes under high South American leaf blight pressure. Industrial Crops and Products, 53, 337–349. https://doi.org/10.1016/j.indcrop.2013.12.033
Castañeda, A. (1997). Zonas aptas para el cultivo de caucho en Colombia. Bogotá, Colombia: Serie Técnica No. 39 - CONIF.
Chee, K. H., & Holliday, P. (1986). South American leaf blight of Hevea rubber. Malaysian Rubber Research and Development Board Monograph, 13, 50.
Clément-Damange, A., Nicolas, D., Legnaté, H., Rivano, F., Le Guen, V., Gnagne, M., & Chapuset, T. (1995). asdasdasHévéa: Stratégies de sélection. Plantations, Recherche, Développement, 2, 5–14.
Confederación Cauchera Colombiana (CCC). (2015). Estado actual del gremio cauchero colombiano. Bogotá, Colombia.
Correa-Pinilla, D. E., Gutiérrez-Vanegas, A. J., Gil-Restrepo, J. P., Martínez-Atencia, J., & de Córdoba-Gaona, O. J. (2022). Agroecological and South American leaf blight escape zones for rubber cultivation in Colombia. Agronomy Journal, 114(5), 2830–2844. https://doi.org/10.1002/agj2.21068
de Santana, A. S., Soares, N. S., & Schröder, C. A. (2018). Competitiveness and efficiency for the rubber tree production system in southern bahia (brazil) through by the policy analysis matrix (PAM). Revista Árvore, 42(6), e420606. https://doi.org/10.1590/1806-90882018000600006
Di Rienzo, J. A., Casanoves, F., Balzarini, M. G., Gonzalez, L., Tablada, M., & Robledo, C. W. (2020). InfoStat. Córdoba, Ar.: Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. http://www.infostat.com.ar
Furtado, Edson Luiz, de Jesus Junior, W. C., & Moraes, W. B. (2020). Forest diseases in Brazil: Status and management. In S. A. Estay (Ed.), Forest Pest and Disease Management in Latin America: Modern Perspectives in Natural Forests and Exotic Plantations (pp. 211–230). Cham: Springer International Publishing. https://doi.org/10.1007/978-3-030-35143-4_14
Furtado, E. L., Menten, J. O. M., & Passos, J. R. (2008). Intensidade do Mal das Folhas em plantas jovens e adultas de seis clones de seringueira na região do Vale do Ribeira. Fitopatologia Brasileira, 33(2), 130–137. https://doi.org/10.1590/S1982-56762008000200007
Gasparotto, L., L., Z., N, J., L., M., & F., R. (1991). Epidemiology of South American leaf blight of rubber tree. Manaus, region. AM. Fitopatologia Brasileira 16 (1): 19–21.
Gasparotto, L., Pereira, J. C. R., & Furtado, E. L. (2012). Doenças da seringueira no Brasil. Embrapa, 2. ed. rev, 255.
Gasparotto, L., & Pereira, J. C. R. (2012). Doenças das folhas. In L. Gasparotto & J. C. R. Pereira (Eds.), Doenças da seringueira no Brasil (2a edição., pp. 35–160). Brasília, DF: Embrapa.
Goncalves, R., Araujo, J., & Macedo, P. (2017). Resistência de campo em Hevea brasiliensis a doença mal-das-folhas- da-seringueira no Acre, Brasil. Embrapa Acre - Artigo em anais de congresso (ALICE). alice.cnptia.embrapa.br/handle/doc/1084512. https://.alice.cnptia.embrapa.br/handle/doc/1084512
Guyot, Jean, & Le Guen, V. (2018). A review of a century of studies on south american leaf blight of the rubber tree. Plant Disease, (Hilton 1955), 1–14. https://doi.org/10.1094/PDIS-04-17-0592-FE
Guyot, J., Condina, V., Doaré, F., Cilas, C., & Sache, I. (2014). Role of ascospores and conidia in the initiation and spread of South American leaf blight in a rubber tree plantation. Plant Pathology, 63(3), 510–518. https://doi.org/10.1111/ppa.12126
Hallé, F., Oldeman, R., & Tomlinson, P. B. (1978). Tropical trees and forests. An Architectural Analysis. Berlin: Springer-Verlag. https://doi.org/10.1007/978-3-642-81190-6
Intituto de Hidrología Meteorolgía y Estudios Ambientales - IDEAM. (2011). Datos meteorológicos del departamento del Caquetá. Bogotá, Colombia.
Hora Júnior, B. T., De Macedo, D. M., Barreto, R. W., Evans, H. C., Raimundo, C., Mattos, R., et al. (2014). Erasing the Past : A New Identity for the Damoclean Pathogen Causing South American Leaf Blight of Rubber. PLoS ONE, 9(8), e104750. https://doi.org/10.1371/journal.pone.0104750
Husson, F., Josse, J., Le, S., & Mazet, J. (2020). FactoMineR: Multivariate Exploratory Data Analysis and Data Mining. R package Version 2.4, 100. https://cran.r-project.org/web/packages/FactoMineR/FactoMineR.pdf
International Rubber Study Group-IRSG. (2022). Latest World Rubber Industry, Rubber statistical Bulletin. Consultation date: October 28, 2022. International Rubber Study Group.
Instituto Geografico Agustin Codazzi - IGAC. (2010). Aspectos ambientales para el ordenamiento territorial de occidente del Departamento del Caquetá (Tomo IV.). Bogotá, Colombia: IGAC.
Instituto Geográfico Agustín Codazzi -IGAC. (2010). Caquetá. Características geodráficas. Bogotá (Colombia): IGAC.
Instituto Geográfico Agustin Codazzi (IGAC). (2014). Estudio general de suelos y zonificación de tierras departamento de Caquetá, escala 1.100.000. Bogotá, DC: Imprenta Nacional de Colombia.
Jaimes, Y. Y., Molina, J. R., & Furtado, E. L. (2015). Clones de Hevea brasiliensis de alta productividad caracterizados por resistencia a Microcyclus ulei en jardin clonal en el magdalena medio colombiano. Summa Phytopathologica, 41(2), 115–120. https://doi.org/10.1590/0100-5405/1985
Jaimes, Y., Rojas, J., Cilas, C., & Furtado, E. L. (2016). Suitable climate for rubber trees affected by the South American Leaf Blight (SALB): Example for identification of escape zones in the Colombian middle Magdalena. Crop Protection, 81, 99–114. https://doi.org/10.1016/j.cropro.2015.12.016
Junqueira, N. T. V., Chaves, G. M., Zambolin, L., Alfenas, A., & Gasparotto, L. (1988). Reação de clones de seringueira a varios isolados de Microcyclus ulei. Pesquisa Agropecuária Brasileira, 23, 877–893.
Kassambara, A., & Mundt, F. (2020). Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R package version 1.0.7, 84. https://cran.r-project.org/web/packages/factoextra/index.html
Le Guen, V., Garcia, D., Mattos, C. R. R., & Clément-Demange, A. (2002). Evaluation of field resistance to Microcyclus ulei of a collection of Amazonian rubber tree (Hevea brasiliensis) germplasm. Crop Breeding and Applied Biotechnology, 2(1), 141–148.
Lieberei, R. (2007). South American leaf blight of the rubber tree (Hevea spp.): New steps in plant domestication using physiological features and molecular markers. Annals of Botany, 100(6), 1125–1142. https://doi.org/10.1093/aob/mcm133
Mattos, C. R., García, D., & Le Guen, V. (2005). Seleção de Clones de Seringueira com Alta Produção e Resistentes ao Mal-Das-Folhas. Ceplac. Comunição Técnico, 28, 1–9.
Mattos, C. R. R., Garcia, D., Pinard, F., & Le Guen, V. (2003). Variabilidade de isolados de Microcyclus ulei no sudeste da Bahia. Fitopatologia Brasileira, 28(5), 502–507. https://doi.org/10.1590/S0100-41582003000500006
Ministerio de Agricultura y Desarrollo Rural (MADR). (2019). Cadena Caucho. Indicadores, apoyos, Colombia: Ministerio de Agricultura y Desarrollo Rural p 13.
Ministerio de Agricultura y Desarrollo Rural-MADR. (2021). Cadena Caucho. Indicadores Generales, Colombia: Ministerio de Agricultura y Desarrollo Rural p 13.
Murad, C. A., & Pearse, J. (2018). Landsat study of deforestation in the Amazon region of Colombia: Departments of Caquetá and Putumayo. Remote Sensing Applications: Society and Environment, 11, 161–171. https://doi.org/10.1016/J.RSASE.2018.07.003
Pinheiro, J., Bates, D., DebRoy, S., & Sarkar, D. (2018). nlme: Linear and Nonlinear Mixed Effects Models. R Package Version, 3(1–131), 1.
R Core Team. (2020). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for statistical Computing. http://www.r-project.org/
Rivano, F. (1997). La maladie Sud-americaine des feuilles de l’hevea. I. Variabilite du pouvoir pathogene de Microcyclus ulei. Plantations Recherch, Developpement, 4(2), 104–114.
Rivano, F., Maldonado, L., Simbaña, B., Lucero, R., Gohet, E., Cevallos, V., & Yugcha, T. (2015). Suitable rubber growing in Ecuador: An approach to South American leaf blight. Industrial Crops and Products, 66, 262–270. https://doi.org/10.1016/j.indcrop.2014.12.034
Rivano, F., Martinez, M., Cevallos, V., & Cilas, C. (2010). Assessing resistance of rubber tree clones to Microcyclus ulei in large-scale clone trials in Ecuador: A less time-consuming field method. European Journal of Plant Pathology, 126(4), 541–552. https://doi.org/10.1007/s10658-009-9563-7
Rivano, F., Mattos, C. R. R., Cardoso, S. E. A., Martinez, M., Cevallos, V., Le Guen, V., & Garcia, D. (2013). Breeding Hevea brasiliensis for yield, growth and SALB resistance for high disease environments. Industrial Crops Production, 44, 659–670. https://doi.org/10.1016/j.indcrop.2012.09.005
Rivano, F., Mattos, C. R. R., Cardoso, S. E. A., Martinez, M., Cevallos, V., Le Guen, V., & Garcia, D. (2013b). Breeding Hevea brasiliensis for yield, growth and SALB resistance for high disease environments. Industrial Crops and Products, 44, 659–670. https://doi.org/10.1016/j.indcrop.2012.09.005
Rivano, F., Vera, J., Cevallos, V., Almeida, D., Maldonado, L., & Flori, A. (2016). Performance of 10 Hevea brasiliensis clones in Ecuador, under South American Leaf Blight escape conditions. Industrial Crops and Products, 94, 762–773. https://doi.org/10.1016/j.indcrop.2016.09.035
Rivera, Z. (2003). Zonas con aptitud edafoclimática para el cultivo de hule (Hevea brasiliensis Muell Arg.) e incidencia de la enfermedad sudamericana de la hoja en el estado de Tabasco, México. Tesis de Maestría. Colegio de Posgraduados. México, DF. pp. 96.
Silva, J. Q., de Gonçalves, P., Filho, J. A. S., & da Costa, R. B. (2010). Agronomical performance and profitability of exploitation systems in four rubber tree clones in São Paulo State. Bragantia, 69(4), 843–854. https://doi.org/10.1590/s0006-87052010000400009
Silva, J. Q., ScaloppiJúnior, E. J., Moreno, R. M. B., de Souza, G. B., de Gonçalves, P. S., & Filho, J. A. S. (2012). Producción y propiedades químicas del caucho en clones de Hevea según los estados fenológicos. Pesquisa Agropecuária Brasileira, 47(8), 1066–1076.
Souza, A. F., Alves, F. R., Junior, W. C. J., Furtado, E. L., & Silva, L. G. (2013). Performance of different rubber tree clones against South American leaf blight ( Microcyclus ulei ). Forest Pathology, 44(3), 211–218. https://doi.org/10.1111/efp.12084
Sterling, A, & Rodríguez, C. H. (2011). Nuevos clones de caucho natural para la Amazonia Colombiana: Énfasis en la resistencia al Mal Suramericano de las Hojas (Microcyclus ulei). Bogotá, Colombia: Instituto Amazónico de Investigaciones Científicas - SINCHI.
Sterling, Armando, & Rodríguez, C. H. (2012). Ampliación de la base genética de caucho natural con proyección para la Amazonia colombiana: fase de evaluación en periodo improductivo a gran escala. Bogotá: Instituto Amazónico de Investigaciones Científicas- Sinchi.
Sterling, Armando, Rodriguez, C., Dussan, I., Correa, J., Vargas, M., Centeno, A., et al. (2012). Evaluación fitosanitaria con énfasis en la resistencia a Microcyclus ulei de diez clones de caucho natural (Hevea brasiliensis) en Campo Clonal a Gran Escala CCGE. In Armando Sterling & C. Rodriguez (Eds.), Ampliación de la base genética de caucho natural con proyección para la Amazonia colombiana: Fase de evaluación en periodo improductivo a gran escala (pp. 79–121). Instituto Amazónico de Investigaciones Científicas SINCHI, Bogotá, Colombia. p. 148.
Sterling, A., & Rodriguez, C. (2018). Estrategias de Manejo para las principales enfermedades y plagas del cultivo del caucho con énfasis en la amazonia colombiana. Bogotá, Colombia: Instituto Amazónico de Investigaciones Científicas SINCHI.
Sterling, A., Galindo-Rodríguez, L. C., Suárez-Córdoba, Y. D., Velasco-Anacona, G., Andrade-Ramírez, T., & Gómez-Torres, A. K. (2019a). Early assessing performance and resistance of Colombian rubber tree genotypes under high South American Leaf Blight pressure in Amazon. Industrial Crops and Products, 141, 111775. https://doi.org/10.1016/j.indcrop.2019.111775
Sterling, A., Martínez-Viuche, E. J., Pimentel-Parra, G. A., Suárez-Córdoba, Y. D., Fonseca-Restrepo, J. A., & Virguez-Díaz, Y. R. (2019b). Dynamics of adaptive responses in growth and resistance of rubber tree clones under South American leaf blight non-escape conditions in the Colombian Amazon. Industrial Crops and Products, 141, 111811. https://doi.org/10.1016/j.indcrop.2019.111811
Sterling, A., Martínez-Viuche, E. J., Suárez-Córdoba, Y. D., Agudelo-Sánchez, A. A., Fonseca-Restrepo, J. A., Andrade-Ramírez, T. K., & Virguez-Díaz, Y. R. (2020). Assessing growth, early yielding and resistance in rubber tree clones under low South American Leaf Blight pressure in the Amazon region Colombia. Industrial Crops and Products, 158, 112958. https://doi.org/10.1016/j.indcrop.2020.112958
Sterling, A., & Melgarejo, L. M. (2014). Variación temporal a Microcyclus uleien los clones de caucho FX 3864 y FX 4098 en condiciones controladas. Revista Colombiana de Biotecnología, 16(2), 158–168. https://doi.org/10.15446/rev.colomb.biote.v16n2.47249
Sterling, A., Pimentel-Parra, G. A., Virguez-Díaz, Y. R., Suárez-Córdoba, Y. D., Hoyos-Duarte, J. D., & Fonseca-Restrepo, J. A. (2021). Long-term resistance in promising rubber tree genotypes as a breeding source for improving South American leaf blight management under high disease incidence in the Colombian Amazon. Crop Protection, 150, 105817. https://doi.org/10.1016/j.cropro.2021.105817
Sterling, A., Rodríguez, N., Quiceno, E., Trujillo, F., Clavijo, A., & Suárez-Salazar, J. C. (2019c). Dynamics of photosynthetic responses in 10 rubber tree (Hevea brasiliensis) clones in Colombian Amazon: Implications for breeding strategies. PLoS ONE, 14(12), e0226254. https://doi.org/10.1371/journal.pone.0226254
Sterling, A., Rodriguez, O., & Quintero, L. (2010). Variabilidad fisiológica de aislamientos de de Microcyclus ulei de la Amazonia colombiana. Momentos De Ciencia, 7(1), 30–35.
Sterling, A., Rodriguez, O. L., Rodriguez, C. H., Martínez, O., Bonilla, N. C., & Dussán, I. (2011). Variabilidad genética de genotipos élites de Hevea brasiliensis mediante el uso de descriptores morfológicos. Revista Colombia Amazónica, 4, 129–142.
Wu, C., Sun, L., Li, Y., & Zeng, R. (2017). Molecular characterization and expression analysis of two farnesyl pyrophosphate synthase genes involved in rubber biosynthesis in Hevea brasiliensis. Industrial Crops and Products, 108(January), 398–409. https://doi.org/10.1016/j.indcrop.2017.06.042
Acknowledgements
The authors would like to thank the project: “Evaluación fitosanitaria y de desempeño agronómico de materiales vegetales élite promisorios de Hevea brasiliensis (potenciales nuevos clones) presentes en el sistema productivo del departamento del Caquetá, resistentes a Pseudocercospora ulei bajo condiciones controladas y naturales”. Contract No. 116-2008I4819-3692 CIAT-SINCHI derived from Agreement No. 054/08 MADR-CIAT, executed by the Temporary Union: Instituto Amazónico de Investigaciones Científicas SINCHI, Universidad de la Amazonía and Asociación de Reforestadores y Cultivadores de Caucho del Caquetá ASOHECA, which made possible the execution and financing of this research.
Funding
Open Access funding provided by Colombia Consortium. This study was funded by Contract No. 116-2008I4819-3692 CIAT-SINCHI derived from Agreement No. 054/08 MADR-CIAT, executed by the Temporary Union: Instituto Amazónico de Investigaciones Científicas SINCHI, Universidad de la Amazonía and Asociación de Reforestadores y Cultivadores de Caucho del Caquetá ASOHECA.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Lyda Constanza Galindo-Rodríguez and Armando Sterling. The first draft of the manuscript was written by Lyda Constanza Galindo, Armando Sterling, Herminton Muñoz Ramirez and Jesica Fonseca and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Human and animal participants
This research did not involve Human and /or Animal Participants.
Informed consent
Informed consent does not apply to this research.
Consent to participate
Not applicable.
Consent for publication
The authors declare that they read and approved the manuscript.
Competing interests
The authors declare that there is no conflict of interest
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Galindo-Rodríguez, L.C., Sterling, A., Muñoz-Ramirez, H. et al. Dynamic analysis of resistance in Colombian elite Hevea brasiliensis genotypes as a breeding strategy for enhancing South American leaf blight management under disease non-escape conditions in the Amazon region. Eur J Plant Pathol (2024). https://doi.org/10.1007/s10658-024-02840-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s10658-024-02840-1