Abstract
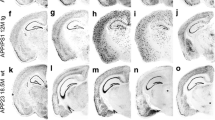
The cadherin superfamily molecules, functioning as cell adhesion molecules, are recognized to play roles in both physiological and pathological processes. The cadherin-based adherent junction (CAJ) is believed to interact with presenilin-1 (PS-1), suggesting that disruptions in CAJ structures might contribute to neurodegeneration, potentially leading to Alzheimer’s Disease (AD). Yet, the specific expression patterns of cadherin superfamily mRNA remain somewhat ambiguous. This research utilized in situ hybridization (ISH) to examine the expression and localization of cadherin mRNA in AD mouse model brains. Long cRNA probes targeting cadherin revealed endogenous mRNA expression in brain sections. Interestingly, senile plaques in the AD mouse brain were also bound to these probes. This binding, however, did not exclusively denote cadherin mRNA, as ISH detected both antisense and sense cRNA probes. Our data suggest that although antisense cRNA probes effectively detected cadherin mRNA expression in AD brain cells, their association with senile plaques may not specifically indicate cadherin mRNA expression.






Similar content being viewed by others
REFERENCES
Iqbal K., Grundke-Iqbal I., Zaidi T., Merz P.A., Wen G.Y., Shaikh S.S., Wisniewski H.M., Alafuzoff I., Winblad B. 1986. Defective brain microtubule assembly in Alzheimer’s disease. Lancet. 2 (8504), 421‒426.
Sherrington R., Rogaev E.I., Liang Y., Rogaeva E.A., Levesque G., Ikeda M., Chi H., Lin C., Li G., Holman K., Tsuda T., Mar L., Foncin J.F., Bruni A.C., Montesi M.P., Sorbi S., Rainero I., Pinessi L., Nee L., Chumakov I., Pollen D., Brookes A., Sanseau P., Polinsky R.J., Wasco W., Da Silva H.A., Haines J.L., Perkicak-Vance M.A., Tanzi R.E., Roses A.D., Fraser P.E., Rommens J.M., St George-Hyslop P.H. 1995. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 375 (6534), 754‒760.
Bagyinszky E., Youn Y.C., An S.S.A., Kim S. 2016. Mutations, associated with early-onset Alzheimer’s disease, discovered in Asian countries. Clin. Int. Aging. 11, 1467‒1488.
Unger M.S., Schernthaner P., Marschallinger J., Mrowetz H., Aigner L. 2018. Microglia prevent peripheral immune cell invasion and promote an anti-inflammatory environment in the brain of APP-PS1 transgenic mice. J. Neuroinflammation. 15 (1), 274.
Weiner M.W., Veitch D.P., Aisen P.S., Beckett L.A., Cairns N.J., Cedarbaum J., Green R.C., Harvey D., Jack C.R., Jagust W., Luthman J., Morris J.C., Petersen R.C., Saykin A.J., Shaw L., Shen L., Schwarz A., Toga A.W., Trojanowski J.Q., Alzheimer’s Disease Neuroimaging I. 2015. 2014 Update of the Alzheimer’s Disease Neuroimaging Initiative: A review of papers published since its inception. Alzheimers Demen. 11 (6), e1‒e120.
Widelitz R. 2005. Wnt signaling through canonical and non-canonical pathways: recent progress. Growth Factors. 23 (2), 111‒116.
Giagtzoglou N., Ly C.V., Bellen H.J. 2009. Cell adhesion, the backbone of the synapse: “Vertebrate” and “invertebrate” perspectives. Cold Spring Harb. Perspect. Biol. 1 (4), a003079.
Wegenast-Braun B.M., Maisch A.F., Eicke D., Radde R., Herzig M.C., Staufenbiel M., Jucker M., Calhoun M.E. 2009. Independent effects of intra- and extracellular a beta on learning-related gene expression. Am. J. Pathol. 175 (1), 271‒282.
Melchior B., Garcia A.E., Hsiung B.K., Lo K.M., Doose J.M., Thrash J.C., Stalder A.K., Staufenbiel M., Neumann H., Carson M.J. 2010. Dual induction of TREM2 and tolerance-related transcript, Tmem176b, in amyloid transgenic mice: Implications for vaccine-based therapies for Alzheimer’s disease. ASN Neuro. 2 (3), 157‒170.
Lehmann S.M., Kruger C., Park B., Derkow K., Rosenberger K., Baumgart J., Trimbuch T., Eom G., Hinz M., Kaul D., Habbel P., Kalin R., Franzoni E., Rybak A., Nguyen D., Veh R., Ninnemann O., Peters O., Nitsch R., Heppner F.L., Golenbock D., Schott E., Ploegh H.L., Wulczyn F.G., Lehnardt S. 2012. An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat. Neurosci. 15 (6), 827‒835.
Neve R.L., Valletta J.S., Li Y., Ventosa-Michelman M., Holtzman D.M., Mobley W.C. 1996. A comprehensive study of the spatiotemporal pattern of beta-amyloid precursor protein mRNA and protein in the rat brain: lack of modulation by exogenously applied nerve growth factor. Brain Res. Mol. Brain Res. 39 (1–2), 185‒197.
Aronov S., Aranda G., Behar L., Ginzburg I. 2001. Axonal tau mRNA localization coincides with tau protein in living neuronal cells and depends on axonal targeting signal. J. Neurosci. 21 (17), 6577‒6587.
Malmqvist T., Anthony K., Gallo J.M. 2014. Tau mRNA is present in axonal RNA granules and is associated with elongation factor 1A. Brain Res. 1584, 22‒27.
Page K., Hollister R., Tanzi R.E., Hyman B.T. 1996. In situ hybridization analysis of presenilin 1 mRNA in Alzheimer disease and in lesioned rat brain. Proc. Natl. Acad. Sci. U. S. A. 93 (24), 14020‒14024.
Irizarry M.C., Locascio J.J., Hyman B.T. 2001. β-Site APP cleaving enzyme mRNA expression in APP transgenic mice—anatomical overlap with transgene expression and static levels with aging. Am. J. Pathol. 158 (1), 173‒177.
Pardue S., White C.L., 3rd, Bigio E.H., Morrison-Bogorad M. 1994. Anomalous binding of radiolabeled oligonucleotide probes to plaques and tangles in Alzheimer disease hippocampus. Mol. Chem. Neuropathol. 22 (1), 1‒24.
Ginsberg S.D., Crino P.B., Lee V.M., Eberwine J.H., Trojanowski J.Q. 1997. Sequestration of RNA in Alzheimer’s disease neurofibrillary tangles and senile plaques. Ann. Neurol. 41 (2), 200‒209.
Radde R., Bolmont T., Kaeser S.A., Coomaraswamy J., Lindau D., Stoltze L., Calhoun M.E., Jäggi F., Wolburg H., Gengler S., Haass C., Ghetti B., Czech C., Hölscher C., Mathews P.M., Jucker M. 2006. Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 7 (9), 940‒946.
Redies C., Engelhart K., Takeichi M. 1993. Differential expression of N- and R-cadherin in functional neuronal systems and other structures of the developing chicken brain. J. Comp. Neurol. 333 (3), 398‒416.
Yamasaki A., Eimer S., Okochi M., Smialowska A., Kaether C., Baumeister R., Haass C., Steiner H. 2006. The GxGD motif of presenilin contributes to catalytic function and substrate identification of gamma-secretase. J. Neurosci. 26 (14), 3821‒3828.
Marcinkiewicz M. 2002. beta APP and furin mRNA concentrates in immature senile plaques in the brain of Alzheimer patients. J. Neuropathol. Exp. Neurol. 61 (9), 815‒829.
Gouras G.K., Almeida C.G., Takahashi R.H. 2005. Intraneuronal Abeta accumulation and origin of plaques in Alzheimer’s disease. Neurobiol. Aging. 26 (9), 1235‒1244.
Friedrich R.P., Tepper K., Roznicke R., Soom M., Westermann M., Reymann K., Kaether C., Fandrich M. 2010. Mechanism of amyloid plaque formation suggests an intracellular basis of A beta pathogenicity. Proc. Natl. Acad. Sci. U. S. A. 107 (5), 1942‒1947.
Ginsberg S.D., Crino P.B., Hemby S.E., Weingarten J.A., Lee V.M.Y., Eberwine J.H., Trojanowski J.Q. 1999. Predominance of neuronal m-RNAs in individual Alzheimer’s disease senile plaques. Ann. Neurol. 45 (2), 174‒181.
Ginsberg S.D., Alldred M.J., Che S.L. 2012. Gene expression levels assessed by CA1 pyramidal neuron and regional hippocampal dissections in Alzheimer’s disease. Neurobiol. Dis. 45 (1), 99‒107.
Uehara Y., Yamada T., Baba Y., Miura S.I., Abe S., Kitajima K., Higuchi M.A., Iwamoto T., Saku K. 2008. ATP-binding cassette transporter G4 is highly expressed in microglia in Alzheimer’s brain. Brain Res. 1217, 239‒246.
Westmark C.J., Malter J.S. 2007. FMRP mediates mGluR(5)-dependent translation of amyloid precursor protein. Plos Biol. 5 (3), 629‒639.
Rogers J.T., Bush A.I., Cho H.H., Smith D.H., Thomson A.M., Friedlich A.L., Lahiri D.K., Leedman P.J., Huang X.D., Cahill C.M. 2008. Iron and the translation of the amyloid precursor protein (APP) and ferritin mRNAs: Riboregulation against neural oxidative damage in Alzheimer’s disease. Biochem. Soc. Transact. 36, 1282‒1287.
Dona F., Houseley J. 2014. Unexpected DNA loss mediated by the DNA binding activity of ribonuclease A. PLoS One. 9 (12), e115008.
Chartrand P., Bertrand E., Singer R.H., Long R.M. 2000. Sensitive and high-resolution detection of RNA in situ. In RNA-Ligand Interactions, Part B: Molecular Biology Methods. Celander D.W., Abelson J.N., Eds. Methods Enzymol. 318, 493‒506.
King O.D., Gitler A.D., Shorter J. 2012. The tip of the iceberg: RNA-binding proteins with prion-like domains in neurodegenerative disease. Brain Res. 1462, 61‒80.
Mathura V.S., Paris D., Ait-Ghezala G., Quadros A., Patel N.S., Kolippakkam D.N., Volmar C.H., Mullan M.J. 2005. Model of Alzheimer’s disease amyloid-beta peptide based on a RNA binding protein. Biochem. Biophys.Res. Commun. 332 (2), 585‒592.
Chen J.W., Newhall J., Xie Z.R., Leckband D., Wu Y.H. 2016. A computational model for kinetic studies of cadherin binding and clustering. Biophys. J. 111 (7), 1507‒1518.
Mills F., Globa A.K., Liu S., Cowan C.M., Mobasser M., Phillips A.G., Borgland S.L., Bamji S.X. 2017. Cadherins mediate cocaine-induced synaptic plasticity and behavioral conditioning. Nat. Neurosci. 20 (4), 540‒549.
McCrea P.D., Maher M.T., Gottardi C.J. 2015. Nuclear signaling from cadherin adhesion complexes. In Cellular Adhesion in Development and Disease. Yap A.S., Ed. Curr. Top. Dev. Biol. 112, 129‒196.
Gutala R.V., Reddy P.H. 2004. The use of real-time PCR analysis in a gene expression study of Alzheimer’s disease post-mortem brains. J. Neurosci. Methods. 132 (1), 101‒107.
Veremeyko T., Starossom S.C., Weiner H.L., Ponomarev E.D. 2012. Detection of microRNAs in microglia by real-time PCR in normal CNS and during neuroinflammation. J. Vis. Exp. 65, 4097.
Acquaah-Mensah G.K., Taylor R.C. 2016. Brain in situ hybridization maps as a source for reverse-engineering transcriptional regulatory networks: Alzheimer’s disease insights. Gene. 586 (1), 77‒86.
Kirouac L., Rajic A.J., Cribbs D.H., Padmanabhan J. 2017. Activation of Ras-ERK signaling and GSK-3 by amyloid precursor protein and amyloid beta facilitates neurodegeneration in Alzheimer’s disease. Eneuro. 4 (2), ENEURO.0149-16.2017.
Benedet A.L., Milà-Alomà M., Vrillon A., Ashton N.J., Pascoal T.A., Lussier F., Karikari T.K., Hourregue C., Cognat E., Dumurgier J., Stevenson J., Rahmouni N., Pallen V., Poltronetti N.M., Salvadó G., Shekari M., Operto G., Gispert J.D., Minguillon C., Fauria K., Kollmorgen G., Suridjan I., Zimmer E.R., Zetterberg H., Molinuevo J.L., Paquet C., Rosa-Neto P., Blennow K., Suárez-Calvet M. 2021. Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the Alzheimer disease continuum. JAMA Neurol. 78 (12), 1471‒1483.
Ali M., Huarte O.U., Heurtaux T., Garcia P., Rodriguez B.P., Grzyb K., Halder R., Skupin A., Buttini M., Glaab E. 2022. Single-cell transcriptional profiling and gene regulatory network modeling in Tg2576 mice reveal gender-dependent molecular features preceding Alzheimer-like pathologies. Mol. Neurobiol. https://doi.org/10.1007/s12035-022-02985-2
ACKNOWLEDGMENTS
This work was supported by the Anatomy I Institute of the University of Jena. The author thanks Dr. Kaether, Prof. Dr. Jucker, and Prof. Dr. Haass and their groups for the mouse model and antibodies and Dr. F. van Roy, Dr. M. Takeichi, Dr. T. Jessell and Dr. H. Cremer for some plasmids. All authors participated in this study and have access to all data in this work.
Funding
This study is supported by the Scientific and Technological Research Project of Henan Provincial Department of Science and Technology (Grant no. 212102310217) and the grant from Funds of the National Natural Science Foundation of China (Grant no. 81401015).
Author information
Authors and Affiliations
Contributions
H.Z. conceived of the study and designed the study; H.Z. and S.D. collected, analyzed the data, and wrote the initial draft of the paper; C.C. was responsible for the project administration and supervision; all authors contributed by carrying out additional analyses and revising the manuscript.
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All studies were conducted in accordance with the principles of biomedical ethics as outlined in the 1964 Declaration of Helsinki and its later amendments. They were also approved by the Zhengzhou University (Zhengzhou, Henan, China), protocol no. 2021-265 dated January 6, 2021.
Each participant in the study provided a voluntary written informed consent after receiving an explanation of the potential risks and benefits, as well as the nature of the upcoming study.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, H., Du, S.J., Gendi, F. et al. Application of Cadherin cRNA Probes in Brains of Alzheimer’s Disease Mouse Model. Mol Biol (2024). https://doi.org/10.1134/S0026893324700134
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S0026893324700134