Abstract
Introduction
Vibration-controlled transient elastography (VCTE) based liver stiffness measurement (LSM) is an excellent ‘rule-out’ test for advanced hepatic fibrosis in liver transplant (LT) recipients, however, its ability to ‘rule-in’ the disease is suboptimal. The study aimed to improve diagnostic performance of LSM in LT recipients.
Methods
Adult LT recipients with a liver biopsy and VCTE were included (N = 150). Sequential covering analysis was performed to create rules to identify patients at low or high risk for advanced fibrosis (stage 3–4).
Results
Advanced hepatic fibrosis was excluded in patients with either LSM < 7.45 kPa (n = 72) or 7.45 ≤ LSM < 12.1 kPa and time from LT < 5.6 years (n = 25). Conversely, likelihood of advanced fibrosis was 95% if patients had LSM > 14.1 and controlled attenuation parameter > 279 dB/m (n = 21). Thus, 118 (79%) were correctly identified and 32 (21%) would have required a biopsy to establish the diagnosis. Compared to previously established LSM based cutoff values of 10.5 kPa (Youden index) and 13.3 kPa (maximized specificity), the false positive rates of sequential covering analysis was 1% compared to 16.5% with LSM ≥ 10.5 kPa and 8.3% with LSM ≥ 13.3 kPa. The true positive rates were comparable at 87% for sequential covering analysis, 93% for LSM ≥ 10.5 kPa and 83% for LSM ≥ 13.3 kPa.
Conclusion
The proposed clinical sequential covering analysis allows for better risk stratification when evaluating for advanced fibrosis in LT recipients compared to LSM alone. Additional efforts are necessary to further reduce the number of patients with indeterminate results in whom a liver biopsy may be required.
Similar content being viewed by others
Introduction
The severity of hepatic fibrosis is a strong predictor of clinical outcomes among liver transplant (LT) recipients [1, 2]. Historically, hepatic fibrosis was quantified via annual protocol liver biopsies, however, this approach exposed patient low risk for advanced fibrosis to unnecessary procedural risk. Moreover, coupled with higher healthcare cost of liver biopsies, annual protocol liver biopsies are no longer used in routine clinical practice in management of LT recipients. This underscores the importance of developing non-invasive fibrosis assessment in LT recipients to identify at risk patients such as those with advanced hepatic fibrosis.
Recent efforts in non-invasive biomarker development have centered around vibration-controlled transient elastography (VCTE) and clinical prediction models (CPM), which can be readily deployed in clinical practice [3, 4]. Both VCTE and CPM have been evaluated in LT recipients and VCTE based liver stiffness measurement, a surrogate for hepatic fibrosis, had outperformed CPMs [5, 6]. While liver stiffness based (LSM)-based fibrosis assessment had high negative predictive value (NPV), its positive predictive value (PPV) remained relatively modest [5]. In non-LT population, the diagnostic performance of LSM has been improved upon by creating LSM-based aggregate scores such as FibroScan-AST (FAST) score, AGILE3 and AGILE4, that incorporate readily available clinical parameters such as age, serum aminotransferases, diabetes status, and platelet counts [7, 8]. However, the performance of such scores in LT recipients did not improve on the diagnostic performance of LSM alone [9]. This is partly due to the fact that many of the parameters used to calculate LSM-based scores such as aminotransferases, platelet counts and diabetes, which can be affected by use of chronic immunosuppression.
The limited performance of LSM-based scores in LT recipients, further highlights key differences in clinical presentation of this population and importance of developing fibrosis scoring systems that consider LT specific physiology. Thus, the aim of the current study was to develop LSM-based scores to improve on the PPV of LSM alone in detecting the presence of advanced hepatic fibrosis (fibrosis stage 3–4) in LT recipients. The study was conducted according to best practices in biomarker development using histology as a reference standard to (1) develop and calibrate LT specific LSM-based score (2) develop and optimize cutoff values that can be readily incorporated into clinical practice and (3) to compare the performance on the new model to LSM alone.
Methods
Study Design
Adult subjects (age ≥ 18 years) were prospectively enrolled into a natural history study of LT recipients at the Hume-Lee Transplant Center at Virginia Commonwealth University [10, 11]. Study participants with qualifying liver biopsy with fibrosis assessment and who underwent VCTE between 1/1/2014 to 2/10/2023 were eligible for inclusion. The analysis was restricted to patients who had a liver biopsy and VCTE within 6 months except for patients with documented cirrhosis on a previous liver biopsy, who only underwent VCTE. The study was approved by the Institutional Review Board (IRB). The manuscript was reviewed and approved by all authors prior to submission.
Patient Population
Adult patients with a liver biopsy and VCTE in the natural history study were used for this analysis. Patients with active use of more than mild alcohol consumption as defined by more than 1 standard drink/day in women and 2 standard drink/day in men were excluded. Additional exclusion criteria included active therapy for HCV, untreated HCV, acute cellular rejection, chronic rejection, cholestatic hepatitis, implantable cardiac devices, pregnancy, ascites, dialysis, and heart failure. Only subjects with well-compensated cirrhosis and no clinical manifestation of portal hypertension were included. Patients with decompensated cirrhosis were excluded as the fibrosis stage is often evident in patients with signs or symptoms of portal hypertension and these models offer little utility in those cases.
Liver Biopsy
The decision to perform a liver biopsy was at the discretion of the treating physician (transplant hepatology or transplant surgery) based on their clinical assessment. All liver biopsies were scored by a histopathologist blinded to the clinical data. Hepatic fibrosis was quantified from stages 0–4 [12]. Advanced fibrosis was defined as fibrosis stage 3–4.
Vibration-Controlled Transient Elastography
All VCTE was performed using the Fibroscan® 502 Touch software (Echosens, Paris, France) as described previously [6]. Briefly, after an overnight fast, subjects were placed in supine position with right arm in maximal abduction and measurements were taken over the right hepatic lobe through the intercostal space [13]. All studies were started using M probe and XL probe was used only if prompted by the 502 Touch software. An LSM exam was considered unreliable if the IQR/Median > 30%, while technical failure was defined as inability to obtain 10 valid measurements.
Plan of Analysis
Summary statistics including medians (first quartile, third quartile), sums, and percentages are presented as appropriate. The sample size of the present study included all patients who underwent a liver biopsy with qualifying VCTE as noted above. Boxplots were constructed for fibrosis vs. LSM, fibrosis vs. CAP and fibrosis vs. time from LT and the significance was determined Jonckheere–Terpstra test. The clinical algorithm was developed using the sequential covering approach to detect presence of at least advanced hepatic fibrosis in LT recipients. The sequential covering approach accomplishes this by learning rules in determining if a LT recipient has advanced hepatic fibrosis. These rules sequentially apply the best thresholds on clinical parameters to predict the presence of advanced fibrosis. As the goal of this sequential covering approach was to allow for easy clinical adaptability, readily available clinical parameters (i.e. age, time from LT, gender, platelet count, aminotransferases, diabetes status) and VCTE based parameters (LSM and CAP) were utilized. The sequential covering analysis produced a “Rule in” zone, “Rule out” zone and an indeterminate zone in which presence or absence of advanced fibrosis cannot be confidently established. The sequential covering analysis was further validated using leave one out cross validation. Diagnostic performance that included sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV) are reported. The TRIPOD checklist is included in the Supplemental Material.
Once the sequential covering approach had been established, it was compared to the diagnostic performance of LSM that has been published previously [6]. The LSM cutoff values used were 10.5 kPa and 13.3 kPa that were established at Youden index and fixed specificity of 90%, respectively [6]. Each approach was used to characterize patients who were appropriately classified as defined by true positives and true negatives. Similarly, misclassification rates that included false positives and false negatives were also determined. Finally, the number of biopsies necessary to help establish the diagnosis was also determined. For the sequential covering approach, this included patients who could not be either ‘ruled in’ or ‘ruled out’ for presence of advanced fibrosis. For LSM alone, since the PPV is relatively low, all patients above the threshold of 10.5 or 13.3 kPa were considered to have required a liver biopsy to help establish the diagnosis.
Two-sided p values of less < 0.05 was considered statistically significant. All statistical analysis was done using SPSS v. 21 (SPSS Inc., Chicago, IL, USA) and R v. 4.2.2.
Results
Description of the Study Cohort
The study cohort consisted of 150 LT recipients with median age of 60 (50, 66) years (Table 1). A total of 86 (57%) were males and 105 (70%) were non-Hispanics whites. The median BMI of the cohort was 28.2 (24.3, 32.6) kg/m2 and 39% were obese. The most common etiology of liver disease requiring LT was Hepatitis C followed by NASH and alcohol induced liver disease. The median time from LT to liver biopsy was 4.9 (1.7, 11.7) years and tacrolimus was commonly used immunosuppression. An XL probe was required in 51 (34%) of patients. The LSM and CAP values are plotted against hepatic fibrosis stages in Figs. 1A and B. Advanced fibrosis (fibrosis stage 3–4) was present in 29 (19%) of patients.
Development of the Models
The VCTE model was developed in stages to optimize the ability of VCTE to ‘rule-in’ and ‘rule-out’ advanced fibrosis. In the first phase of model building, the NPV of VCTE was leveraged to exclude patients unlikely to have any significant fibrosis. Thus, using sequential covering analysis, an LSM value of 7.45 kPa was used as the entry point into the clinical algorithm (Fig. 2). A value of LSM less than 7.45 kPa resulted in exclusion of 72 (48%) patients in the cohort.
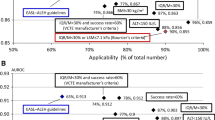
A The proposed sequential covering analysis for identification of liver transplant recipients with advanced fibrosis. AF advanced fibrosis, CAP controlled attenuation parameter, LSM liver stiffness measurement, LT liver transplant. B The relationship between LSM, CAP and time from liver transplantation as predictor of advanced hepatic fibrosis. C Visual representation of performance of sequential covering analysis in identifying liver transplant recipients with advanced fibrosis
The next node was an LSM threshold of 12.1 kPa. Of the 40 patients with LSM less than 12.1, only 3 subjects had advanced fibrosis. By introducing time from LT as the next node in the sequential covering, patients who had a LT within 5.6 years were unlikely to have advanced fibrosis, leading to correct identification of another 25 (17%) of patients. Thus, LSM < 7.45 kPa or LSM between 7.45 and 12.1 kPa and receiving a LT within 5.6 years correctly identified 65% of the study cohort with the absence of advanced fibrosis.
The remaining cohort of 53 patients who had LSM > 12.1 kPa and had received a LT at least 5.6 years prior were now enriched with advanced fibrosis (n = 29). In this cohort, an LSM threshold of 14.8 kPa was introduced and 29 patients had LSM greater than 14.8 kPa with PPV of 80%. The next node introduced a CAP value of 279 dB/m as the threshold. Thus, use of LSM > 14.8 kPa and CAP value 279 dB/m or higher lead to identification of 20 out of 21 patients with advanced fibrosis with a PPV of 95%. This approached leveraged the relationship between LSM, CAP and time from LT as key positive predictor of LT (Fig. 2B).
In patients with LSM between 12.1 and 14.8 kPa, 6 out of 24 had advanced fibrosis. Similarly, patients LSM > 12.1 and CAP ≤ 279 kPa, 3 out of 8 patients had advanced fibrosis. Thus, the diagnosis of advanced fibrosis could not be established in 32 (21%) patients. This group of patients was defined as the indetermediate zone.
In summary, the implementation of the proposed sequential covering analysis lead to correct characterization of 65% of patients who were ruled out for advanced fibrosis with 100% accuracy (Fig. 2C) and 14% of patients who were ruled in for advanced fibrosis with 95% positive predictive value. The intermediate zone consisted of 21% of the cohort with a 28% prevalence of advanced hepatic fibrosis. The developed sequential covering analysis approach was validated using leave 1-out cross validation with similar diagnostic performance.
Comparison of the Proposed Sequential Approach to LSM Alone
The performance of sequential approach to LSM models was evaluated using previously published LSM cutoff values that were specific to LT recipients [6]. First, the LSM cutoff value of 10.5 kPa was used that was calculated at Youden Index (maximal sensitivity and specificity). The LSM cutoff value of 10.5 kPa had PPV of 57% and NPV of 98% (Table 2). Of the 150 patients, LSM value of 10.5 kPa correctly identified 128 (85%) patients (true positives + true negatives/total patients). There were 20 (13%) patients who were incorrectly identified as having advanced fibrosis.
An LSM cutoff value of 13.3 was also utilized as this was established at 90% specificity and thus, may reduce the need for unnecessary biopsy. The LSM value of greater than 13.3 kPa had PPV of 71%, NPV of 96% and correctly identified 135 (90%) patients. The false positive rates for sequential covering analysis (excluding indeterminate ranges) was significantly lower than LSM cutoff values ≥ 10.5 kPa and ≥ 13.3 kPa (1% vs. 16.5% vs. 8.3%) (Fig. 3). The true positive rates were 87%, 93% and 83% for sequential covering analysis, LSM ≥ 10.5 kPa and LSM ≥ 13.3 kPa, respectively.
Discussion
The LSM based assessment of patients has become a mainstay of identifying patients at risk of advanced hepatic fibrosis and recent studies highlight the utility of LSM in LT recipients [6, 9, 14]. The diagnostic performance of LSM for detection of advanced hepatic fibrosis in LT recipients is excellent, owing to its high NPV [6]. Thus, VCTE based LSM is a ‘rule-out’ test as its PPV is relatively modest and high LSM values requires further validation with a liver biopsy. The present study utilized sequential use of clinical and VCTE-based parameters (i.e. LSM and CAP) to improve the diagnostic performance of VCTE for detection of advanced hepatic fibrosis and thereby further reducing the need for liver biopsy in LT recipients. There is limited data using elastography for risk stratification in LT recipients and these studies utilized dichotomous cutoff values. Two-dimensional shear wave elastography (2D-SWE) has previously been associated have higher mortality rates in the post-LT population, however, 2D-SWE is not as widely used in clinical practice as VCTE [18]. This underscores the importance of developing risk stratification employing VCTE.
The entry point in the clinical decision making for VCTE based algorithm employed a relatively low LSM cutoff value to eliminate patients who are less likely to have advanced fibrosis with perfect accuracy. While hepatic fibrosis progression in LT recipients is usually accelerated, it is remains rare relatively early after LT [2, 14]. The optimal cutoff value for time from LT was determined to be 5.6 years by modeling patient data from the study cohort. No patients below this cutoff had advanced fibrosis. Thus, by combining intermediate LSM values with time from LT, a significant number of LT recipients can be further eliminated. These two decision rules allow ‘ruling out’ of advanced fibrosis among LT with 100% accuracy. These findings supplement previously published literature that showed high NPV and discriminatory value of LSM for ruling out advanced hepatic fibrosis.
The PPV value of LSM to detect advanced hepatic fibrosis improves as LSM value increases [15]. We leveraged this concept to establish a higher threshold (i.e. 14.8 kPa) where the likelihood of advanced hepatic fibrosis was considerably higher than LSM alone based on prior published reports [6, 9]. Moreover, it is well established among patients with nonalcoholic fatty liver disease (NAFLD) that progression to cirrhosis [and potentially advanced hepatic fibrosis] is associated with reduction in severity of hepatic fat [16, 17]. While the pathophysiological basis for this observation needs to be better defined, it does allow for incorporation of presence and severity of liver fat as a surrogate for exclusion of patients who may not have yet developed advanced hepatic fibrosis. Thus, using a low CAP value that is readily obtained from VCTE to exclude patients who may not have advanced fibrosis improved the PPV of LSM > 14.8 kPa from 85 to 95% when LSM > 14.8 kPa was combined with CAP ≤ 279 dB/m. Incorporations of these physiological processes helped improve the PPV of VCTE and therefore allows the use of VCTE as a ‘rule-in’ test in select cohort of LT recipients with advanced hepatic fibrosis. Finally, within the indeterminate zone, the prevalence of advanced hepatic fibrosis was considerably higher (nearly 1 in 3 patients), thus improving the overall yield of a liver biopsy.
The diagnostic performance of the proposed sequential approach improves on only utilizing LSM alone for risk stratification. However, there remained a smaller cohort of patients who could not be either confidently ‘ruled in’ or ‘ruled out’ for advanced hepatic fibrosis. These were patients who either had advanced hepatic fibrosis with LSM value < 14.8 kPa or elevated LSM plus high CAP value. A conscious decision was made not to develop additional rules (i.e. nodes) to risk stratify these patients further as the number of patients meeting these criteria were relatively small and would lead to overfitting of the model, making the algorithm less generalizable. However, the proposed approach was able to reduce the number of liver biopsies needed if LSM alone was used.
Strengths and Limitations
A major limitation of VCTE in clinical practice is sub-optimal PPV, thereby, relegating VCTE largely as a ‘rule-out’ test [6]. In the present study, we used best practices in biomarker development to propose a VCTE-based algorithm for detection of advanced hepatic fibrosis in LT recipients that not only maximizes NPV but also PPV in a select group of patients in whom a liver biopsy can be largely avoided. The parameters used in the algorithm are either readily available (i.e. time for LT) or can be obtained from VCTE (LSM and CAP) that minimizes the need for excessive testing and time delay in establishing the diagnosis. Thus, the proposed algorithm allows for rapid integration into clinical practice for more precise risk stratification in LT recipients.
The study results must be evaluated in the context of study limitation. The present study, was not able to provide clarity to a sub-set of patients, in whom a liver biopsy would be needed to establish the diagnosis. Due to relatively smaller sample size, the study is unable to develop a disease specific algorithm, however, this requires larger sample size. The present study provides the foundational data needed to prospectively design such a study. As the number of patients with stage 2 fibrosis was relatively modest, the study is unable to provide a similar algorithm for risk stratification of patients with at least moderate hepatic fibrosis after LT. However, presence of advanced hepatic fibrosis in LT recipients has been negatively associated with both graft and patient health and thus, was used in this pilot study to optimize VCTE based risk stratification strategies. Additionally, patients were chosen for biopsy based on hepatologist judgement. As the study was retrospective, the sequence in which patients underwent VCTE and liver biopsy was not standardized. While these can introduce potential bias, the present study provides novel conceptual data on designing prospective study to develop clinically useful VCTE based algorithm for risk stratification.
In conclusion, detection of advanced hepatic fibrosis in LT recipients is challenging and VCTE has proven to be a useful tool for risk-stratification, particularly as a ‘rule-out’ test. The present study, utilizes VCTE and clinical parameters to develop a novel clinical algorithm to improve on the diagnostic performance of VCTE alone for detection of advanced hepatic fibrosis in LT recipients. While this strategy still requires further validation, incorporation of readily available clinical parameters in the algorithm allows for rapid integration into clinical practice to improve the clinical care of LT recipients.
References
Burra P, Senzolo M, Adam R et al. Liver transplantation for alcoholic liver disease in Europe: a study from the ELTR (European Liver Transplant Registry). Am J Transplant. 2010;10:138–148. https://doi.org/10.1111/j.1600-6143.2009.02869.x.
Rinella ME, Satapathy SK, Brandman D et al. Factors impacting survival in those transplanted for NASH cirrhosis: data from the NailNASH Consortium. Clin Gastroenterol Hepatol. 2022. https://doi.org/10.1016/J.CGH.2022.02.028.
Siddiqui MS, Vuppalanchi R, Van Natta ML et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2018. https://doi.org/10.1016/j.cgh.2018.04.043.
Siddiqui MS, Yamada G, Vuppalanchi R et al. Diagnostic accuracy of noninvasive fibrosis models to detect change in fibrosis stage. Clin Gastroenterol Hepatol. 2019;17:1877-1885.e5. https://doi.org/10.1016/j.cgh.2018.12.031.
Siddiqui MS, Bui AT, Syed T, Tseng M, Hassouneh R, Bhati CS. Performance of vibration-controlled transient elastography and clinical prediction models in liver transplant recipients. Clin Gastroenterol Hepatol. 2022. https://doi.org/10.1016/J.CGH.2022.02.023.
Siddiqui MS, Idowu MO, Stromberg K, et al. 2021. Diagnostic performance of vibration-controlled transient elastography in liver transplant recipients. Clin Gastroenterol Hepatol. 19:367–374.
Newsome PN, Sasso M, Deeks JJ et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:362–373. https://doi.org/10.1016/S2468-1253(19)30383-8.
Sanyal AJ, Foucquier J, Younossi ZM et al. Enhanced diagnosis of advanced fibrosis and cirrhosis in individuals with NAFLD using FibroScan-based Agile scores. J Hepatol. 2023. https://doi.org/10.1016/J.JHEP.2022.10.034.
Arshad T, Bhati CS, Bui AT et al. Evaluation of liver stiffness measurement based scores in liver transplant recipients. Liver Transpl. 2022. https://doi.org/10.1002/LT.26568.
Siddiqui MBS, Patel S, Arshad T et al. The relationship between hypoadiponectinemia and cardiovascular events in liver transplant recipients. Transplantation. 2019. https://doi.org/10.1097/TP.0000000000002714.
Siddiqui MB, Arshad T, Patel S et al. Small dense low-density lipoprotein cholesterol predicts cardiovascular events in liver transplant recipients. Hepatology. 2019. https://doi.org/10.1002/hep.30518.
Kleiner DE, Brunt EM, Van Natta M et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. https://doi.org/10.1002/hep.20701.
Vuppalanchi R, Siddiqui MS, Van Natta ML et al. Performance characteristics of vibration-controlled transient elastography for evaluation of non-alcoholic fatty liver disease. Hepatology. 2017. https://doi.org/10.1002/hep.29489.
Bhati C, Idowu MO, Sanyal AJ et al. Long term outcomes in patients undergoing liver transplantation for nonalcoholic steatohepatitis related cirrhosis. Transplantation. 2017;101:1867–1874. https://doi.org/10.1097/TP.0000000000001709.
Boursier J, Vergniol J, Guillet A et al. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J Hepatol. 2016;65:570–578. https://doi.org/10.1016/j.jhep.2016.04.023.
Caldwell SH, Lee VD, Kleiner DE et al. NASH and cryptogenic cirrhosis: a histological analysis. Ann Hepatol. 2009;8:346–352.
Shadab Siddiqui M, Harrison SA, Abdelmalek MF et al. Case definitions for inclusion and analysis of endpoints in clinical trials for NASH through the lens of regulatory science. Hepatology. 2017. https://doi.org/10.1002/hep.29607.
Fallahzadeh M, Asrani S, Vahhab E et al. Prediction of long-term morbidity and mortality after liver transplantation using two-dimensional shear wave elastography compared with liver biopsy. Liver Transpl. 2022. https://doi.org/10.1002/lt26450.
Funding
The project was supported by NIH R01 DK129564-01.
Author information
Authors and Affiliations
Contributions
Study design: MSS, ATB, DV, TA, MM. Patient recruitment: VP, VK, DB, SL, AS. Data collection: DV, TA, HK, AB, SG, AA. Statistical analysis: ATB. Manuscript preparation: MSS, ATB, DV, TA. Critical review of manuscript: All authors.
Corresponding author
Ethics declarations
Conflict of interest
None: DV, TA, HK, AB, SG, AA, VP, VK, DB, SL, AS, MM. MSS: Sagimet (DSMB); AMRA (Advising). ATB: Zydus (Consulting).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Arshad, T., Vainer, D., Khan, H. et al. Development of Clinical Algorithm Utilizing Vibration-Controlled Transient Elastography to Detect Advanced Hepatic Fibrosis in Liver Transplant Recipients. Dig Dis Sci (2024). https://doi.org/10.1007/s10620-024-08366-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10620-024-08366-0