Abstract
Background
Infliximab and vedolizumab are widely used to treat Crohn’s disease (CD) and ulcerative colitis (UC).
Aims
This systematic review and network meta-analysis evaluated comparative efficacy of various regimens for intravenous or subcutaneous infliximab and vedolizumab during maintenance treatment in CD and UC.
Methods
Parallel-group randomized controlled trials (RCTs) were identified by a systematic literature review (CRD42022383401) and included if they evaluated therapeutics of interest for maintenance treatment of adults with moderate-to-severe luminal CD or UC and assessed clinical remission between Weeks 30 and 60. Clinical remission rates in CD or UC and mucosal healing rates in UC were analyzed in a Bayesian network meta-analysis model. Endoscopic outcomes in CD were synthesized by proportional meta-analysis.
Results
Overall, 13 RCTs were included in the analyses. All vedolizumab studies randomized induction responders to maintenance treatment; infliximab studies used a treat-through design. Subcutaneous infliximab 120 mg every 2 weeks had the highest odds ratio (OR) [95% credible interval] versus placebo for clinical remission during the maintenance phase (CD: 5.90 [1.90–18.2]; UC: 5.45 [1.94–15.3]), with surface under the cumulative ranking curve (SUCRA) values of 0.91 and 0.82, respectively. For mucosal healing in UC, subcutaneous infliximab 120 mg every 2 weeks showed the highest OR (4.90 [1.63–14.1]), with SUCRA value of 0.73, followed by intravenous vedolizumab 300 mg every 4 weeks (SUCRA value, 0.70). Endoscopic outcomes in CD were better with subcutaneous infliximab 120 mg every 2 weeks than intravenous infliximab 5 mg/kg every 8 weeks.
Conclusions
Subcutaneous infliximab showed a favorable efficacy profile for achieving clinical remission and endoscopic outcomes during maintenance treatment in CD or UC.
Graphical Abstract

Similar content being viewed by others
Introduction
Inflammatory bowel disease (IBD) refers to a heterogeneous group of chronic inflammatory disorders affecting the digestive tract, of which the principal phenotypes are Crohn’s disease (CD) and ulcerative colitis (UC) [1]. Globally, the burden of IBD is increasing, with the most recent systematic assessment showing an increase in age-standardized prevalence from 79.5 per 100,000 people in 1990 to 84.3 per 100,000 in 2017 [2].
During the past 15 or so years, biologic treatment options have revolutionized therapy for moderate-to-severe IBD [3]. However, as the range of treatment options has expanded to include not only the tumor necrosis factor inhibitors (TNFis), but also the biologics vedolizumab (VDZ) and ustekinumab, and small molecules such as Janus kinase (JAK) inhibitors and sphingosine-1-phosphate (S1P) receptor modulators, the complexity of treatment-related decisions has increased [4]. Infliximab (IFX) and VDZ are considered effective biologic treatment options for patients with moderate-to-severe IBD, and both are available in Europe as intravenous (IV) and subcutaneous (SC) formulations with various dose adjustment strategies [5,6,7,8,9]. Recently, SC IFX has been approved by the US Food and Drug Administration (FDA) for the maintenance treatment of moderate-to-severe CD and UC and SC VDZ for moderate-to-severe UC [10,11,12]. To date, no head-to-head randomized controlled trials (RCTs) have evaluated IFX and VDZ for the treatment of patients with IBD [13].
In the absence of head-to-head data from prospective RCTs, network meta-analyses (NMAs), which use data from multiple RCTs with common comparators, can be an important source of information by providing indirect evidence on the comparative aggregate efficacy of different treatments in the IBD field [13, 14]. Indeed, systematic reviews and NMAs of data from RCTs contribute to evidence-based healthcare decision-making, for example when developing clinical practice guidelines and reimbursement policies [15].
Several systematic reviews and NMAs have assessed the efficacy of IFX and VDZ [16,17,18]; however, limited comparative efficacy results are available for SC formulations of these agents, given their recent regulatory approval in Europe and the US for the IBD indication (UC only for VDZ SC). To address this evidence gap, we conducted an NMA to evaluate the comparative efficacy of IFX and VDZ during maintenance treatment for TNFi-naïve CD and UC, including comparison of the SC formulation. To our knowledge, this analysis is the first to comprehensively assess the SC administration route for IFX and VDZ as separate treatment arms in a TNFi-naïve population.
Methods
The systematic literature review was performed according to a prospectively registered study protocol (PROSPERO number CRD42022383401; https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=383401) [19].
Search Strategy
We performed systematic electronic searches of PubMed and Embase to identify potentially relevant studies reported as full-text reports. Search strategies employed Medical Subject Headings and free-text terms (Supplementary materials). Additionally, we conducted hand searches of relevant gray literature sources, including the European Crohn’s and Colitis Organisation website and the European Medicines Agency website. All searches were conducted for 1997 to December 1, 2022.
Inclusion and Exclusion Criteria
Study Design
Parallel-group randomized (placebo- or active-) controlled trials were eligible for inclusion. We included studies that evaluated IV or SC IFX (reference product or biosimilar) or VDZ for maintenance treatment (≥ 22 weeks) and that assessed clinical remission at a timepoint between 30 and 60 weeks. Studies comparing clinical outcomes between a reference product and its biosimilar were excluded.
Outcomes
The prespecified outcome of interest was clinical remission rate (as defined in the included studies, e.g., Crohn’s Disease Activity Index [CDAI] score of ≤ 150 for patients with CD, or Mayo score of ≤ 2 and no subscore of > 1 for patients with UC). In addition, exploratory analyses were conducted for endoscopic outcomes, as defined in the included studies; in patients with CD, endoscopic remission was defined as either an absolute Simple Endoscopic Score for Crohn’s Disease (SES-CD) of ≤ 2 or an SES-CD subscore of ≤ 2, and mucosal healing was defined as an absence of all ulcers; in patients with UC, mucosal healing was defined as an absolute endoscopic subscore of 0 or 1 (based on the Mayo scoring system).
Participants
Two cohorts of patients were included and analyzed separately: TNFi-naïve adults (aged ≥ 18 years) with moderate-to-severe CD and TNFi-naïve adults with moderate-to-severe UC. Pediatric patients (aged < 18 years), patients who had previously received TNFis, and patients with either fistulizing CD or acute severe UC, were excluded. For analysis of endoscopic outcomes in CD, all patients were included, regardless of previous TNFi exposure, due to data availability.
Study Selection
Two authors (DC and D-HK) independently screened the titles and abstracts of the retrieved records (i.e., full-text articles published in peer-reviewed journals) against the predefined eligibility criteria to identify potentially relevant studies for inclusion (noting reasons for exclusion). Full-text publications for studies identified as potentially relevant were sourced and reviewed independently by two authors (DC and D-HK) to determine inclusion/exclusion. Disagreements were resolved by discussion or through arbitration by a third author if necessary. Multiple reports of the same study were collated, so that studies were the unit of interest for this review. The screening and full-text review process was documented to generate a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart [20].
Quality Assessment
Risk of bias for the included studies was evaluated using the Cochrane risk of bias tool version 1.0 [21, 22]. Briefly, potential sources of bias were rated as high, low, or unclear for the following seven domains: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective outcome reporting; and other bias. Assessments were completed independently by two authors (DC and D-HK), with disagreements resolved by discussion or arbitration by a third author if necessary.
Statistical Methods
Clinical remission rate data were analyzed in separate Bayesian NMA fixed-effect models for the CD and UC cohorts. A Bayesian NMA fixed-effect model was also used to analyze mucosal healing data for the UC cohort. For all analyses, interventions were split by biologic (IFX or VDZ), dosage regimen (dose and frequency), and route of administration (IV or SC).
Clinical remission data (CD and UC) and mucosal healing data (UC) were synthesized in the Bayesian network models to estimate the odds ratio (OR) of each active comparator achieving clinical remission compared with placebo; for each comparison, ORs were reported with associated 95% credible intervals (CrIs). The relative effects of the interventions were used to calculate rank probabilities for each regimen (where rank 1 represents the best treatment option). To extract quantitative summaries of rank probabilities, surface under the cumulative ranking curve (SUCRA) values were calculated using the sum of the cumulative rank probabilities [23, 24], where higher SUCRA scores correlate with better efficacy.
As it was not possible to synthesize endoscopic outcomes in CD by using an NMA due to absence of common intervention among studies, mucosal healing and endoscopic remission data (CD) were synthesized using proportional meta-analyses per each regimen so that they can be compared narratively. In addition, a hierarchical algorithm was applied to enable comparative analysis of the endoscopic outcome assessment: mucosal healing defined by the absence of all ulcers was first utilized or, if not reported, endoscopic remission defined by absolute SES-CD of ≤ 2 or SES-CD subscore of ≤ 2 were utilized for the analysis. Endoscopic data were synthesized to generate pooled proportions with 95% confidence intervals (CIs) by treatment.
Statistical analyses were performed using R version 4.2.1 with metafor [25], meta [26], and gemtc [27, 28] package version 2.6–0.
Results
Search Results
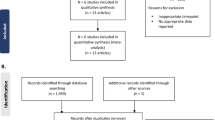
A PRISMA flow diagram summarizing the flow of information for studies enrolling patients with CD is presented in Fig. 1A. We identified a total of 5,809 records through the searches. After removal of duplicates, 5,132 records were screened and 5,035 records were excluded. Overall, 101 full-text publications were reviewed against the eligibility criteria and 94 publications were excluded. A total of seven studies (nine publications) were included in the qualitative and quantitative syntheses, as follows:
-
IFX (four studies): NCT00207662 (ACCENT I) [29], NCT00094458 (SONIC) [30], NCT02883452 (CT-P13 SC 1.6 study Part 1) [31], NCT02883452 (CT-P13 SC 1.6 study Part 2) [32, 33].
-
VDZ (three studies): NCT00783692 (GEMINI 2) [34, 35], NCT02038920 [36], NCT02611817 (VISIBLE 2) [37].
PRISMA flow diagram for A Crohn’s disease and B ulcerative colitis cohorts. ECCO, European Crohn’s and Colitis Organisation; EMA, European Medicines Agency; SmPC, Summary of Product Characteristics; VDZ, vedolizumab. aAdditional sources were the ECCO website (n = 1 article: 2020 ECCO guideline for Crohn’s disease medical treatment) and the EMA website (n = 4 articles: CT-P13 EPAR EMA/376884/2020; CT-P13 EPAR EMA/CHMP/548703/2019; CT-P13 SmPC [last updated Nov 25, 2022]; VDZ SmPC [last updated Oct 13, 2022]). bAdditional sources were the ECCO website (n = 1 article: 2022 ECCO guideline for ulcerative colitis medical treatment) and the EMA website (n = 3 articles: CT-P13 EPAR EMA/376884/2020; CT-P13 SmPC [last updated Nov 25, 2022]; VDZ SmPC [last updated Oct 13, 2022])
The flow of information for studies enrolling patients with UC is summarized in the PRISMA flow diagram presented in Fig. 1B. We identified a total of 4,194 records through the searches. After removal of duplicates, 3,514 records were screened and 3,423 records were excluded. Overall, 98 full-text publications were reviewed against the eligibility criteria and 91 publications were excluded. A total of seven studies (nine publications) were included in the qualitative and quantitative syntheses, as follows:
-
IFX (four studies): NCT00036439 (ACT 1) [38], NCT00096655 (ACT 2) [38], Jiang et al. [39], NCT02883452 (CT-P13 SC 1.6 study Part 2) [32, 33].
-
VDZ (three studies): NCT00783718 (GEMINI 1) [40, 41], NCT02039505 (CCT-101) [42], NCT02611830 (VISIBLE 1) [43].
Study Characteristics
Studies Contributing to the CD Analyses
The design and eligibility criteria of the seven studies contributing data to the CD analyses were generally consistent (Table 1). All were multicenter studies (six global, one Japanese) with a treatment duration of 50–60 weeks (corresponding to the timepoint for assessment of clinical remission and endoscopic outcomes). Studies with a treatment duration of 22–60 weeks were also allowed for the comparison of endoscopic outcomes. The four IFX studies used a treat-through design, whereby all patients who completed the induction phase were eligible for maintenance treatment (although ACCENT I was a treat-through study, clinical remission was only evaluated in Week 2 responders). In contrast, the three VDZ studies re-randomized patients who responded to induction (response defined as a ≥ 70-point decrease in CDAI score at Week 6/10) to subsequently receive maintenance treatment. Eligibility criteria were a minimum disease duration of 6–12 weeks and a CDAI score of 220–400/450; prior TNFi treatment was not permitted in any of the IFX studies but was permitted in the VDZ studies, with the proportion of patients with prior TNFi use ranging from approximately 50–80% across study arms. Only data for TNFi-naïve patients were included in the present analyses, except for assessment of endoscopic remission from VISIBLE 2, which included TNFi-experienced patients. Patients in the IFX studies received IFX IV 5 mg/kg every 8 weeks (Q8W), IFX IV 5 mg/kg Q8W + azathioprine (AZA) 2.5 mg/kg/day, IFX IV 10 mg/kg Q8W, IFX SC 120 mg every 2 weeks (Q2W), or IFX SC 120/240 mg Q2W (according to bodyweight) as maintenance intervention, and placebo, AZA 2.5 mg/kg/day, or IFX IV 5 mg/kg Q8W as comparator (in Part 2 of the CT-P13 SC 1.6 study, patients who initially received maintenance CT-P13 IV 5 mg/kg Q8W were switched to receive CT-P13 SC 120/240 mg Q2W from Week 30). Patients in the VDZ studies received VDZ IV 300 mg Q8W, VDZ IV 300 mg every 4 weeks (Q4W), or VDZ SC 108 mg Q2W as maintenance intervention and placebo as comparator.
A total of 973 participants were assigned to the relevant maintenance treatment arms of the included studies. Baseline characteristics were not routinely reported for the subset of TNFi-naïve patients in each of the included studies; therefore, it is not possible to summarize patient characteristics for the specific TNFi-naïve population contributing data to the present analyses. However, in the overall study populations of the included studies (i.e., across arms and including patients who had previously received treatment with a TNFi), mean/median age ranged from 32.6 to 38.6 years, 25.0 to 69.2% of participants were female, and mean/median disease duration was 2.2 to 9.6 years (Table 1).
Studies Contributing to the UC Analyses
The design and eligibility criteria of the seven studies contributing data to the UC analyses were generally consistent (Table 2). All the included studies were global, multicenter studies except for one single-center study conducted in China and one conducted in Japan [39, 42]. All studies had a duration of between 22 and 60 weeks (corresponding to the timepoint for assessment of clinical remission and mucosal healing). The four IFX studies used a treat-through design while in the VDZ studies, only patients who responded to induction at Week 6 (GEMINI 2; VISIBLE 1) or Week 10 (CCT-101) were subsequently re-randomized to receive maintenance treatment (response defined as a reduction in Mayo score of ≥ 3 points and a decrease of ≥ 30% from baseline score, plus a decrease of ≥ 1 point on the rectal bleeding scale or an absolute rectal bleeding score of ≤ 1). Eligibility criteria were moderate-to-severe UC as defined by a Mayo score of 6–12 points and a Mayo endoscopic subscore of ≥ 2; the GEMINI 1 and VISIBLE 1 studies also required ≥ 15 cm of involved colon. Prior TNFi treatment was not permitted in any of the IFX studies but was permitted in the VDZ studies, with the proportion of patients with prior TNFi use ranging from approximately 35–50% across study arms (only data for TNFi-naïve patients were included in the present analyses). Patients in the IFX studies received IFX IV 3.5 mg/kg Q8W, IFX IV 5 mg/kg Q8W, IFX IV 10 mg/kg Q8W, or IFX SC 120/240 mg Q2W (according to bodyweight) as maintenance intervention, and either placebo or IFX IV 5 mg Q8W as comparator (in Part 2 of the CT-P13 SC 1.6 study, patients who initially received maintenance CT-P13 IV 5 mg/kg Q8W were switched to receive CT-P13 SC 120/240 mg Q2W from Week 30). Patients in the VDZ studies received VDZ IV 300 mg Q8W, VDZ IV 300 mg Q4W, or VDZ SC 108 mg Q2W as maintenance intervention and placebo as comparator.
A total of 1330 participants were assigned to the relevant maintenance treatment arms of the included studies. As above, it was not possible to summarize patient characteristics for the specific TNFi-naïve population contributing data to the present analyses. However, in the overall study populations of the included studies (i.e., across arms and including patients who had previously received treatment with a TNFi), mean/median age ranged from 33.0 to 44.0 years, 33 to 49% of participants were female, and mean/median disease duration was 4.3 to 8.7 years (Table 2).
Risk of Bias in the Included Studies
The risk of bias assessment is summarized in Supplementary Fig. 1. Across the 49 assessments for the studies contributing data to the CD analyses (seven studies, seven domains), 33 were considered to be at low risk of bias, 12 to have an unclear risk of bias, and four to be at high risk of bias; Part 1 and Part 2 of the CT-P13 SC 1.6 study were considered to have a high risk of bias for blinding of participants and personnel, and blinding of outcome assessment due to the open-label design.
Across the 49 assessments for the studies contributing to the UC analyses (seven studies, seven domains), 28 were considered to be at low risk of bias, 18 to have an unclear risk of bias, and three were considered at high risk of bias; Part 2 of the CT-P13 SC 1.6 study was considered to have a high risk of bias for blinding of participants and personnel, and blinding of outcome assessment due to the open-label design, and the VISIBLE 1 study was considered to have a high risk of bias due to selective reporting.
Comparative Efficacy Between Treatments
Clinical Remission Rates in Patients with CD
The NMA for clinical remission rates in patients with moderate-to-severe CD included seven treatments and eight direct comparisons (Fig. 2A). VDZ IV 300 mg Q8W versus placebo, IFX IV 5 mg/kg Q8W versus placebo, and IFX IV 5 mg/kg Q8W versus IFX SC 120 mg Q2W were the direct comparisons most commonly evaluated in the included studies.
Evidence network diagrams for clinical remission in TNFi-naïve patients with moderate-to-severe A Crohn’s disease or B ulcerative colitis; and for C mucosal healing in TNFi-naïve patients with moderate-to-severe UC. Line thickness is weighted according to the number of studies evaluating each treatment regimen (in terms of dosage and administration route for each biologic). IFX, infliximab; IV, intravenous; PBO, placebo; QnW, every n weeks; SC, subcutaneous; TNFi, tumor necrosis factor inhibitor; VDZ, vedolizumab
ORs versus placebo for achieving clinical remission during the maintenance phase are presented in Fig. 3A. Clinical remission rates were significantly higher than placebo for all biologics, dosage regimens, and routes of administration examined, with the exception of VDZ SC 108 mg Q2W. IFX SC 120 mg Q2W had the highest OR versus placebo for clinical remission during the maintenance phase (5.90; 95% CrI, 1.90–18.2), while VDZ SC 108 mg Q2W had the lowest OR versus placebo (1.28; 95% CrI, 0.69–2.42).
Clinical remission rates during IFX or VDZ maintenance therapy in TNFi-naïve patients with moderate-to-severe A Crohn’s disease or B ulcerative colitis; and C mucosal healing rates in TNFi-naïve patients with moderate-to-severe UC. CI, confidence interval; Crl, credible interval; IFX, infliximab; IV, intravenous; PBO, placebo; QnW, every n weeks; SC, subcutaneous; TNFi, tumor necrosis factor inhibitor; VDZ, vedolizumab. aOnly responders by the end of the induction phase were re-randomized and assessed in the maintenance phase
Rank probabilities for achieving clinical remission in patients with CD are presented in Supplementary Fig. 2A. When the treatments were ranked according to SUCRA, IFX SC 120 mg Q2W ranked highest (SUCRA value, 0.91), followed by IFX IV 10 mg/kg Q8W (0.81), then VDZ IV 300 mg Q8W (0.61) (Fig. 4A). Placebo ranked last (i.e., rank 7; SUCRA value, 0.04) and VDZ SC 108 mg Q2W ranked sixth (SUCRA value, 0.16).
Summary of SUCRA values for clinical remission during maintenance therapy with IFX or VDZ in TNFi-naïve patients with moderate-to-severe A Crohn’s disease or B ulcerative colitis; and C for mucosal healing during maintenance therapy with IFX or VDZ in TNFi-naïve patients with moderate-to-severe ulcerative colitis. Higher scores correspond to higher ranking for achieving clinical remission. CD, Crohn’s disease; IFX, infliximab; IV, intravenous; NA, not available; PBO, placebo; QnW, every n weeks; SC, subcutaneous; SUCRA, surface under the cumulative ranking curve; TNFi, tumor necrosis factor inhibitor; UC, ulcerative colitis; VDZ, vedolizumab. aOnly responders by the end of the induction phase were re-randomized and assessed in the maintenance phase. bNo studies evaluating IFX IV 3.5 mg/kg Q8W in patients with CD were identified
Clinical Remission Rates in Patients with UC
Eight treatments and 11 comparisons were included in the NMA for clinical remission rates in patients with moderate-to-severe UC (Fig. 2B). VDZ IV 300 mg Q8W versus placebo and IFX IV 5 mg/kg Q8W versus placebo were the direct comparisons most commonly evaluated in the included studies.
ORs versus placebo for achieving clinical remission during the maintenance phase are presented in Fig. 3B. Clinical remission rates were significantly higher than placebo for all biologics, dosage regimens, and routes of administration evaluated. IFX SC 120 mg Q2W had the highest OR versus placebo for clinical remission during the maintenance phase (5.65; 95% CrI, 1.75–17.0).
Rank probabilities for achieving clinical remission in patients with UC are presented in Supplementary Fig. 2B. When the interventions were ranked according to the SUCRA, IFX SC 120 mg Q2W ranked highest (SUCRA value, 0.81) and VDZ SC 108 mg Q2W ranked second (SUCRA value, 0.68) (Fig. 4B). Placebo ranked last (i.e., rank 8; SUCRA value, 0.00) and IFX IV 5 mg/kg Q8W ranked seventh (SUCRA value, 0.36).
Endoscopic Outcomes in Patients with CD
Proportional meta-analyses were conducted for IFX SC 120 mg Q2W and IFX IV 5 mg/kg Q8W in patients with moderate-to-severe CD (Supplementary Fig. 3A and B). For treatments with only one study included (i.e., IFX IV 10 mg/kg Q8W and VDZ SC 108 mg Q2W), the single proportion was drawn in a forest plot (Supplementary Fig. 3C and D).
IFX SC 120 mg Q2W showed a higher proportion ratio of 0.51 (95% CI, 0.21–0.81) than IFX IV 5 mg/kg Q8W (0.38 [95% CI, 0.24–0.52]) for achieving endoscopic endpoints.
Mucosal Healing Rates in Patients with UC
Eight treatments and 11 direct comparisons were included in the NMA for mucosal healing rate in patients with moderate-to-severe UC (Fig. 2C). The overall spectrum of timepoints at which mucosal healing was assessed spanned from the earliest follow-up point of Week 22 in the 1.6 Part 2 study to Week 60 in the CCT-101 study.
ORs versus placebo for achieving mucosal healing during the maintenance phase are presented in Fig. 3C. IFX SC 120 mg Q2W had the highest rank (OR, 4.90; 95% CrI, 1.63–14.1), followed by VDZ IV 300 mg Q4W (OR, 4.31; 95% CrI, 2.29–8.38), VDZ SC 108 mg Q2W (OR, 4.23; 95% CrI, 1.99–9.12), VDZ IV 300 mg Q8W (OR, 3.87; 95% CrI, 2.39–6.18), IFX IV 10 mg/kg Q8W (OR, 3.53; 95% CrI, 2.42–5.19), and IFX IV 3.5 mg/kg Q8W (OR, 3.03; 95% CrI, 1.37–6.88); IFX IV 5 mg/kg Q8W ranked last (OR, 2.87; 95% CrI, 2.00–4.18).
Rank probabilities for achieving mucosal healing in patients with UC are presented in Supplementary Fig. 2C. When the interventions were ranked according to the SUCRA (Fig. 4C), IFX SC 120 mg Q2W ranked highest (SUCRA value, 0.73) and VDZ IV 300 mg Q4W ranked second (SUCRA value, 0.70).
Discussion
We conducted meta-analyses to compare multiple IFX and VDZ dosage regimens and administration routes in terms of clinical remission and endoscopic outcomes during maintenance phase in TNFi-naïve patients with moderate-to-severe CD or UC. In patients with CD, all treatments except VDZ SC 108 mg Q2W were found to be more effective than placebo in terms of achieving clinical remission. Across the seven treatments evaluated, IFX SC 120 mg Q2W ranked first, followed by IFX IV 10 mg/kg Q8W; placebo ranked last and VDZ SC 108 mg Q2W ranked second to last. In patients with UC, all treatments were found to be more effective than placebo in terms of achieving clinical remission or mucosal healing. IFX SC 120 mg Q2W ranked first and VDZ SC 108 mg Q2W ranked second; as expected, placebo ranked last.
Although IFX SC has been developed and used in the IBD field in Europe since 2020, only a few studies have evaluated its use in terms of comparative effectiveness in the context of the whole therapeutic armamentarium. While our study does not evaluate IFX SC against all possible therapeutics, comparative data for various dosage regimens and formulations of VDZ could provide insights into IFX SC's potential position. VDZ was selected as the most clinically relevant comparator for the UC indication given its positioning as a first-/early-line biologic for maintenance treatment [5], which was based on the observed superiority of VDZ over adalimumab for achievement of clinical remission and endoscopic improvement in the VARSITY study [44]. While ustekinumab could also be a logical choice of comparator, ustekinumab is not ubiquitously reimbursed as a first-line biologic for UC. Likewise, VDZ has been identified as the most frequently used first-line biologic behind IFX and adalimumab in CD [45], and it was our intention to compare across modes of action. Additionally, as is the case for UC, ustekinumab is not ubiquitously reimbursed as a first-line biologic for the treatment of moderate-to-severe CD. JAK inhibitors (e.g., upadacitinib) and S1P receptor modulators were not considered in the present NMA due to concerns related to the risk of major adverse cardiovascular events, thrombosis, malignancies, and death with JAK inhibitors [46], and relatively low uptake of S1P receptor modulators. Moreover, regarding JAK inhibitors, upadacitinib is approved by the FDA to be used after failure of TNFis, which does not align with our scope of comparing the first-line therapy. Finally, the focus of the present study on TNF-naïve patients was based on the preponderance of data for IFX in TNF-naïve patients and a corresponding lack of data in TNF-exposed populations.
While CD is a multifactorial disease, with many biological players interacting to determine disease course and treatment response, TNF is a principal cytokine driver of the underlying pathology [47, 48]. The central role for TNF in CD pathogenesis might explain the potentially better performance of IFX IV regimens in CD compared with in UC. In the meantime, across indications, the well-documented higher stable serum IFX levels achieved with SC dosing [33, 49] might be more effective for maintenance of treatment effect compared with the more variable serum levels associated with IV dosing. In contrast, the higher peak serum IFX levels achieved with IV dosing may be more important for induction of response.
Although several systematic reviews and NMAs have assessed the efficacy of IFX and VDZ, most do not evaluate individual dosage regimens or different formulations (i.e., IV versus SC), nor do they focus on the use of these biological agents for maintenance treatment. For example, Lasa and colleagues performed an NMA based on data from Phase III trials of biologics and small-molecule drugs for UC [16]. However, the primary outcome was induction of clinical remission and the interventions considered were not separated by dosage regimen [16]. Singh et al. conducted a systematic review and meta-analysis of biologic therapies for moderate-to-severe CD [18]. The results of the analysis showed that compared with placebo, IFX had a higher OR for maintenance of clinical remission than VDZ. Again, the analyses did not consider individual dosage regimens or formulations separately and the analyses were restricted to CD [18]. A more recent NMA included comparison of specific dosage regimens for all biological therapies and small molecules that have progressed to Phase III trials for patients with luminal CD [50]. For maintenance of clinical remission, the result of the NMA aligns with our result that IFX IV 10 mg/kg Q8W and IFX SC 120 mg Q2W were more favorable than VDZ when compared with placebo. However, the analyses were limited to CD and only assessed clinical outcomes and safety profiles of each drug [50]. Thus, the comparative efficacy of SC formulations of IFX and VDZ as maintenance treatment for moderate-to-severe CD and UC has not been comprehensively explored.
The present body of work builds upon a previous systematic review and meta-analysis, which demonstrated superior efficacy during induction with IFX versus VDZ, and comparable efficacy during the maintenance phase [18, 50]. However, IV and SC formulations were not considered separately [17] and thus our work represents an addition to the existing evidence base. Our findings that IFX SC 120 mg Q2W was ranked first for maintenance of clinical remission (in both CD and UC) is contrary to a recent consensus opinion piece that positioned VDZ over IFX for maintenance of efficacy in patients with UC [13]. This discrepancy may have arisen due to the IV and SC formulations for each agent having been grouped together, reinforcing the importance of considering formulations and dosage regimens separately, as herein.
The present findings that IFX SC 120 mg Q2W showed favorable efficacy over VDZ are all the more remarkable given that the included studies for VDZ selectively re-randomized responders at the end of the induction phase [34, 36, 37, 41,42,43]. Notably, a statistically significant difference in response to induction with VDZ compared with placebo was not observed in all of the VDZ studies. For example, while a statistically significant difference in response rate favoring VDZ over placebo was observed at Week 6 in the GEMINI 1 study [41], the Phase III RCT in Japanese patients failed to show a statistically significant between-group difference in clinical response rates following induction with VDZ or placebo [42]. IFX studies have generally used a treat-through design in which both responders and non-responders in induction phase were randomized to interventions. Indeed, only one IFX study, ACCENT I, evaluated clinical remission during the maintenance phase in Week 2 responders only [29], while in the pivotal study of IFX SC, both responders and non-responders were randomized for maintenance treatment (with Week 6 response used as a stratification factor) [30]. Given that initial responses to IFX or VDZ therapies may predict long-term responses (i.e., outcomes during the maintenance phase) [51, 52], a greater difference in comparative efficacy of IFX IV and SC versus VDZ might be expected if the comparison was conducted using the same study design. Nevertheless, despite the difference in patient population during maintenance phase between IFX and VDZ studies causing a potential source of bias, IFX SC 120 mg Q2W ranked higher than VDZ regimens for the achievement of clinical remission in both patient populations (CD and UC).
To our knowledge, this is the first study to compare endoscopic outcomes with IFX and VDZ for both IV and SC formulations of each agent using meta-analyses. For CD, retrospective studies or post hoc analyses were previously the main source of information on comparative endoscopic efficacy with IFX and VDZ. For example, the EVOLVE study suggested comparable efficacy between IFX and VDZ IV over a 24-month period [53], while a recent post hoc analysis using patient-level data from IFX and VDZ IV studies suggested better efficacy with IFX IV compared with VDZ IV for achieving one-year endoscopic healing [54]. In the present analyses, heterogeneity among studies in terms of outcome definitions, limited sample sizes, and absence of common interventions among studies hindered comparative evaluation of endoscopic outcomes using NMA. With the caveat that the following interpretation should be regarded with caution given the narrative comparison and heterogeneity among treatments, the proportional meta-analyses were conducted in an attempt to provide initial evidence of comparative efficacy of IFX and VDZ regimens in terms of endoscopic outcomes. The pooled proportions implied potentially better efficacy of IFX SC than IFX IV.
For UC, multiple NMAs have previously compared endoscopic outcomes (e.g., mucosal healing) with IFX and VDZ [51,52,53,58]. A series of NMAs by Vickers et al. [55], Trigo-Vincente et al. [56], and Lu et al. [57], and meta-analyses by Cholapranee et al. [58], offered consistent findings, collectively suggesting that VDZ IV might possess a higher mucosal healing rate compared with IFX IV. This also aligns with the findings of our NMA, which also found VDZ IV to have a numerically higher OR compared with IFX IV. Moreover, in addition to confirming previous findings, our study provides further insights by demonstrating the relative performance of the Sc forms of each drug, IFX SC and VDZ SC, consistently demonstrating a high position for IFX SC in terms of clinical remission and mucosal healing rates in patients with UC.
Regarding other endoscopic outcomes such as endoscopic remission or response, RCTs included in our analysis did not provide the necessary data to make analyses feasible. Although limited, previous studies have compared IFX IV and VDZ IV regarding such outcomes. A post hoc analysis using individual patient data suggested better efficacy with IFX IV in achieving both one-year endoscopic improvement and endoscopic remission compared with VDZ IV [59], while a retrospective cohort study by Pabla and colleagues showed that VDZ was associated with higher rates of endoscopic remission and response compared with anti-TNF agents [60]. With the growing importance of endoscopic efficacy, further studies are required to confirm these findings.
Strengths of our study include the comprehensive search strategies and assessment of the risk of bias, which used validated methodology [20,21,22]. In addition, the primary outcome of clinical remission can be considered an important outcome for patients and, as such, is the main endpoint from a regulatory perspective [61,62,63,64]. Therefore, the findings of the present NMA can be considered clinically relevant and of interest to both patients and clinicians and may inform first-line biologic treatment of moderate-to-severe disease. Other strengths include the well-defined study population (TNFi-naïve) and the comparison strategy which, as discussed above, includes multiple dosage regimens and formulations, with results for IV and SC formulations reported separately.
Although the rationale for restricting the analyses to include only IFX and VDZ has been discussed, this focus also represents a potential limitation. Given the rapidly expanding number of therapeutic options available for CD and UC, direct comparisons are warranted, while future indirect comparisons should include a broader range of therapies, with careful evaluation of different dosage regimens and formulations to address the lack of direct evidence. Other limitations include variability among the included studies regarding the timepoint for the efficacy evaluation, and variability with respect to re-randomization according to induction response, as potential sources of bias. In addition, the included studies were conducted over an approximately 20-year period (since 1999 for ACCENT I study on CD), during which time the diagnostic and treatment landscape has evolved substantially. This disparity in time period is further compounded by the fact that, for example, the terms used to assess endoscopic improvement in CD have changed over time, making it difficult to analyze them together. Hence, given the temporal heterogeneity, the interpretation of indirect comparison should be made with caution. Another point to consider is that we drew the current conclusion mainly based on the point estimates of relative effect size of each comparator—given the width of CrIs, especially for IFX SC due to relatively small size of its reference study, potential uncertainty should be considered in parallel and future studies are warranted of larger size with additional data to further confirm the current findings. In addition, no safety outcomes were considered for this analysis, which may limit the understanding of clinical utility. However, IFX and VDZ have established safety profiles and have previously been shown to be well tolerated in CD and UC populations.
Conclusions
Within the limitations of indirect comparisons, IFX SC showed a favorable efficacy profile for achieving clinical remission during maintenance treatment in TNFi-naïve patients with CD or UC when compared with IFX IV or VDZ IV/SC dosage regimens evaluated. IFX SC also showed favorable efficacy for achieving mucosal healing in TNFi-naïve patients with UC, while additional studies are required to further determine comparative endoscopic efficacy in patients with CD.
References
Szymanska S, Matuszczyk M, Osuch M et al. Inflammatory bowel disease - one entity with many molecular faces. Prz Gastroenterol. 2019;14:228–232.
GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17–30.
Cote-Daigneault J, Bouin M, Lahaie R, Colombel JF, Poitras P. Biologics in inflammatory bowel disease: what are the data? United European Gastroenterol J. 2015;3:419–428.
Al-Bawardy B, Shivashankar R, Proctor DD. Novel and emerging therapies for inflammatory bowel disease. Front Pharmacol. 2021;12:651415.
Raine T, Bonovas S, Burisch J et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis. 2022;16:2–17.
Torres J, Bonovas S, Doherty G et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2020;14:4–22.
Remicade summary of product characteristics, 2022. Available at: https://www.ema.europa.eu/en/documents/product-information/remicade-epar-product-information_en.pdf. Accessed November 21, 2023.
Remsima summary of product characteristics, 2023. Available at: https://www.ema.europa.eu/en/documents/product-information/remsima-epar-product-information_en.pdf. Accessed November 21, 2023.
Entyvio summary of product characteristics, 2023. Available at: https://www.ema.europa.eu/en/documents/product-information/entyvio-epar-product-information_en.pdf. Accessed November 21, 2023.
Zymfentra biologics license application approval letter, 2023. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2023/761358Orig1s000ltr.pdf. Accessed November 21, 2023.
Zymfentra prescribing information, 2023. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761358s000lbl.pdf. Accessed November 21, 2023.
Entyvio prescribing information, 2023. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/125476s057lbl.pdf. Accessed November 21, 2023.
Juillerat P, Grueber MM, Ruetsch R, Santi G, Vuillemoz M, Michetti P. Positioning biologics in the treatment of IBD: a practical guide - which mechanism of action for whom? Curr Res Pharmacol Drug Discov. 2022;3:100104.
Rouse B, Chaimani A, Li T. Network meta-analysis: an introduction for clinicians. Intern Emerg Med. 2017;12:103–111.
Jansen JP, Fleurence R, Devine B et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011;14:417–428.
Lasa JS, Olivera PA, Danese S, Peyrin-Biroulet L. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:161–170.
Peyrin-Biroulet L, Arkkila P, Armuzzi A et al. Comparative efficacy and safety of infliximab and vedolizumab therapy in patients with inflammatory bowel disease: a systematic review and meta-analysis. BMC Gastroenterol. 2022;22:291.
Singh S, Murad MH, Fumery M et al. Comparative efficacy and safety of biologic therapies for moderate-to-severe Crohn’s disease: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:1002–1014.
Choi D, Kim D-H. CRD42022383401: systematic review and network meta-analysis of the clinical remission of infliximab and vedolizumab in patients with Crohn’s disease and ulcerative colitis. PROSPERO. 2022.
Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Higgins JP, Altman DG, Gotzsche PC et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Chichester (UK): Cochrane, 2022.
Mbuagbaw L, Rochwerg B, Jaeschke R et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev. 2017;6:79.
Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–171.
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid. Based Ment. Health. 2019;22:153–160.
van Valkenhoef G, Lu G, de Brock B et al. Automating network meta-analysis. Res Synth Methods. 2012;3:285–299.
van Valkenhoef G, Dias S, Ades AE, Welton NJ. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods. 2016;7:80–93.
Hanauer SB, Feagan BG, Lichtenstein GR et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549.
Colombel JF, Sandborn WJ, Reinisch W et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395.
Reinisch W, Jang BI, Borzan V et al. DOP62 A novel formulation of CT-P13 (infliximab biosimilar) for subcutaneous administration: 1-year result from a Phase I open-label randomised controlled trial in patients with active Crohn’s disease. J Crohns Colitis. 2019;13:S066–S067.
Remsima: extension of indication variation assessment report, 2020. Available at: https://www.ema.europa.eu/en/documents/variation-report/remsima-h-c-2576-ii-0082-epar-assessment-report-variation_en.pdf. Accessed November 21, 2023.
Schreiber S, Ben-Horin S, Leszczyszyn J et al. Randomized controlled trial: subcutaneous vs intravenous infliximab CT-P13 maintenance in inflammatory bowel disease. Gastroenterology. 2021;160:2340–2353.
Sandborn WJ, Feagan BG, Rutgeerts P et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–721.
Sands BE, Sandborn WJ, Van Assche G et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease in patients naive to or who have failed tumor necrosis factor antagonist therapy. Inflamm Bowel Dis. 2017;23:97–106.
Watanabe K, Motoya S, Ogata H et al. Effects of vedolizumab in Japanese patients with Crohn’s disease: a prospective, multicenter, randomized, placebo-controlled Phase 3 trial with exploratory analyses. J Gastroenterol. 2020;55:291–306.
Vermeire S, D’Haens G, Baert F et al. Efficacy and safety of subcutaneous vedolizumab in patients with moderately to severely active Crohn’s disease: results from the VISIBLE 2 randomised trial. J Crohns Colitis. 2022;16:27–38.
Rutgeerts P, Sandborn WJ, Feagan BG et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476.
Jiang XL, Cui HF, Gao J, Fan H. Low-dose infliximab for induction and maintenance treatment in Chinese patients with moderate to severe active ulcerative colitis. J Clin Gastroenterol. 2015;49:582–588.
Feagan BG, Rubin DT, Danese S et al. Efficacy of vedolizumab induction and maintenance therapy in patients with ulcerative colitis, regardless of prior exposure to tumor necrosis factor antagonists. Clin Gastroenterol Hepatol. 2017;15:229–239.
Feagan BG, Rutgeerts P, Sands BE et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710.
Motoya S, Watanabe K, Ogata H et al. Vedolizumab in Japanese patients with ulcerative colitis: a Phase 3, randomized, double-blind, placebo-controlled study. PLoS ONE. 2019;14:e0212989.
Sandborn WJ, Baert F, Danese S et al. Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. Gastroenterology. 2020;158:562–572.
Sands BE, Peyrin-Biroulet L, Loftus EV Jr et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med. 2019;381:1215–1226.
Jensen KJ, Jensen CB, Wennerstrom C, Burisch J, Petersen J. Drug utilization of biologic therapy in Crohn’s disease and ulcerative colitis: a population-based Danish cohort study 2015–2020. Scand J Gastroenterol. 2023;28:726–736.
Núñez P, Quera R, Yarur AJ. Safety of Janus kinase inhibitors in inflammatory bowel diseases. Drugs. 2023;83:299–314.
Pagnini C, Cominelli F. Tumor necrosis factor’s pathway in Crohn’s disease: potential for intervention. Int J Mol Sci. 2021;22:10273.
Peyrin-Biroulet L, Sandborn WJ, Panaccione R et al. Tumour necrosis factor inhibitors in inflammatory bowel disease: the story continues. Therap Adv Gastroenterol. 2021;22:17562848211059954.
Schreiber S, D’Haens GR, Cummings F et al. P0472 Switching from intravenous to subcutaneous infliximab in patients with active inflammatory bowel disease: post-hoc analysis of pre/post switch outcomes from a multicentre, randomised controlled pivotal trial. Abstract and poster presented at UEG Week 2021. United European Gastroenterol J. 2021;9:553–554.
Barberio B, Gracie DJ, Black CJ, Ford AC. Efficacy of biological therapies and small molecules in induction and maintenance of remission in luminal Crohn’s disease: systematic review and network meta-analysis. Gut. 2023;72:264–274.
Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimising response to anti-TNF therapy in Crohn’s disease - algorithm for practical management. Aliment Pharmacol Ther. 2016;43:30–51.
Meserve J, Dulai P. Predicting response to vedolizumab in inflammatory bowel disease. Front Med (Lausanne). 2020;7:76.
Bressler B, Mantzaris G, Silverberg M et al. P621 Real-world effectiveness and safety of vedolizumab and anti-TNF in biologic-naive Crohn’s disease patients: results from the EVOLVE study. J Crohns Colitis. 2019;13:S427–S428.
Narula N, Wong ECL, Dulai PS, Marshall JK, Jairath V, Reinisch W. Comparative effectiveness of biologics for endoscopic healing of the ileum and colon in Crohn’s disease. Am J Gastroenterol. 2022;117:1106–1117.
Vickers AD, Ainsworth C, Mody R et al. Systematic review with network meta-analysis: comparative efficacy of biologics in the treatment of moderately to severely active ulcerative colitis. PLoS One. 2016;11:e0165435.
Trigo-Vicente C, Gimeno-Ballester V, Garcia-Lopez S, Lopez-Del Val A. Systematic review and network meta-analysis of treatment for moderate-to-severe ulcerative colitis. Int J Clin Pharm. 2018;40:1411–1419.
Lu X, Jarrett J, Sadler S, Tan M, Dennis J, Jairath V. Comparative efficacy of advanced treatments in biologic-naive or biologic-experienced patients with ulcerative colitis: a systematic review and network meta-analysis. Int J Clin Pharm. 2023;45:330–341.
Cholapranee A, Hazlewood GS, Kaplan GG, Peyrin-Biroulet L, Ananthakrishnan AN. Systematic review with meta-analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn’s disease and ulcerative colitis controlled trials. Aliment Pharmacol Ther. 2017;45:1291–1302.
Narula N, Wong ECL, Marshall JK, Colombel JF, Dulai PS, Reinisch W. Comparative efficacy for infliximab vs vedolizumab in biologic naive ulcerative colitis. Clin Gastroenterol Hepatol. 2022;20:1588–1597.
Pabla BS, Alex Wiles C, Slaughter JC et al. Safety and efficacy of vedolizumab versus tumor necrosis factor alpha antagonists in an elderly IBD population: a single institution retrospective experience. Dig Dis Sci. 2022;67:3129–3137.
Ulcerative colitis: developing drugs for treatment guidance for industry [draft], 2022. Available at: https://www.fda.gov/media/158016/download. Accessed November 21, 2023.
Crohn's disease: developing drugs for treatment guidance for industry [draft], 2022. Available at: https://www.fda.gov/media/158001/download. Accessed November 21, 2023.
Guideline on the development of new medicinal products for the treatment of ulcerative colitis (Rev 1), 2018. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-development-new-medicinal-products-treatment-ulcerative-colitis-revision-1_en.pdf. Accessed November 21, 2023.
Guideline on the development of new medicinal products for the treatment of Crohn's disease (Rev 2), 2018. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-development-new-medicinal-products-treatment-crohns-disease-revision-2_en.pdf. Accessed November 21, 2023.
Acknowledgments
Medical writing support, including development of a draft outline and subsequent drafts in consultation with the authors, collating author comments, copyediting, fact-checking, and referencing, was provided by Emma Evans, DPhil, CMPP, at Aspire Scientific Limited (Bollington, UK). Funding for medical writing support for this article was provided by Celltrion Healthcare (Incheon, Republic of Korea).
Funding
Open access funding provided by Medical University of Vienna. Analysis was funded by Celltrion Healthcare Co., Ltd (Incheon, Republic of Korea).
Author information
Authors and Affiliations
Contributions
Conceptualization: Laurent Peyrin-Biroulet and Walter Reinisch; methodology: Laurent Peyrin-Biroulet, HyunSoo Park, Dasom Choi, Dong-Hyeon Kim, and Walter Reinisch; data curation, validation, formal analysis and investigation, and visualization: HyunSoo Park, Dasom Choi, and Dong-Hyeon Kim; supervision: Laurent Peyrin-Biroulet and Walter Reinisch. All authors contributed to the drafting and critical revision of the manuscript and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Laurent Peyrin-Biroulet has received personal fees from AbbVie, Allergan, Alma, Amgen, Applied Molecular Transport, Arena, Biogen, BMS, Boehringer Ingelheim, Celgene, Celltrion, Enterome, Enthera, Ferring, Fresenius Kabi, Galapagos, Genentech, Gilead, Hikma, Index Pharmaceuticals, Inotrem, Janssen, Lilly, MSD, Mylan, Nestlé, Norgine, Oppilan Pharma, OSE Immunotherapeutics, Pfizer, Pharmacosmos, Roche, Samsung Bioepis, Sandoz, Sterna, Sublimity Therapeutics, Takeda, Theravance, Tillotts, and Vifor. Peter Bossuyt has received research grants from AbbVie, Amgen, Celltrion, Mylan, Pfizer, and Takeda; lecture fees from AbbVie, Celltrion, Janssen, Lilly, and Takeda; and consulting fees from AbbVie, Arena, BMS, Celltrion, Dr Falk, Galapagos, Janssen, Lilly, Pentax, PSI-CRO, Roche, Takeda, and Tetrameros. Dominik Bettenworth is on the advisory board or has acted as a consultant for AbbVie, Amgen, Arena, Atheneum, BNG Service GmbH, BMS, CED Service GmbH, Celltrion, DGVS, Diaplan, Else Kröner-Fresenius Foundation, Falk Foundation, Galapagos, GuidePoint, Impulze, Ferring, Janssen, Lilly, Medical Tribune, MedTriX, MSD, Mylan, Onkowissen, Pharmacosmos, Pfizer, Roche, Sandoz, Takeda, Tetrameros, Thieme, Tillotts, UCB Biopharma, Viatris, and Vifor Pharma. Edward Loftus has acted as a consultant for AbbVie, Alvotech, Amgen, Arena, Avalo Therapeutics, Boehringer Ingelheim, BMS, CALIBR, Celltrion, Eli Lilly, Fresenius Kabi, Genentech, Gilead, GlaxoSmithKline, Gossamer Bio, Iota Biosciences, Iterative Scopes, Janssen, KSL Diagnostics, Morphic, Ono Pharma, Pfizer, Protagonist, Scipher, Sun Pharma, Surrozen, Takeda, and UCB; received research support from AbbVie, AstraZeneca, BMS, Genentech, Gilead, Gossamer Bio, Janssen, Pfizer, Takeda, Theravance, and UCB; and is a shareowner of Exact Sciences. Suzanne Anjie declares no conflicts of interest. Geert D’Haens has received consultancy fees from AbbVie, Agomab, AM Pharma, AMT, Arena, AstraZeneca, BMS, Boehringer Ingelheim, Celltrion, Eli Lilly, Exeliom Biosciences, Exo Biologics, Galapagos, Gilead, GlaxoSmithKline, Gossamer Bio, Immunic, Index Pharmaceuticals, Johnson & Johnson, Kaleido, Origo, Pfizer, Polpharma, ProciseDx, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonist, and Roche; speaker fees from AbbVie, Arena, BMS, Galapagos, Gilead, Pfizer, and Takeda; and served on a Data Monitoring Board for AstraZeneca, Galapagos, and Seres. Masayuki Saruta has received honoraria from AbbVie, EA Pharma, Gilead, Janssen, Mitsubishi Tanabe, and Takeda; writing fees from EA Pharma; grants for commissioned/joint research from EPS Corporation; and scholarship grants from EA Pharma, Kissei Pharmaceutical, Mochida Pharmaceutical, and Zeria Pharmaceutical. Perttu Arkkila has been an advisory board member of Celltrion and Janssen, is a stockholder of Orion Pharma, and has attended educational events organized and funded by Takeda. HyunSoo Park is an employee of Celltrion Healthcare Co., Ltd. Dasom Choi is an employee of Celltrion Healthcare Co., Ltd. Dong-Hyeon Kim is an employee of Celltrion Healthcare Co., Ltd. Walter Reinisch has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AbbVie, Celltrion, Falk Pharma, Ferring, Janssen, MEDICE, Mitsubishi Tanabe, MSD, Pfizer, Pharmacosmos, PLS Education, Roche, Shire, Takeda, Therakos, and Vifor; served as a consultant for AbbVie, Algernon, Amgen, Arena, Astellas, AstraZeneca, Bioclinica, Boehringer Ingelheim, BMS, Calyx, Celgene, Celltrion, Eli Lilly, Ernst & Young, Falk Pharma, Ferring, Fresenius, Galapagos, Gatehouse Bio, Genentech, Gilead, Grünenthal, ICON, Index Pharma, Inova, Intrinsic Imaging, Janssen, Johnson & Johnson, Landos Biopharma, LivaNova, Mallinckrodt, MedAhead, MedImmune, Mitsubishi Tanabe, MSD, Nash Pharmaceuticals, Nestlé, Novartis, OMass, Otsuka, Parexel, Periconsulting, Pfizer, Pharmacosmos, Prometheus, Protagonist, Provention Bio, Quell Therapeutics, Robarts Clinical Trials, Roche, Sandoz, Schering-Plough, Second Genome, Seres Therapeutics, Setpoint Medical, Sigmoid, Sublimity, Takeda, Teva Pharma, Therakos, Theravance, Vifor, and Zealand; received support for attending meetings and/or travel from AbbVie, Janssen, and Takeda; and served on a Data Safety Monitoring Board or advisory board for OSE Pharma.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
PRISMA Statement
The authors have read the PRISMA 2020 Checklist, and the manuscript was prepared and revised according to the PRISMA 2020 Checklist.
Prior Presentation
Selected results were presented as a poster at the 18th Congress of the European Crohn’s and Colitis Organisation (March 1–4, 2023, Copenhagen, Denmark). Encores were presented at the 35th Belgian Week of Gastroenterology (March 8–10, 2023, Antwerp, Belgium), the Digestive Disease Days Spring 2023 Meeting (March 22–23, 2023, Veldhoven, The Netherlands), the 11th Annual Meeting of the Asian Organization for Crohn’s and Colitis (April 10–12, 2023, Busan, Korea), and the Digestive Disease Week (May 6–9, 2023, Chicago, IL, USA).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Peyrin-Biroulet, L., Bossuyt, P., Bettenworth, D. et al. Comparative Efficacy of Subcutaneous and Intravenous Infliximab and Vedolizumab for Maintenance Treatment of TNF-naive Adult Patients with Inflammatory Bowel Disease: A Systematic Literature Review and Network Meta-analysis. Dig Dis Sci (2024). https://doi.org/10.1007/s10620-023-08252-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10620-023-08252-1