Abstract
Behavioral addictions (BA) and substance use disorders (SUDs) share core features, including impaired control and craving, leading to significant personal and societal impacts. Previous research has identified the pre-supplementary motor area (pre-SMA) as a critical node in GD-related neurocircuitry, making it a potential target for interventions also in SUDs. Theta-burst stimulation (TBS) offers a non-invasive method to modulate pre-SMA activity. This study included 58 participants diagnosed with GD or SUDs. They underwent bilateral pre-SMA continuous TBS (cTBS) sessions targeting craving, impulsivity, and addiction severity. Standardized scales and questionnaires were employed to assess the outcomes. cTBS parameters included 20 daily sessions with 80% resting motor threshold (RMT). Both GD and SUD groups exhibited significant reductions in addiction severity and craving following cTBS. Impulsivity decreased significantly in SUD but not in GD. The study’s findings underscore the potential of pre-SMA TBS as an adjunctive treatment for GD and SUDs. The observed improvements in addiction severity and craving emphasize the shared neuronal mechanisms underlying these disorders. However, the nuanced differences, especially in impulsivity, indicate the need for further research to tailor interventions precisely.
Similar content being viewed by others
Behavioral addictions (BA), characterized by excessive and compulsive engagement in non-substance-related activities (Grant et al., 2010; Robbins & Clark, 2015), have garnered increasing attention in the field of psychiatry due to the increasing prevalence and changing epidemiology of BA (Abbott, 2020) and the consequent interference with the quality of life of the patients (Potenza et al., 2019). Gambling disorder (GD), a prototypical BA, shares striking parallels with substance use disorders (SUDs) (Griffiths, 2017). Key shared features include impaired control over the behavior, craving, tolerance, withdrawal, and continued engagement despite adverse consequences (Alavi et al., 2012; Leeman & Potenza, 2012; Rash et al., 2016), all of which can lead to profound personal, social, and financial disruptions (Rodriguez-Monguio et al., 2018; Browne et al., 2017). Neuroscientific research has advanced our understanding of the neuronal substrates underpinning both behavioral and substance addictions (Murch & Clark, 2016; Paulus, 2022). In a Research Domain Criteria (RDoC) perspective, a framework for research on mental disorders that includes multiple dimensions with a strong focus on neurocircuitries (Insel et al., 2010), GD patients exhibit deficits in the domain of “positive valence systems,” particularly in the “approach motivation” and “reward learning” constructs, as well as in the “cognitive systems,” primarily in the “cognitive control” construct (Pallanti et al., 2021).
Among the neuronal regions implicated, the pre-supplementary motor area (pre-SMA) has emerged as a critical node in the neurocircuitry of addiction (Lohse et al., 2023; Kaufman et al., 2003). The pre-SMA is considered a non-primary motor area (Woolsey et al., 1952; Matelli et al., 1985, 1991; Barbas & Pandya, 1987; Matsuzaka & Tanji;, 1996, Zilles et al., 1995; Zilles et al., 1996, Geyer et al., 2000), and is involved in higher-level aspects of motor behavior such as motor selection and inhibition, which is of critical relevance in current behavioral investigations and psychiatric interventions. This array of functions is reflected by pre-SMA’s distinct structural connectivity (Eickhoff et al., 2011; Picard & Strick, 1996; Rizzolatti & Luppino, 2001; Nachev et al., 2005, 2007, 2008; Shima et al., 1996). This cortical area is located in the mesial aspect of the caudal part of each frontal lobe and rostral to the SMA and the primary motor cortex as illustrated in Fig. 1. Its structural connectome is complex, including short- and medium-range fibers as well as long association fiber tracts such as the cingulum bundle (CB). A critically important structural connection of pre-SMA is with the subthalamic nucleus via the hyperdirect pathway. This structural connection makes the pre-SMA a cortical target (or “portal”) with direct access to the striatal circuitry via the subthalamic nucleus (STN). This is particularly relevant in the treatment of SUD and behavioral addictions such as gambling disorder, considering the association of the corticostriatal circuitry in compulsive behaviors (see, e.g., Robbins et al., 2019; Greenberg et al., 2010; Fineberg et al., 2010; Fettes et al., 2017; Alexander et al., 1986; Milad & Rauch, 2012; Graybiel & Raucht, 2022). Overall, given its important connections and bio-behavioral functions, the pre-SMA has become recently a very important neuroanatomical target for neuromodulation treatments in psychiatry.
Pre-supplementary motor area (pre-SMA) and its structural connectome. Pre-SMA is located in the mesial aspect of each hemisphere and occupies the anterior portion of Brodmann’s architectonic area 6 (BA6) (Rushmore et al., 2022 – HOA ComPaRe paper). In the Talairach coordinate system (Talairach & Tournoux, 1988), the pre-SMA lies approximately between an anterior coronal plane at the callosal genu and a posterior coronal plane at the anterior commissure (AC). Its inferior border is the cingulate sulcus and extends to the hemispheric margin superiorly. Pre-SMA’s morphology and topographical location are illustrated in A on a medial magnetic resonance imaging (MRI) view of a human subject’s hemisphere, whereas similar information is shown in B using an inflated cortical surface representation of T1-weighted MRI data. A schematic diagram of pre-SMA’s structural circuitry is illustrated in C; this circuitry includes short- and medium-range fibers (s.r.f; m.r.f.) and long association fiber tracts such as the cingulum bundle (CB). An important structural connection of pre-SMA is with the subthalamic nucleus via the hyperdirect pathway (h-d.p.)
Recent studies have shown that alterations in pre-SMA activity are associated with the severity of addiction-related behaviors across addiction types (Huang & Ma, 2023; Bell et al., 2014; Lohse et al., 2023), in light of the causal link emerged between the level of pre-SMA activity and the propensity for impulsive risk-taking behavior in the context of gambling behaviors (Lohse et al., 2023).
Theta-burst stimulation (TBS) is a brief form of repetitive transcranial magnetic stimulation (rTMS) that holds promise in targeting specific brain regions and altering neuronal activity patterns (Huang et al., 2005), by inducing long-term potentiation (LTP) and long-term depression (LTD)–like phenomena (Suppa et al., 2016). TBS can be intermittent (iTBS) or continuous (cTBS). iTBS involves administering brief TBS trains at intervals, maintaining a prevailing excitatory effect and eliciting LTP. In contrast, cTBS is administered continuously for a duration sufficient to enable the inhibitory effect to surpass the facilitatory effect, leading to LTD (Suppa et al., 2016). As such, TBS offers a potential avenue for modulating pre-SMA function and, by extension, influencing addictive behaviors. The application of TBS to addiction treatment represents a novel and innovative approach, with the potential to mitigate the devastating impact of both behavioral addictions like GD and SUDs, as it has been shown in major depressive disorder (MDD) and obsessive compulsive disorder (OCD) (Blumberger et al., 2018; Williams et al., 2021; Cole et al., 2022). In a randomized-controlled trial, Pallanti et al. (2023) have investigated the efficacy of continuous theta-burst stimulation (cTBS) in gambling disorder when targeting the pre-SMA, showing a significant reduction of craving and gambling severity only in the active stimulation group. These findings provide a model for exploring its utility across different addiction types. Furthermore, Pallanti et al. (2022) showed the beneficial effects of cTBS over the pre-SMA in SUDs in comorbidity with schizophrenia.
However, at our knowledge, no study directly investigated the effects of cTBS in SUDs and compare the efficacy of such a protocol in BA and SUDs, with the aim not only to understand the treatment potential of this intervention but also to investigate whether targeting the pre-SMA could yield to different outcomes in these two groups.
In this naturalistic study, we seek to explore the therapeutic effects of pre-SMA cTBS on individuals diagnosed with GD and SUDs, comparing the outcomes between these two distinct forms of addiction. By utilizing a comprehensive battery of outcome measures, we aim to shed light on the potential divergent and shared neuronal mechanisms underlying these addiction types. We expect to observe an improvement in symptoms’ severity in both groups, with specific affected domains. Considering the lack of FDA-approved treatment, through this research, we hope to contribute to the development of targeted interventions that can improve the lives of individuals grappling with the complex challenges of addiction.
Methods
Participants
The data of patients aged 18–55 with a diagnosis of SUD (polysubstance use, cocaine, cannabis) or GD, who underwent rTMS, were extracted from databases containing information on patients of the psychiatric clinic at the Istituto di Neuroscienze, Florence (Italy). In addition, a previous rTMS treatment, concomitant alcohol or drug use (all patients of both conditions were abstinent from 1 month before the start of the intervention), a diagnosis of MDD, bipolar disorder, psychotic or neurological disorder, illiteracy, or cognitive impairment were all exclusion criteria, with the aim to remove potential confounding variables. All diagnoses were based on criteria of the DSM-5. All patients underwent a safety screening for TMS, to exclude history of epilepsy, seizures or increased risk of seizures for any reason, history of any metal in the head (outside the mouth) or metallic implant, and known or suspected pregnancy or lactation. rTMS was added to ongoing pharmacological treatments, which were unchanged during 1 month prior to the start of TBS protocol and remained stable throughout the duration of the neurostimulation sessions. The data of 58 patients were included for the analysis (Mage = 37, SDage = 11.64, nmales = 35 (60%), nfemales = 23 (40%)). After the complete description of the study to participants, written informed consent was obtained from each one for the inclusion of their data in this study.
Procedure and TMS Protocols
cTBS was administered with the MagVenture MagPro R30 stimulator with add-on theta-burst option (MagVenture INC.) using a Cool D-B80 figure-of-eight coil. cTBS consists of bursts of 3 pulses separated by 20 ms (i.e., 50 Hz) delivered repeatedly at theta frequency on the pre-SMA bilaterally (Fig. 1).
Stimulus intensities were set at 80% of resting motor threshold (RMT). Two trains of 600 pulses (a total of 1200 pulses) were administered with an 8-s intertrain interval. The protocol consisted of 20 daily sessions. The bilateral pre-SMA was targeted using individual MRI and a neuronavigation system (SofTaxic Optic 2.0). The TMS coil was held by a mechanical arm. RMT was defined as the minimum pulse strength needed to elicit a response in a resting target muscle (abductor pollicis brevis) in 5/10 trials using single-pulse TMS administered to the contralateral primary motor cortex.
Assessment
Participants’ addiction severity was assessed using standardized scales: the Addiction Severity Index (ASI; McLellan et al., 1980), a structured interview (Cronbach’s α = 0.82), which assesses the severity of a person’s substance abuse (e.g., “How much money would you say you spent during the past 30 days on drugs?”), assigning a severity index on a scale from 0 to 9; and the Visual Analog Scale (VAS) for craving intensity, which is a single-item scale (Cronbach’s α = 0.91) in which the patient is asked to indicate the point on a line that represents your current state with respect to a substance “With zero (0) meaning ‘No Craving At All’ and 10 meaning ‘Strongest Craving Ever’” (Mottola, 1993). Additionally, UPPS-P Impulsive Behavior Scale, a 59-item scale (e.g. “When I am upset I often act without thinking”) with a maximum score of 236 (Cronbach’s α = 0.86), which evaluates impulsivity across multiple domains (Whiteside & Lynam, 2001) was administered, considering that impulsivity is one of the main features of the addicted clinical picture (Dawe & Loxton, 2004; Lee et al., 2019) and that both impulsive and compulsive dimensions involve motor/response disinhibition (Robbins et al., 2012), which is associated with pre-SMA (Obeso et al., 2017). Sheehan Disability Scale (SDS; Sheehan, 1983), a 3-item scale (e.g. “The symptoms have disrupted your ability to function at work”) assessing work, social, and family disability with a maximum score of 30 (Cronbach’s α = 0.83), was used to assess functional impairment and disability across multiple life domains, in order to identify whether addiction severity is related to a higher degree of impairment in everyday activities.
These assessments were conducted at baseline and post-treatment.
Statistical Analysis
The baseline demographic and clinical characteristics of the sample were tabulated with descriptive statistics. Parametric (t test) and non-parametric (Wilcoxon) tests were used according to variables’ distribution (tested with the Shapiro–Wilk test) to analyze changes in scores over time and the interaction effect “group × time.” For all statistical analyses, the alpha level of significance was set at 0.05. All the statistical analyses were performed with the statistical programming language R (version 4.0.5).
Results
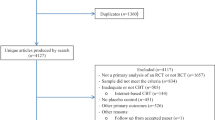
Of the 58 subjects included in the study, 25 had GD and 33 SUD. Our study demonstrated significant improvements in both BA and SUD groups following cTBS to the bilateral pre-SMA. Both groups (see Figs. 2 and 3) showed a significant reduction in ASI scores (GD: V = 170, p = 0.0023, r = 0.43; SUD: V = 345, p = 0.005, r = 0.23), indicating a decrease in overall addiction severity, in craving intensity, as measured by VAS, (GD: V = 226.5, p = 0.001, r = 0.45; SUD: V = 245, p = 0.023, r = 0.16) and in CPFQ (p < 0.01).
Impulsivity (see Fig. 4), measured with UPPR-S decreased significantly in SUD (t = 5.86, p < 0.001, d = 1) but not in GD (t = 1.25, p > 0.05). When a mixed-effect ANOVA was run to assess the interaction effect “group × time,” it was not significant for ASI and CPFQ scores, while a significant interaction effect was observed for craving (F = 4.47, p = 0.037) and UPPR-S (F = 4.77, p = 0.031).
Discussion
In this comparative study examining the impact of cTBS on the pre-SMA in gambling disorder versus SUDs, the treatment demonstrated effectiveness in both disorders, albeit with distinct outcomes. In both conditions, there was a reduction in addiction severity, indicating that pre-SMA cTBS might have a broad influence on addictive behaviors irrespective of the specific addiction type. Craving also diminished, with a more pronounced improvement observed in BA, suggesting that pre-SMA cTBS may modulate craving-related neural circuits relevant to both BA and SUD. However, alternative mechanisms may be implicated in SUD-related craving. Notably, impulsivity showed improvement only in SUD, not in GD. The efficacy of pre-SMA cTBS in GD corroborates prior findings (Pallanti et al., 2023). While this study presents novel insights into the effects of this protocol on SUD, earlier research indicated preliminary findings in schizophrenia with comorbid SUD (Pallanti et al., 2022).
Implications for a Shared Framework for Addiction Treatment
The observed reduction in addiction severity for both disorders underscores a promising common therapeutic dimension for cTBS in addressing addictive behaviors across the spectrum. This suggests that TBS may possess the potential to alleviate the adverse consequences of both BA and SUDs, aligning with a growing body of research indicating overlapping neuronal circuits in these conditions (Balodis & Potenza, 2020). Approaching this from an RDoC perspective aids in identifying the neurobiological factors underlying the two disorders. The deficits evident in both disorders are linked to the RDoC domains of positive valence systems (specifically approach motivation and reward learning) and cognitive control (particularly the construct of response inhibition), respectively (Pallanti et al., 2021), with the latter associated with the functioning of the pre-SMA. Our results, in this sense, suggested a shared framework for shared domains common to different forms of addiction, with one of this emerged through the targeting of response inhibition and the cortical portal represented by the pre-SMA.
Impulsivity in BA and SUD
The absence of improvement in impulsivity in GD aligns with the findings of Pallanti et al. (2023). However, noteworthy is the observed reduction in impulsivity reported in the SUD group in this study. It is crucial to highlight distinctions that have surfaced when comparing impulsivity in SUD and GD (Leeman & Potenza, 2012). Additionally, prior reports have indicated that individuals with BA tend to display lower levels of impulsivity compared to those with SUD, potentially linked to the neurotoxic effects of drug exposure (Albein-Urios et al., 2012).
Cognitive Rigidity and Addiction
These results warrant interpretation within a broader context that considers cognitive rigidity in both SUD and GD patients. Shared neural indicators, such as diminished ventrolateral prefrontal cortex (vlPFC) activation associated with cognitive inflexibility, have been observed in both gambling and cocaine addictions (Verdejo-Garcia et al., 2015). However, the effects linked to cocaine use extend to a wider range of task-related dysregulation patterns, manifesting as irregular signaling in dorsolateral and dorsomedial PFC regions. A study utilizing a reversal learning task as a measure of cognitive flexibility deficits identified cognitive rigidity as a key dimension of addiction disorders, encompassing aspects of both impulsivity and compulsivity (Izquierdo & Jentsch, 2012; Lee et al., 2019; Winstanley et al., 2010). While addiction models suggest a shift from initial impulsivity to dependence-related compulsivity, it is noted that impulsivity retains a role even in the later phases of the disorder (Winstanley et al., 2010; Lee et al., 2019). In this sense, new models of addiction should not focus on the distinctions of impulsivity and compulsivity, but on the cognitive rigidity dimension.
The Evolutionary Roots of Craving: Toward a New Model of Addictive Behaviors
The most significant clinical insight from this study lies in the notable improvement in craving, a pivotal aspect of addiction. A comprehensive understanding of addiction and craving necessitates delving into the evolutionary origins of craving (Liedtke et al., 2011) and acknowledging its dual polarities. One polarity characterizes craving as a response to the need to take risks in a negative contextual setting, associated with impulsive behaviors. The other polarity involves craving and compulsivity aimed at enhancing resources in a favorable context. Phenomenologically, these two behavioral modules can be differentiated. The human inclination to use drugs has capitalized on these evolutionarily relevant mechanisms of gratification (Liedtke et al., 2011).
Beyond the transition from ventral striatum activation to dorsal striatum activation during dependence (Everitt & Robbins, 2013), the role of context and ancient survival mechanisms becomes pivotal. Consequently, the activation of a gratification component can potentially be distinguished at three moments: activation preceding the behavior, activation following the behavior, and activation associated with context uncertainty. These forms may be linked to distinct reward circuits (Koob & Volkow, 2010). This discussion aims to enhance existing models of addictive behaviors and treatment strategies by proposing the consideration that recovery from addiction does not entail the absence of craving—a evolutionarily necessary phenomenon—but rather a reduction in craving below a threshold where it becomes controllable.
Brain and Potential Therapeutic Mechanisms
The reported improvements may be attributed to the utilization of two distinct brain mechanisms. Firstly, our results could stem from the direct modulation of pre-SMA activity, leading to an enhancement in inhibitory control. This improvement, in turn, contributes to a reduction in behaviors associated with gambling or substance intake, as well as a decrease in related thoughts. Secondly, these outcomes may be elucidated by the modulation of other brain regions interconnected with the pre-SMA. It is crucial to underscore the significance of the pre-SMA structural connectome’s anatomy, particularly its direct anatomical link with the subthalamic nucleus (STN) through the hyperdirect pathway projection. Additionally, its association connectivity via short-range, middle-range, and long fiber tracks plays a critical role (refer to, e.g., Makris et al., 2023). The hyperdirect pathway acts as a direct mechanism for modulating corticostriatal circuitry through transcranial magnetic stimulation (TMS) stimulation of the pre-SMA. Given the involvement of the striatum in pathological mechanisms of gambling (Pallanti et al., 2010; Hollander et al., 2005) and substance use (Kravitz et al., 2015), this pathway may be implicated in the observed results. To validate these hypotheses and provide insight into the neurofunctional changes underlying the observed therapeutic differences between the two groups, future neuroimaging studies should be conducted.
Limitations and Future Research
The TMS device here used did not allow to target specifically the left or right pre-SMA; indeed, bilateral pre-SMA was stimulated. It is not clear whether our results could have been affected by this. In the future, network-informed targeting (Lioumis & Rosanova, 2022) will be more helpful to decide whether lateral or bilateral targeting is optimal and multi-locus TMS (mTMS) to simultaneously modulate more than one cortical structure (Nieminen et al., 2022). Moreover, the 80% RMT here adopted is a low intensity. Future studies should dose intensity with TMS–EEG, since neuronal perturbations may depend on the regional and personal cytoarchitecture (Rosanova et al., 2009), with the aim also to reduce the number of sessions needed to have the desired result (Casarotto et al., 2022; Lioumis & Rosanova, 2022). Another limitation is associated with the retrospective design of the study. Retrospective studies depend on preexisting data, which may be susceptible to recall bias and constrained by the availability of historical records. Additionally, given that the study was not sham-controlled to mitigate bias, it remains possible that unblinding or placebo effects could have influenced the outcomes. Moreover, the potential impact of comorbidity with ADHD should be considered. Many individuals with substance use or gambling show attentional deficits (Luo & Levin, 2017), which relate to executive functioning alterations, impulsivity, and emotional dysregulation.
Conclusions
The promising results of this study highlight the potential of pre-SMA TBS as an adjunctive treatment for both GD and SUDs. By targeting shared neuronal mechanisms related to craving, cognitive control, and impulsivity, cTBS offers a novel approach to addressing behavioral and substance addictions.
References
Alavi, S. S., Ferdosi, M., Jannatifard, F., Eslami, M., Alaghemandan, H., & Setare, M. (2012). Behavioral addiction versus substance addiction: Correspondence of psychiatric and psychological views. International Journal of Preventive Medicine, 3(4), 290.
Albein-Urios, N., Martinez-González, J. M., Lozano, Ó, Clark, L., & Verdejo-García, A. (2012). Comparison of impulsivity and working memory in cocaine addiction and pathological gambling: Implications for cocaine-induced neurotoxicity. Drug and Alcohol Dependence, 126(1–2), 1–6.
Alexander, G. E., DeLong, M. R., & Strick, P. L. (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience, 9(1), 357–381.
Balodis, I. M., & Potenza, M. N. (2020). Common neurobiological and psychological underpinnings of gambling and substance-use disorders. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 99, 109847.
Barbas, H., & Pandya, D. N. (1987). Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. The Journal of Comparative Neurology, 256, 211–288.
Bell, R. P., Garavan, H., & Foxe, J. J. (2014). Neural correlates of craving and impulsivity in abstinent former cocaine users: Towards biomarkers of relapse risk. Neuropharmacology, 85, 461–470.
Blumberger, D. M., Vila-Rodriguez, F., Thorpe, K. E., Feffer, K., Noda, Y., Giacobbe, P., Knyahnytska, Y., Kennedy, S. H., Lam, R. W., Daskalakis, Z. J., & Downar, J. (2018). Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): A randomised non-inferiority trial. Lancet (London England), 391(10131), 1683–1692.
Browne, M., Rawat, V., Greer, N., Langham, E., Rockloff, M., & Hanley, C. (2017). What is the harm? Applying a public health methodology to measure the impact of gambling problems and harm on quality of life. Journal of Gambling Issues, 36, 28–50.
Casarotto, S., Fecchio, M., Rosanova, M., Varone, G., D’Ambrosio, S., Sarasso, S., Pigorini, A., Russo, S., Comanducci, A., Ilmoniemi, R. J., & Massimini, M. (2022). The rt-TEP tool: Real-time visualization of TMS-evoked potentials to maximize cortical activation and minimize artifacts. Journal of Neuroscience Methods, 370, 109486.
Cole, E. J., Phillips, A. L., Bentzley, B. S., Stimpson, K. H., Nejad, R., Barmak, F., Veerapal, C., Khan, N., Cherian, K., Felber, E., Brown, R., Choi, E., King, S., Pankow, H., Bishop, J. H., Azeez, A., Coetzee, J., Rapier, R., Odenwald, N., … Williams, N. R. (2022). Stanford neuromodulation therapy (SNT): A double-blind randomized controlled trial. The American Journal of Psychiatry, 179(2), 132–141.
Dawe, S., & Loxton, N. J. (2004). The role of impulsivity in the development of substance use and eating disorders. Neuroscience & Biobehavioral Reviews, 28(3), 343–351.
Eickhoff, S. B., Bzdok, D., Laird, A. R., Roski, C., Caspers, S., Zilles, K., & Fox, P. T. (2011). Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. NeuroImage, 57(3), 938–949.
Everitt, B. J., & Robbins, T. W. (2013). From the ventral to the dorsal striatum: Devolving views of their roles in drug addiction. Neuroscience & Biobehavioral Reviews, 37(9), 1946–1954.
Fettes, P., Schulze, L., & Downar, J. (2017). Cortico-striatal-thalamic loop circuits of the orbitofrontal cortex: Promising therapeutic targets in psychiatric illness. Frontiers in Systems Neuroscience, 11, 25.
Fineberg, N. A., Potenza, M. N., Chamberlain, S. R., Berlin, H. A., Menzies, L., Bechara, A., & Hollander, E. (2010). Probing compulsive and impulsive behaviors, from animal models to endophenotypes: A narrative review. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 35(3), 591–604.
Geyer, S., Matelli, M., Luppino, G., & Zilles, K. (2000). Functional neuroanatomy of the primate isocortical motor system. Anatomy and Embryology, 202, 443–474.
Grant, J. E., Potenza, M. N., Weinstein, A., & Gorelick, D. A. (2010). Introduction to behavioral addictions. The American Journal of drug and Alcohol Abuse, 36(5), 233–241.
Graybiel, A. M., & Raucht, S. L. (2022). Toward a neurobiology review of obsessive-compulsive disorder. Obsessive-compulsive disorder and Tourette's syndrome (pp. 119–123). Routledge.
Greenberg, B. D., Rauch, S. L., & Haber, S. N. (2010). Invasive circuitry-based neurotherapeutics: Stereotactic ablation and deep brain stimulation for OCD. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 35(1), 317–336.
Griffiths, M. D. (2017). Behavioural addiction and substance addiction should be defined by their similarities not their dissimilarities. Addiction, 112(10), 1718–1720.
Hollander, E., Sood, E., Pallanti, S., Baldini-Rossi, N., & Baker, B. (2005). Pharmacological treatments of pathological gambling. Journal of Gambling Studies, 21(1), 99–110.
Huang, D., & Ma, Y. Y. (2023). Increased excitability of layer 2 cortical pyramidal neurons in the supplementary motor cortex underlies high cocaine-seeking behaviors. Biological Psychiatry, 94(11), 875–887.
Huang, Y. Z., Edwards, M. J., Rounis, E., Bhatia, K. P., & Rothwell, J. C. (2005). Theta burst stimulation of the human motor cortex. Neuron, 45(2), 201–206.
Insel, T., Cuthbert, B., Garvey, M., Heinssen, R., Pine, D. S., Quinn, K., Sanislow, C., & Wang, P. (2010). Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. The American Journal of Psychiatry, 167(7), 748–751.
Izquierdo, A., & Jentsch, J. D. (2012). Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology (Berl), 219(2), 607–620. https://doi.org/10.1007/s00213-011-2579-7
Kaufman, J. N., Ross, T. J., Stein, E. A., & Garavan, H. (2003). Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. Journal of Neuroscience, 23(21), 7839–7843.
Koob, G. F., & Volkow, N. D. (2010). Neurocircuitry of addiction. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 35(1), 217–238.
Kravitz, A. V., Tomasi, D., LeBlanc, K. H., Baler, R., Volkow, N. D., Bonci, A., & Ferré, S. (2015). Cortico-striatal circuits: Novel therapeutic targets for substance use disorders. Brain Research, 1628, 186–198.
Lee, R. S., Hoppenbrouwers, S., & Franken, I. (2019). A systematic meta-review of impulsivity and compulsivity in addictive behaviors. Neuropsychology Review, 29, 14–26.
Leeman, R. F., & Potenza, M. N. (2012). Similarities and differences between pathological gambling and substance use disorders: A focus on impulsivity and compulsivity. Psychopharmacology (Berl), 219, 469–490.
Liedtke, W. B., McKinley, M. J., Walker, L. L., Zhang, H., Pfenning, A. R., Drago, J., & Denton, D. A. (2011). Relation of addiction genes to hypothalamic gene changes subserving genesis and gratification of a classic instinct, sodium appetite. Proceedings of the National Academy of Sciences, 108(30), 12509–12514.
Lioumis, P., & Rosanova, M. (2022). The role of neuronavigation in TMS-EEG studies: Current applications and future perspectives. Journal of Neuroscience Methods, 380, 109677. https://doi.org/10.1016/j.jneumeth.2022.109677
Lohse, A., Løkkegaard, A., Siebner, H. R., & Meder, D. (2023). Linking impulsivity to activity levels in pre-supplementary motor area during sequential gambling. Journal of Neuroscience, 43(8), 1414–1421.
Luo, S. X., & Levin, F. R. (2017). Towards precision addiction treatment: New findings in co-morbid substance use and attention-deficit hyperactivity disorders. Current Psychiatry Reports, 19, 1–6.
Makris, N., Rushmore, R., Kaiser, J., Albaugh, M., Kubicki, M., Rathi, Y., & Kennedy, D. N. (2023). A proposed human structural brain connectivity matrix in the Center for Morphometric Analysis Harvard-Oxford Atlas Framework: A historical perspective and future direction for enhancing the precision of human structural connectivity with a novel neuroanatomical typology. Developmental Neuroscience, 45(2), 1–1.
Matelli, M., Luppino, G., & Rizzolatti, G. (1991). Architecture of superior and mesial area 6 and the adjacent cingulate cortex in the macaque monkey. The Journal of Comparative Neurology, 311(4), 445–462.
Matelli, M., Luppino, G., & Rizzolatti, G. (1985). Patterns of cytochrome oxidase activity in the frontal agranular cortex of the macaque monkey. Behavioural Brain Research, 18, 125–136.
Matsuzaka, Y., & Tanji, J. (1996). Changing directions of forthcoming arm movements: Neuronal activity in the presupplementary and supplementary motor area of monkey cerebral cortex. Journal of Neurophysiology, 76(4), 2327–2342.
McLellan, A. T., Luborsky, L., Woody, G. E., & O’BRIEN, C. P. (1980). An improved diagnostic evaluation instrument for substance abuse patients: The Addiction Severity Index. The Journal of Nervous and Mental Disease, 168(1), 26–33.
Milad, M. R., & Rauch, S. L. (2012). Obsessive-compulsive disorder: Beyond segregated cortico-striatal pathways. Trends in Cognitive Sciences, 16(1), 43–51.
Mottola, C. A. (1993). measurement strategies: the visual analogue scale. Decubitus, 6(5), 56–58.
Murch, W. S., & Clark, L. (2016). Games in the brain: Neural substrates of gambling addiction. The Neuroscientist, 22(5), 534–545.
Nachev, P., Rees, G., Parton, A., Kennard, C., & Husain, M. (2005). Volition and conflict in human medial frontal cortex. Current Biology, 15(2), 122–128.
Nachev, P., Wydell, H., O’neill, K., Husain, M., & Kennard, C. (2007). The role of the pre-supplementary motor area in the control of action. NeuroImage, 36, T155–T163.
Nachev, P., Kennard, C., & Husain, M. (2008). Functional role of the supplementary and pre-supplementary motor areas. Nature Reviews Neuroscience, 9(11), 856–869.
Nieminen, J. O., Sinisalo, H., Souza, V. H., Malmi, M., Yuryev, M., Tervo, A. E., Stenroos, M., Milardovich, D., Korhonen, J. T., Koponen, L. M., & Ilmoniemi, R. J. (2022). Multi-locus transcranial magnetic stimulation system for electronically targeted brain stimulation. Brain Stimulation, 15(1), 116–124. https://doi.org/10.1016/j.brs.2021.11.014
Obeso, I., Wilkinson, L., Teo, J. T., Talelli, P., Rothwell, J. C., & Jahanshahi, M. (2017). Theta burst magnetic stimulation over the pre-supplementary motor area improves motor inhibition. Brain Stimulation, 10(5), 944–951.
Pallanti, S., Bernardi, S., Allen, A., Chaplin, W., Watner, D., DeCaria, C. M., & Hollander, E. (2010). Noradrenergic function in pathological gambling: blunted growth hormone response to clonidine. Journal of Psychopharmacology (Oxford, England), 24(6), 847–853.
Pallanti, S., Marras, A., & Makris, N. (2021). A research domain criteria approach to gambling disorder and behavioral addictions: Decision-making, response inhibition, and the role of cannabidiol. Frontiers in Psychiatry, 12, 634418.
Pallanti, S., Di Ponzio, M., Makris, N., & Kubicki, M. (2022). Theta-burst stimulation over the pre-supplementary motor area in schizophrenia and comorbid substance use disorder: Preliminary clinical data. Annals of Psychiatry and Treatment, 6(1), 028–032.
Pallanti, S., Camprodon, J. A., Di Ponzio, M., & Makris, N. (2023). Efficacy of continuous theta burst stimulation to the pre-supplementary motor area in gambling disorder: A randomized double-blind controlled trial. International Journal of Mental Health and Addiction.
Paulus, M. P. (2022). Neural substrates of substance use disorders. Current Opinion in Neurology, 35(4), 460–466.
Picard, N., & Strick, P. L. (1996). Motor areas of the medial wall: A review of their location and functional activation. Cerebral Cortex, 6(3), 342–353.
Potenza, M. N., Balodis, I. M., Derevensky, J., & grant, J. E., Petry, N. M., Verdejo_Garcia, A., & Yip, S. W. (2019). Gambling disorder. Nature reviews. Disease primers, 5(1), 51.
Rash, C. J., Weinstock, J., & Van Patten, R. (2016). A review of gambling disorder and substance use disorders. Substance Abuse and Rehabilitation, 7, 3–13.
Rizzolatti, G., & Luppino, G. (2001). The cortical motor system. Neuron, 31(6), 889–901.
Robbins, T. W., & Clark, L. (2015). Behavioral addictions. Current Opinion in Neurobiology, 30, 66–72.
Robbins, T. W., Gillan, C. M., Smith, D. G., de Wit, S., & Ersche, K. D. (2012). Neurocognitive endophenotypes of impulsivity and compulsivity: Towards dimensional psychiatry. Trends in Cognitive Sciences, 16(1), 81–91.
Robbins, T. W., Vaghi, M. M., & Banca, P. (2019). Obsessive-compulsive disorder: Puzzles and prospects. Neuron, 102(1), 27–47.
Rodriguez-Monguio, R., Brand, E., & Volberg, R. (2018). The economic burden of pathological gambling and co-occurring mental health and substance use disorders. Journal of Addiction Medicine, 12(1), 53–60.
Rosanova, M., Casali, A., Bellina, V., Resta, F., Mariotti, M., & Massimini, M. (2009). Natural frequencies of human corticothalamic circuits. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29(24), 7679–7685. https://doi.org/10.1523/JNEUROSCI.0445-09.2009
Rushmore, R. J., Bouix, S., Kubicki, M., Rathi, Y., Yeterian, E., & Makris, N. (2022). HOA2. 0-ComPaRe: A next generation Harvard-Oxford Atlas comparative parcellation reasoning method for human and macaque individual brain parcellation and atlases of the cerebral cortex. Frontiers in Neuroanatomy, 16, 1035420.
Sheehan, D. V. (1983). Sheehan disability scale. Handbook of Psychiatric Measures, 2(2), 100–102.
Shima, K., Mushiake, H., Saito, N., & Tanji, J. (1996). Role for cells in the presupplementary motor area in updating motor plans. Proceedings of the National Academy of Sciences, 93(16), 8694–8698.
Suppa, A., Huang, Y. Z., Funke, K., Ridding, M. C., Cheeran, B., Di Lazzaro, V., Ziemann, U., & Rothwell, J. C. (2016). Ten years of theta burst stimulation in humans: established knowledge. Unknowns and prospects. Brain Stimulation, 9(3), 323–335.
Talairach, J., & Tournoux, P. (1988). Co-planar stereotaxic atlas of the human brain. Thieme.
Verdejo-Garcia, A., Clark, L., Verdejo-Roman, J., Albein-Urios, N., Martinez-Gonzalez, J. M., Gutierrez, B., & Soriano-Mas, C. (2015). Neural substrates of cognitive flexibility in cocaine and gambling addictions. The British Journal of Psychiatry, 207(2), 158–164.
Whiteside, S. P., & Lynam, D. R. (2001). The five factor model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences, 30(4), 669–689. https://doi.org/10.1016/S0191-8869(00)00064-7
Williams, N. R., Sudheimer, K. D., Cole, E. J., Varias, A. D., Goldstein-Piekarski, A. N., Stetz, P., Lombardi, A., Filippou-Frye, M., van Roessel, P., Anderson, K., McCarthy, E. A., Wright, B., Sandhu, T., Menon, S., Jo, B., Koran, L., Williams, L. M., & Rodriguez, C. I. (2021). Accelerated neuromodulation therapy for obsessive-compulsive disorder. Brain Stimulation, 14(2), 435–437.
Winstanley, C. A., Olausson, P., Taylor, J. R., & Jentsch, J. D. (2010). Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcoholism: Clinical and Experimental Research, 34(8), 1306–1318.
Woolsey, C. N., Settlage, P. H., Meyer, D. R., Sencer, W., Pinto Hamuy, T., & Travis, A. M. (1952). Patterns of localization in precentral and supplementary motor areas and their relation to the concept of a premotor area. Research Publications - Association for Research in Nervous and Mental Disease, 30, 238–264.
Zilles, K., Schlaug, G., Matelli, M., Luppino, G., Schleicher, A., Qü, M., & Roland, P. E. (1995). Mapping of human and macaque sensorimotor areas by integrating architectonic, transmitter receptor, MRI and PET data. Journal of Anatomy, 187(Pt 3), 515.
Zilles, K., Schlaug, G., Geyer, S., Luppino, G., Matelli, M., Qu, M., Schleicher, A., & Schormann, T. (1996). Anatomy and transmitter receptors of the supplementary motor areas in the human and nonhuman primate brain. Advances in Neurology, 70, 29–43.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pallanti, S., Levola, J., Lioumis, P. et al. Treatment of Behavioral Addictions and Substance Use Disorders: a Focus on the Effects of Theta-Burst Stimulation Over the Pre-SMA. Int J Ment Health Addiction (2024). https://doi.org/10.1007/s11469-024-01261-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s11469-024-01261-9