Article contents
Natromelansonite, Na3Zr[Si7AlO19]⋅4–5H2O, a new member of the rhodesite mero-plesiotype series from Mont Saint-Hilaire, Quebec, Canada
Published online by Cambridge University Press: 15 January 2024
Abstract
Natromelansonite, Na3Zr[Si7AlO19]⋅4–5H2O, was found at the Poudrette (Demix) quarry, Mont Saint-Hilaire, Quebec, Canada in a highly altered pegmatite together with a clay mineral, steacyite, polylithionite and rhodochrosite. It occurs as an outer zone of tabular crystals to 0.1 × 0.3 × 1 mm in size flattened on (001). The inner zone is made of melansonite. The mineral is grey with white powder colour and vitreous lustre. The cleavage is parallel to {010}, perfect. The Mohs hardness is 3.5. The mineral has green fluorescence under short-wave ultraviolet light. The Dcalc is 2.31 g/cm3. The infrared spectrum is reported. The composition (wt.%, average of 8 analyses) is Na2O 10.08, K2O 1.72, CaO 0.24, BaO 0.32, MnO 0.10, Al2O3 6.86, Y2O3 0.35, Yb2O3 0.55, SiO2 56.23, ZrO2 14.78, H2O 9.52, total 100.75. The empirical formula calculated on the basis of O = 23 apfu and H = 8 apfu is Na(□Na0.38Ca0.02Mn0.01)2(Na0.70K0.28Ba0.02)Σ1.00(Zr0.91Y0.02Yb0.02)Σ0.95(Si7.08Al1.02)Σ8.10O19⋅4H2O. The mineral is monoclinic, P21/m, a = 6.5156(3) Å, b = 24.061(1) Å, c = 6.9759(6) Å, β = 90.453(5)° and V = 1093.61(9) Å3 and Z = 2. The strongest reflections of the powder X-ray diffraction pattern [d,Å(I)(hkl)] are: 12.02(100)(020), 6.97(89)(001), 6.51(39)(100), 3.416(37)(160), 3.062(42)($\bar{1}$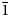 61, 102, 161 and $\bar{1}$
61, 102, 161 and $\bar{1}$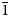 12), 3.018(38)(230 and 042), 2.864(40)(240 and 132). The crystal structure, solved and refined from single-crystal X-ray diffraction data (R1 = 0.042), is based on a double sheet of tetrahedra (T) and a sheet of octahedra (O) that alternate along the [010] direction forming a TOT structure typical for members of the rhodesite mero-plesiotype series.
12), 3.018(38)(230 and 042), 2.864(40)(240 and 132). The crystal structure, solved and refined from single-crystal X-ray diffraction data (R1 = 0.042), is based on a double sheet of tetrahedra (T) and a sheet of octahedra (O) that alternate along the [010] direction forming a TOT structure typical for members of the rhodesite mero-plesiotype series.
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2024. Published by Cambridge University Press on behalf of The Mineralogical Society of the United Kingdom and Ireland
Footnotes
Associate Editor: Sergey V Krivovichev
References
- 1
- Cited by




