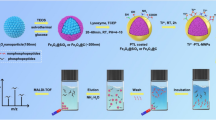
Abstract
A facile and mild method based on self-assembled lysozyme (LYZ) to fabricate bifunctional MNPs@UIO-66-Arg core-shell-satellite nanocomposites (CSSNCs) is reported for the high-efficiency enrichment of phosphopeptides. Under physiological conditions, LYZ rapidly self-assembled into a robust coating on Fe3O4@SiO2 magnetic nanoparticles (MNPs) with abundant surface functional groups, which effectively mediate heterogeneous nucleation and growth of UIO-66 nanocrystals. Well-defined MNPs@UIO-66 CSSNCs with stacked pores, showing high specific surface area (333.65 m2 g− 1) and low mass transfer resistance, were successfully fabricated by fine-tuning of the reaction conditions including reaction time and acetic acid content. Furthermore, the UIO-66 shells were further modified with arginine to obtain bifunctional MNPs@UIO-66-Arg CSSNCs. Thanks to the unique morphology and synergistic effect of Zr-O clusters and guanidine groups, the bifunctional MNPs@UIO-66-Arg CSSNCs exhibited outstanding enrichment performance for phosphopeptides, delivering a low limit of detection (0.1 fmol), high selectivity (β-casein/BSA, mass ratio 1:2000), and good capture capacity (120 mg g− 1). The mechanism for phosphopeptides capture may attribute to the hydrogen bonds, electrostatic interactions, and Zr–O–P bonds between phosphate groups in peptides and guanidyl/Zr-O clusters on bifunctional MNPs@UIO-66-Arg CSSNCs. In addition, the small stacking pores on the core-shell-satellite architecture may selectively capture phosphopeptides with low molecular weight, eliminating interference of other large molecular proteins in complex biological samples.
Graphical Abstract








Similar content being viewed by others
References
Hu J, Guo YT, Li YM (2006) Research progress in protein post-translational modification. Sci Bull 51(6):633–645
Xiong FF, Jia JX, Ma JT, Jia Q (2022) Glutathione-functionalized magnetic thioether-COFs for the simultaneous capture of urinary exosomes and enrichment of exosomal glycosylated and phosphorylated peptides. Nanoscale 14(3):853–864
Zhong HY, Xiao X, Zheng S, Zhang WY, Ding MJ, Jiang HY, Huang LL, Kang J (2013) Mass spectrometric analysis of mono- and multi-phosphopeptides by selective binding with NiZnFe2O4 magnetic nanoparticles. Nat Commun 4:1656
Luo B, Chen Q, He J, Li ZY, Lan F, Wu Y (2019) Boronic acid-functionalized magnetic metal-organic frameworks via a dual-ligand strategy for highly efficient enrichment of phosphopeptides and glycopeptides. ACS Sustain Chem Eng 7(6):6043–6052
Ma WF, Zhang Y, Li LL, Zhang YT, Yu M, Guo J, Lu HJ, Wang CC (2013) Ti4+-immobilized magnetic composite microspheres for highly selective enrichment of phosphopeptides. Adv Funct Mater 23(1):107–115
Li YL, Sun NR, Hu XF, Deng CH (2019) Recent advances in nanoporous materials as sample preparation techniques for peptidome research. TracTrend Anal Chem 120:115658
Low TY, Mohtar MA, Lee PY, Omar N, Zhou HJ, Ye ML (2021) Widening the bottleneck of phosphoproteomics: evolving strategies for phosphopeptide enrichment. Mass Spectrom Rev 40(4):309–333
Sun NR, Wu H, Shen XZ, Deng CH (2019) Nanomaterials in proteomics. Adv Funct Mater 29(26):1900253
Shi S, Zhang WT, Wu HF, Li YC, Ren XY, Li M, Liu J, Sun J, Yue TL, Wang JL (2020) In situ cascade derivation toward a hierarchical layered double hydroxide magnetic absorbent for high-performance protein separation. ACS Sustain Chem Eng 8(12):4966–4974
Wang Y, Wei YY, Gao PC, Sun S, Du Q, Wang ZF, Jiang Y (2021) Preparation of Fe3O4@PMAA@Ni microspheres towards the efficient and selective enrichment of histidine-rich proteins. ACS Appl Mater Inter 13(9):11166–11176
Luo B, Yan S, Zhang HN, Zhou J, Lan F, Ying BW, Wu Y (2021) Metal-organic framework-derived hollow and hierarchical porous multivariate metal-oxide microspheres for efficient phosphoproteomics analysis. ACS Appl Mater Inter 13(29):34762–34772
Pu CL, Zhao HL, Hong YY, Wang ZX, Zheng Y, Lan MB (2021) Hierarchical dendritic mesoporous TiO2 nanocomposites for highly selective enrichment of endogenous phosphopeptides. ACS Sustain Chem Eng 9(17):5818–5826
Gao CH, Bai J, He YT, Zheng Q, Ma WD, Lei ZX, Zhang MY, Wu J, Fu FF, Lin Z (2019) Postsynthetic functionalization of Zr4+-immobilized core-shell structured magnetic covalent organic frameworks for selective enrichment of phosphopeptides. ACS Appl Mater Inter 11(14):13735–13741
He YT, Zheng Q, Lin Z (2021) Ti4+-immobilized hierarchically porous zirconium-organic frameworks for highly efficient enrichment of phosphopeptides. Microchim Acta 188(5):150
Alpert AJ, Hudecz O, Mechtler K (2015) Anion-exchange chromatography of phosphopeptides: weak anion exchange versus strong anion exchange and anion-exchange chromatography versus electrostatic repulsion-hydrophilic interaction chromatography. Anal Chem 87(9):4704–4711
Yu LZ, He J, Yan S, Liu YC, Lan F, Wu Y (2022) Magnetic MXene/PAMAM composites with flexible dimensional regulation for highly effective enrichment of phosphopeptides. ACS SustainChem Eng 10(7):2494–2508
Possemato AP, Paulo JA, Mulhern D, Guo A, Gygi SP, Beausoleil SA (2017) Multiplexed phosphoproteomic profiling using titanium dioxide and immunoaffinity enrichments reveals complementary phosphorylation events. J Proteome Res 16(4):1506–1514
Rush J, Moritz A, Lee KA, Guo A, Goss VL, Spek EJ, Zhang H, Zha XM, Polakiewicz RD, Comb MJ (2005) Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat Biotechnol 23(1):94–101
Hong YY, Pu CL, Zhao HL, Sheng QY, Zhan QL, Lan MB (2017) Yolk-shell magnetic mesoporous TiO2 microspheres with flowerlike NiO nanosheets for highly selective enrichment of phosphopeptides. Nanoscale 9(43):16764–16772
Jiang DD, Li XQ, Jia Q (2018) Design of two-dimensional layered double hydroxide nanosheets embedded with Fe3O4 for highly selective enrichment and isotope labeling of phosphopeptides. ACS Sustain Chem Eng 7(1):421–429
Yu LZ, Luo B, Zhou XX, Liu YC, Lan F, Wu Y (2021) In situ controllable fabrication of two-dimensional magnetic Fe3O4/TiO2@Ti3C2Tx composites for highly efficient phosphopeptides enrichment. ACS Appl Mater Inter 13(46):54665–54676
Chen JW, Li K, Yang J, Gu JL (2021) Bimetallic ordered large-pore mesoMOFs for simultaneous enrichment and dephosphorylation of phosphopeptides. ACS Appl Mater Inter 13(50):60173–60181
Hu ZJ, Chen ZY, Chen XW, Wang JH (2022) Advances in the adsorption/enrichment of proteins/peptides by metal-organic frameworks-affinity adsorbents. TracTrend in Anal Chem 153:116627
Cao LC, Zhao YM, Chu ZY, Zhang XM, Zhang WB (2020) Core-shell magnetic bimetallic MOF material for synergistic enrichment of phosphopeptides. Talanta 206:120165
Huan WW, Xing MY, Cheng C, L J (2018) Facile fabrication of magnetic metal-organic framework nanofibers for specific capture of phosphorylated peptides. ACS Sustain Chem Eng 7(2):2245–2254
Pan YN, Zhang CH, Xiao RL, Zhang LY, Zhang WB (2021) Dual-functionalized magnetic bimetallic metal-organic framework composite for highly specific enrichments of phosphopeptides and glycopeptides. Anal Chim Acta 1158:338412
Cai GR, Yan P, Zhang LL, Zhou HC, Jiang HL (2021) Metal-organic framework-based hierarchically porous materials: synthesis and applications. Chem Rev 121(20):12278–12326
Zhong M, Kong LJ, Zhao K, Zhang YH, Li N, Bu XH (2021) Recent progress of nanoscale metal-organic frameworks in synthesis and battery applications. Adv Sci 8(4):2001980
Deng Q, Wu J, Chen Y, Zhang Z, Wang Y, Fang G, Wang S, Zhang Y (2014) Guanidinium functionalized superparamagnetic silica spheres for selective enrichment of phosphopeptides and intact phosphoproteins from complex mixtures. J Mater Chem B 2(8):1048–1058
Liu R, Zhao J, Han Q, Hu X, Wang D, Zhang X, Yang P (2018) One-step assembly of a biomimetic biopolymer coating for particle surface engineering. Adv Mater 30(38):1802851
Wang SZ, McGuirk CM, Ross MB, Wang SY, Chen PC, Xing H, Liu Y, Mirkin CA (2017) General and direct method for preparing oligonucleotide-functionalized metal-organic framework nanoparticles. J Am Chem Soc 139(29):9827–9830
Luo B, Yang MG, Jiang PP, Lan F, Wu Y (2018) Multi-affinity sites of magnetic guanidyl-functionalized metal-organic framework nanospheres for efficient enrichment of global phosphopeptides. Nanoscale 10(18):8391–8396
Wang SZ, McGuirk CM, d’Aquino A, Mason JA, Mirkin CA (2018) Metal-organic framework nanoparticles. Adv Mater 30(37):1800202
Zhou JQ, Liang YL, He XW, Chen LX, Zhang YK (2017) Dual-functionalized magnetic metal-organic framework for highly specific enrichment of phosphopeptides. ACS Sustain Chem Eng 5(12):11413–11421
Chen YJ, Xiong ZC, Peng L, Gan YY, Zhao YM, Shen J, Qian JH, Zhang LY, Zhang WB (2015) Facile preparation of core-shell magnetic metal-organic framework nanoparticles for the selective capture of phosphopeptides. ACS Appl Mater Inter 7(30):16338–16347
Yan S, Luo B, He J, Lan F, Wu Y (2021) Phytic acid functionalized magnetic bimetallic metal-organic frameworks for phosphopeptide enrichment. J Mater Chem B 9(7):1811–1820
Li JY, Zhang S, Gao W, Hua Y, Lian HZ (2020) Guanidyl-functionalized magnetic bimetallic MOF nanocomposites developed for selective enrichment of phosphopeptides. ACS Sustain Chem Eng 8(44):16422–16429
Zhu XY, Gu JL, Yang J, Wang Z, Li YS, Zhao LM, Zhao WR, Shi JL (2015) Zr-based metal-organic frameworks for specific and size-selective enrichment of phosphopeptides with simultaneous exclusion of proteins. J Mater Chem B 3(20):4242–4248
Xiong ZC, Chen YJ, Zhang LY, Ren J, Zhang QQ, Ye ML, Zhang WB, Zou HF (2014) Facile synthesis of guanidyl-functionalized magnetic polymer microspheres for tunable and specific capture of global phosphopeptides or only multiphosphopeptides. ACS Appl Mater Inter 6(24):22743–22750
Acknowledgements
This work was supported by the Natural Science Foundation Project of Shaanxi Province (No. 2020JZ-24) and the Fundamental Research Funds for the Central Universities (GK201801006).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Li, N., Li, J. et al. Bifunctional MNPs@UIO-66-Arg core-shell-satellite nanocomposites for enrichment of phosphopeptides. Microchim Acta 191, 211 (2024). https://doi.org/10.1007/s00604-024-06177-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-024-06177-8