Abstract
Purpose
This study aims to compare the fecal metabolome in post pull-through HD with and without HAEC patients and healthy young children using nuclear magnetic resonance (NMR) spectroscopy.
Methods
Fresh fecal samples were collected from children under 5 years of age in both post-pull-through HD patients and healthy Thai children. A total of 20 fecal samples were then analyzed using NMR spectroscopy.
Results
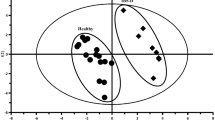
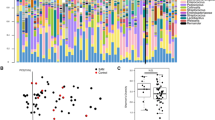
Thirty-four metabolites identified among HD and healthy children younger than 5 years were compared. HD samples demonstrated a significant decrease in acetoin, phenylacetylglutamine, and N-acetylornithine (corrected p value = 0.01, 0.04, and 0.004, respectively). Succinate and xylose significantly decreased in HD with HAEC group compared to HD without HAEC group (corrected p value = 0.04 and 0.02, respectively). Moreover, glutamine and glutamate metabolism, and alanine, aspartate, and glutamate metabolism were the significant pathways involved, with pathway impact 0.42 and 0.50, respectively (corrected p value = 0.02 and 0.04, respectively).
Conclusion
Differences in class, quantity, and metabolism of protein and other metabolites in young children with HD after pull-through operation were identified. Most of the associated metabolic pathways were correlated with the amino acids metabolism, which is required to maintain intestinal integrity and function.








Similar content being viewed by others
Data availability
Data can be accessed at Open Science Framework (https://osf.io/bvs96/).
References
Nicholson JK, Lindon JC (2008) Metabonomics. Nature 455:1054–1056
Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S (2012) Host-gut microbiota metabolic interactions. Science 336:1262–1267. https://doi.org/10.1126/science.1223813
Chang PV, Hao L, Offermanns S, Medzhitov R (2014) The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci 111:2247–2252. https://doi.org/10.1073/pnas.1322269111
Demehri FR, Frykman PK, Cheng Z, Ruan C, Wester T, Nordenskjöld A, Kawaguchi A et al (2016) Altered fecal short chain fatty acid composition in children with a history of Hirschsprung-associated enterocolitis. J Pediatr Surg 51:81–86. https://doi.org/10.1016/j.jpedsurg.2015.10.012
Phetcharaburanin J, Lees H, Marchesi JR, Nicholson JK, Holmes E, Seyfried F, Li JV (2016) Systemic characterization of an obese phenotype in the zucker rat model defining metabolic axes of energy metabolism and host-microbial interactions. J Proteome Res 15:1897–1906. https://doi.org/10.1021/acs.jproteome.6b00090
Sengupta A, Ghosh S, Basant A, Malusare S, Johri P, Pathak S, Sharma S, Sonawat HM (2011) Global host metabolic response to Plasmodium vivax infection: a 1H NMR based urinary metabonomic study. Malar J 10:1–13. https://doi.org/10.1186/1475-2875-10-384
Thompson GN, Walter JH, Bresson JL, Ford GC, Lyonnet SL, Chalmers RA, Saudubray JM et al (1990) Sources of propionate in inborn errors of propionate metabolism. Metabolism 39:1133–1137. https://doi.org/10.1016/0026-0495(90)90084-P
Wang Y, Christopher BA, Wilson KA, Muoio D, McGarrah RW, Brunengraber H, Zhang GF (2018) Propionate-induced changes in cardiac metabolism, notably CoA trapping, are not altered by l-carnitine. Am J Physiol Endocrinol Metab 315:E622–E633
Chen L, Zhou L, Chan ECY, Neo J, Beuerman RW (2011) Characterization of the human tear metabolome by LC–MS/MS. J Proteome Res 10:4876–4882. https://doi.org/10.1021/pr2004874
Larsson JMH, Karlsson H, Sjövall H, Hansson GC (2009) A complex, but uniform O-glycosylation of the human MUC2 mucin from colonic biopsies analyzed by nano LC/MSn. Glycobiology 19:756–766. https://doi.org/10.1093/glycob/cwp048
Ten-Doménech I, Ramos-Garcia V, Piñeiro-Ramos JD, Gormaz M, Parra-Llorca A, Vento M, Kuligowski J, Quintás G (2020) Current practice in untargeted human milk metabolomics. Metabolites 10:43. https://doi.org/10.3390/metabo10020043
Pini Prato A, Gentilino V, Giunta C, Avanzini S, Parodi S, Mattioli G, Martucciello G, Jasonni V (2008) Hirschsprung’s disease: 13 Years’ experience in 112 patients from a single institution. Pediatr Surg Int 24:175–182. https://doi.org/10.1007/s00383-007-2089-1
Pastor AC, Osman F, Teitelbaum DH, Caty MG, Langer JC (2009) Development of a standardized definition for Hirschsprung’s-associated enterocolitis: a Delphi analysis. J Pediatr Surg 44:251–256. https://doi.org/10.1016/j.jpedsurg.2008.10.052
EssamElhalaby BA, Coran AG, Blane CE, Hirschl RB, Teitelbaum Ann Arbor DH (1995) Enterocolitis associated with Hirschsprung’s disease: a clinical-radiological characterization based on 168 patients. J Pediatr Surg 30:76–83
Menezes M, Puri P (2006) Long-term outcome of patients with enterocolitis complicating Hirschsprung’s disease. Pediatr Surg Int 22:316–318. https://doi.org/10.1007/s00383-006-1639-2
Minford JL, Ram A, Turnock RR, Lamont GL, Kenny SE, Rintala RJ, Lloyd DA, Baillie CT (2004) Comparison of functional outcomes of Duhamel and transanal endorectal coloanal anastomosis for Hirschsprung’s disease. J Pediatr Surg 39:161–165. https://doi.org/10.1016/j.jpedsurg.2003.10.004
Suita S, Taguchi T, Ieiri S, Nakatsuji T (2005) Hirschsprung’s disease in Japan: analysis of 3852 patients based on a nationwide survey in 30 years. J Pediatr Surg 40:197–202. https://doi.org/10.1016/j.jpedsurg.2004.09.052
Marty TL, Seo T, Sullivan JJ, Matlak ME, Black RE, Johnson DG (1995) Rectal irrigations for the prevention of postoperative enterocolitis in Hirschsprung’s disease. J Pediatr Surg 30:652–654. https://doi.org/10.1016/0022-3468(95)90681-9
Neuvonen MI, Korpela K, Kyrklund K, Salonen A, de Vos W, Rintala RJ et al (2018) Intestinal microbiota in Hirschsprung disease. J Pediatr Gastroenterol Nutr 67:594–600. https://doi.org/10.1097/MPG.0000000000001999
Prato PA, Cavalieri D, Bartow-McKenney C, Hudspeth K, Mosconi M, Rossi V, Avanzini S et al (2019) A metagenomics study on Hirschsprung’s disease associated enterocolitis: biodiversity and gut microbial homeostasis depend on resection length and patient’s clinical history. Front Pediatr 1:326. https://doi.org/10.3389/fped.2019.00326
Aworanti O, Hung J, McDowell D, Martin I, Quinn F (2013) Are routine dilatations necessary post pull-through surgery for Hirschsprung disease? Eur J Pediatr Surg 23:383–388. https://doi.org/10.1055/s-0033-1333635
Roorda D, Abeln ZA, Oosterlaan J, Van Heurn LW, Derikx JM (2019) Botulinum toxin injections after surgery for Hirschsprung disease: systematic review and meta-analysis. World J Gastroenterol 25:3268–3280. https://doi.org/10.3748/wjg.v25.i25.3268
AladdeinMattar BF, Coran AG, Teitelbaum Ann Arbor DH (2003) MUC-2 mucin production in Hirschsprung’s disease: possible association with enterocolitis development. J Pediatr Surg. https://doi.org/10.1053/jpsu.2003.50071
Aslam A, Spicer RD, Corfield AP (1997) Children with Hirschsprung’s disease have an abnormal colonic mucus defensive barrier independent of the bowel innervation status. J Pediatr Surg 32:1206–1210
Cheng Z, Zhao L, Dhall D, Ruegger PM, Borneman J, Frykman PK, Zani A et al (2018) Bacterial microbiome dynamics in post pull-through Hirschsprung-associated enterocolitis (HAEC): an experimental study employing the endothelin receptor B-null mouse model. Front Surg. https://doi.org/10.3389/fsurg.2018.00030
Nakamura H, O’Donnell AM, Marayati NF, Tomuschat C, Coyle D, Puri P (2019) Altered expression of inflammasomes in Hirschsprung’s disease. Pediatr Surg Int 35:15–20. https://doi.org/10.1007/s00383-018-4371-9
Shen DH, Shi CR, Chen JJ, Yu SY, Wu Y, Yan WB (2009) Detection of intestinal bifidobacteria and lactobacilli in patients with Hirschsprung’s disease associated enterocolitis. World J Pediatr. https://doi.org/10.1007/s12519-009-0038-x
Plekhova V, De Paepe E, Van Renterghem K, Van Winckel M, Hemeryck LY, Vanhaecke L (2021) Disparities in the gut metabolome of post-operative Hirschsprung’s disease patients. Sci Rep 11:1–10. https://doi.org/10.1038/s41598-021-95589-0
Tang W, Su Y, Yuan C, Zhang Y, Zhou L, Peng L, Wang P et al (2020) Prospective study reveals a microbiome signature that predicts the occurrence of post-operative enterocolitis in Hirschsprung disease (HSCR) patients. Gut Microbes 00:1–13. https://doi.org/10.1080/19490976.2020.1711685
Gratton J, Phetcharaburanin J, Mullish BH, Williams HRT, Thursz M, Nicholson JK et al (2016) Optimized sample handling strategy for metabolic profiling of human feces. Anal Chem 88:4661–4668. https://doi.org/10.1021/acs.analchem.5b04159
Wishart DS, Feunang YD, Marcu A, Guo AC, Liang K, Vázquez-Fresno R, Sajed T et al (2018) HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res 46:D608–D617. https://doi.org/10.1093/nar/gkx1089
Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, Djoumbou Y et al (2012) HMDB 3.0—the human metabolome database in 2013. Nucleic Acids Res 41:D801–D807. https://doi.org/10.1093/nar/gks1065
Wishart DS, Knox C, Guo AC, Eisner R, Young N, Gautam B, Hau DD et al (2009) HMDB: a knowledgebase for the human metabolome. Nucleic Acids Res 37:D603–D610. https://doi.org/10.1093/nar/gkn810
Wishart DS, Tzur D, Knox C, Eisner R, Guo AC, Young N, Cheng D et al (2007) HMDB: the human metabolome database. Nucleic Acids Res 35:D521–D526. https://doi.org/10.1093/nar/gkl923
Maukonen J (2012) Characterization of the human predominant fecal microbiota: with special focus on the clostridial clusters IV and XIVa. Dissertation, the aalto university school of chemical technology Finland
Shimshoni E, Ghini V, Solomonov I, Luchinat C, IritSagi PT (2020) Integrated metabolomics and proteomics of symptomatic and early pre-symptomatic states of colitis. BioRxiv. https://doi.org/10.1101/2020.03.22.002196
Garner CE, Smith S, de Lacy CB, White P, Spencer R, Probert CSJ, Ratcliffe NM (2007) Volatile organic compounds from feces and their potential for diagnosis of gastrointestinal disease. FASEB J 21:1675–1688. https://doi.org/10.1096/fj.06-6927com
Walker V, Mills GA, Hall MA, Lowes JA (1989) Carbohydrate fermentation by gut microflora in preterm neonates. Arch Dis Child 64:1367–1373. https://doi.org/10.1136/adc.64.10_spec_no.1367
Austin KM (2012) The pathogenesis of Hirschsprung’s disease-associated enterocolitis. Semin Pediatr Surg 21:319–327. https://doi.org/10.1053/j.sempedsurg.2012.07.006
Moldave K, Meister A (1957) Synthesis of phenykacetylglutamine by human tissue. J Biol Chem 229:463–476
Nemet I, Saha PP, Gupta N, Zhu W, Romano KA, Skye SM, Cajka T et al (2020) A cardiovascular disease-linked gut microbial metabolite acts via adrenergic receptors. Cell 180:862-877.e22. https://doi.org/10.1016/j.cell.2020.02.016
Yu F, Feng X, Li X, Luo Y, Wei M, Zhao T, Xia J (2021) Gut-derived metabolite phenylacetylglutamine and white matter hyperintensities in patients with acute ischemic stroke. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2021.675158
Goedert JJ, Sampson JN, Moore SC, Xiao Q, Xiong X, Hayes RB, Ahn J et al (2014) Fecal metabolomics: assay performance and association with colorectal cancer. Carcinogenesis 35:2089–2096. https://doi.org/10.1093/carcin/bgu131
Ohkohchi N, Himukai M, Igarashi Y, Kasai M (1986) Mechanism of D-xylose transport in human small intestine. J Pediatr Gastroenterol Nutr 5:372–378. https://doi.org/10.1097/00005176-198605000-00006
Ehrenpreis ED, Salvino M, Craig RM (2001) Improving the serum D-xylose test for the identification of patients with small intestinal malabsorption. J Clin Gastroenterol 33:36–40. https://doi.org/10.1097/00004836-200107000-00009
Lerch MM, Braun J, Harder M, Hofstaädter F, Schumpelick V, Matern S, Biochem D (1989) Postoperative adaptation of the small intestine after total colectomy and J-pouch-anal anastomosis. Dis Colon Rectum 32:600–608. https://doi.org/10.1007/BF02554181
Macias-Ceja DC, Ortiz-Masiá D, Salvador P, Gisbert-Ferrándiz L, Hernández C, Hausmann M, Rogler G, Esplugues JV et al (2018) Succinate receptor mediates intestinal inflammation and fibrosis. Mucosal Immunol 121(12):178–187. https://doi.org/10.1038/s41385-018-0087-3
Ooi M, Nishiumi S, Yoshie T, Shiomi Y, Kohashi M, Fukunaga K, Nakamura S et al (2011) GC/MS-based profiling of amino acids and TCA cycle-related molecules in ulcerative colitis. Inflamm Res 60:831–840. https://doi.org/10.1007/S00011-011-0340-7/FIGURES/2
Banerjee A, Herring CA, Chen B, Kim H, Simmons AJ, Southard-Smith AN, Allaman MM et al (2020) Succinate produced by intestinal microbes promotes specification of tuft cells to suppress ileal inflammation. Gastroenterology 159:2101-2115.e5. https://doi.org/10.1053/j.gastro.2020.08.029
Tedelind S, Westberg F, Kjerrulf M, Vidal A (2007) Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol 28:2826–2832
Segain JP, Raingeard de la Blétière D, Bourreille A, Leray V, Gervois N, Rosales C et al (2000) Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut 47:397–403
Wu G (2010) Functional amino acids in growth, reproduction, and health. Adv Nutr 1:31–37. https://doi.org/10.3945/an.110.1008
Wu G, Wu Z, Dai Z, Yang Y, Wang W, Liu C, Wang B et al (2013) Dietary requirements of “nutritionally non-essential amino acids” by animals and humans. Amino Acids 44:1107–1113. https://doi.org/10.1007/s00726-012-1444-2
Yan Z, Poroyko V, Gu S, Zhang Z, Pan L, Wang J et al (2014) Characterization of the intestinal microbiome of Hirschsprung’s disease with and without enterocolitis. Biochem Biophys Res Commun 445:269–274. https://doi.org/10.1016/j.bbrc.2014.01.104
Chong PP, Chieng D-S, Lee Yean Low AH, Shamsudin MN, HFS and KPN, (2006) Recurrent candidaemia in a neonate with Hirschsprung’s disease: fluconazole resistance and genetic relatedness of eight Candida tropicalis isolates. J Med Microbiol 55:423–428. https://doi.org/10.1099/jmm.0.46045-0
Mellon AF, Deshpande SA, Mathers JC, Bartlett K (2000) Effect of oral antibiotics on intestinal production of propionic acid. Arch Dis Child 82:169–172. https://doi.org/10.1136/adc.82.2.169
Mullish BH, McDonald JAK, Thursz MR, Marchesi JR (2018) Antibiotic-associated disruption of microbiota composition and function in cirrhosis is restored by fecal transplant. Hepatology 68:1205. https://doi.org/10.1002/hep.30037
Lee E, Shin A, Jeong K-W, Jin B, Jnawali HN, Shin S et al (2014) Role of phenylalanine and valine10 residues in the antimicrobial activity and cytotoxicity of piscidin-1. PLoS ONE 9:e114453. https://doi.org/10.1371/journal.pone.0114453
Jiang M, Gong Q-Y, Lai S-S, Cheng Z-X, Chen Z-G, Zheng J et al (2019) Phenylalanine enhances innate immune response to clear ceftazidime-resistant Vibrio alginolyticus in Danio rerio. Fish Shellfish Immunol 84:912–919. https://doi.org/10.1016/j.fsi.2018.10.071
Allison C, Macfarlane GT (1989) Influence of pH, nutrient availability, and growth rate on amine production by Bacteroides fragilis and Clostridium perfringens. Appl Environ Microbiol 55:2894–2898. https://doi.org/10.1128/aem.55.11.2894-2898.1989
Acknowledgements
This work was supported by grants from Faculty of Medicine, Khon Kaen University (Grant No. IN63253) to Kanokrat Thaiwatcharamas and Thailand Science Research and Innovation (TSRI) to Watcharin Loilome.
Funding
Faculty of Medicine, Khon Kaen University, IN63253, Thailand Science Research and Innovation.
Author information
Authors and Affiliations
Contributions
K.T., W.L., J.P., P.T., and P.K. made substantial contributions to the conception and design of the work. K.T. and J.P. made substantial contributions to the acquisition of data, analysis, and interpretation of data. J.P. and P.H. made substantial contributions to the analysis of data and the creation of new software used in the work. K.T., J.P., S.C., and W.L. drafted the work or revised it critically for important intellectual content. K.T. wrote the main manuscript text. J.P. and P.H. prepared figures 1-6. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thaiwatcharamas, K., Loilome, W., Ho, P.N. et al. Children with Hirschsprung disease exhibited alterations in host–microbial co-metabolism after pull-through operation. Pediatr Surg Int 40, 87 (2024). https://doi.org/10.1007/s00383-024-05667-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s00383-024-05667-3