Abstract
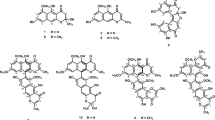
Photochemical reactions are powerful tools for synthesizing organic molecules. The input of energy provided by light offers a means to produce strained and unique molecules that cannot be assembled using thermal protocols, allowing for the production of immense molecular complexity in a single chemical step. Furthermore, unlike thermal reactions, photochemical reactions do not require active reagents such as acids, bases, metals, or enzymes. Photochemical reactions play a central role in green chemistry. This article reports the isolation and structure determination of four new compounds (1–4) from the photoreaction products of the Polyozellus multiplex MeOH ext. The structures of the new compounds were elucidated using MS, IR, comprehensive NMR measurements and microED. The four compounds were formed by deacetylation of polyozellin, the main secondary metabolite of P. multiplex, and addition of singlet oxygen generated by sunlight. To develop drugs for treating Alzheimer’s disease (AD) on the basis of the amyloid cascade hypothesis, the compounds (1–4) obtained by photoreaction were evaluated for BACE1 inhibitory activity. The hydrolysates (5 and 6) of polyozellin, the main secondary metabolites of P. multiplex, were also evaluated. The photoreaction products (3 and 4) and hydrolysates (5 and 6) of polyozellin showed BACE1 inhibitory activity (IC50: 2.2, 16.4, 23.3, and 5.3 μM, respectively).
Graphical abstract






Similar content being viewed by others
References
Newman DJ, Cragg GM (2020) Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod 83:770–803. https://doi.org/10.1021/acs.jnatprod.9b01285
Pye CR, Bertin MJ, Lokey RS, Gerwick WH, Linington RG (2017) Retrospective analysis of natural products provides insights for future discovery trends. Proc Natl Acad Sci USA 114:5601–5606. https://doi.org/10.1073/pnas.1614680114
Galloway WRJD, Isidoro-Liobet A, Spring DR (2010) Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat Com 80. https://doi.org/10.1038/ncomms1081
Kikuti H, Oshima Y (2018) Developments toward the production of diverse natural-product-like compounds: diversity-oriented synthesis and diversity-enhanced extracts. Heterocycles 96:1509–1527. https://doi.org/10.3987/REV-18-885
Kikuti H, Kawai K, Nakashiro Y, Yonezawa T, Kawaji K, Kodama E, Oshima Y (2019) Construction of a meroterpenoid-like compounds library based on diversity-enhanced extracts. Chem Eur J 25:1106–1112. https://doi.org/10.1002/chem.201805417
Suzuki Y, Ichinohe K, Sugawara A, Kida S, Murase S, Zhang J, Yamada O, Hattori T, Oshima Y, Kikuti H (2021) Development of indole alkaloid-type dual immune checkpoint inhibitors against CTLA-4 and PD-L1 based on diversity-enhanced extracts. Front Chem 9:766107. https://doi.org/10.3389/fchem.2021.766107
Ciana CL, Bochet CG (2007) Clean and easy photochemistry. Chimia 61:650–654. https://doi.org/10.2533/chimia.2007.650
Kärkäs MD, Porco JA, Stephenson CRJ (2016) Photochemical approaches to complex chemotypes: applications in natural product synthesis. Chem Rev 116:9683–9747. https://doi.org/10.1021/acs.chemrev.5b00760
Hoffmann N (2012) Photochemical reactions of aromatic compounds and the concept of the photon as a traceless reagent. Photochem Photobiol Sci 11:1613–1641. https://doi.org/10.1039/c2pp25074h
Ito S, White FJ, Okunishi E, Aoyama Y, Yamano A, Sato H, Ferrara JD, Jasnowski M, Meyer M (2021) Structure determination of small molecule compounds by an electron diffractometer for 3DED/MicroED. Cryst Eng Comm 23:8622–8630. https://doi.org/10.1039/D1CE01172C
Gemmi M, Mugnaioli E, Gorelik TE, Kolb U, Palatinus L, Boullay P, Hovmoller S, Abrahams JP (2019) 3D Electron diffraction: the nanocrystallography revolution. ACS Cent Sci 5:1315–1329. https://doi.org/10.1021/acscentsci.9b00394
Takahashi S, Kawano T, Nakajima N, Suda Y, Usukhbayar N, Kimura K, Koshino H (2018) Synthesis of polyozellin, a prolyl oligopeptidase inhibitor, and its structural revision. Bioorg Med Chem Lett 28:930–933. https://doi.org/10.1016/j.bmcl.2018.01.054
Nakabayashi S, Ishikura A, Fujihara K, Hirabayashi S, Koike S, Sasaki H, Ogasawara Y, Koyama K, Kinoshita K (2022) Inhibition of amyloid-β aggregation by p-terphenyls from the mushroom Polyozellus multiplex and their neuroprotective effects. Heterocycles 104:2025–2036. https://doi.org/10.3987/COM-22-14711
Chon SH, Yang EJ, Lee T, Song KS (2016) β-Secretase (BACE1) inhibitory and neuroprotective effects of p-terphenyls from Polyozellus multiplex. Food Funct 9:3834–3842. https://doi.org/10.1039/C6FO00538A
Clinger JA, Zhang Y, Liu Y, Miller MD, Hall RE, Lanen SGV, Phillips GN Jr, Thorson JS, Elshahawi SI (2021) Structure and function of a dual reductase–dehydratase enzyme system involved in p-terphenyl biosynthesis. ACS Chem Biol 16:2816–2824. https://doi.org/10.1021/acschembio.1c00701
Hainder W, Xu W, Liu M, Wu Y, Thang Y, Wei M, Wang C, Lu L (2020) Structure-activity relationships and potent cytotoxic activities of terphenyllin derivatives from a small compound library. Chem Biodivers 17:e2000207. https://doi.org/10.1002/cbdv.202000207
Zhou G, Zhu T, Che Q, Zhang G, Li D (2021) Structural diversity and biological activity of natural p-terphenyls. Mar Life Sci Technol 4:62–73. https://doi.org/10.1007/s42995-021-00117-8
Li W, Li X, Lou H (2018) Structural and biological diversity of natural p-terphenyls. J Asian Nat Prod Res 20:1–13. https://doi.org/10.1080/10286020.2017.1381089
Zhang X, Mou X, Mao N, Hao J, Liu M, Zheng J, Wang C, Gu Y, Shao C (2018) Design, semisynthesis, α-glucosidase inhibitory, cytotoxic, and antibacterial activities of p-terphenyl derivatives. Eur J Med Chem 146:232–244. https://doi.org/10.1016/j.ejmech.2018.01.057
Liu J (2006) Natural terphenyls: developments since 1877. Chem Rev 106:2209–2223. https://doi.org/10.1021/cr050248c
Gunasekera SP, Gunasekera M, Gunawardana GP, McCarthy P, Burres N (1990) Two new bioactive cyclic peroxides from the marine sponge Plakortis angulospiculatus. J Nat Prod 53:669–674. https://doi.org/10.1021/np50069a021
Jiménez-Romero C, Ortiz I, Vincente J, Vera B, Rodríguez AD, Nam S, Jove R (2010) Bioactive cycloperoxides isolated from the Puerto Rican sponge Plakortis halichondrioides. J Nat Prod 73:1694–1700. https://doi.org/10.1021/np100461t
Ghogare AA, Greer A (2016) Using singlet oxygen to synthesize natural products and drugs. Chem Rev 116:9994–10034. https://doi.org/10.1021/acs.chemrev.5b00726
Sheldrick GM (2015) SHELXT—integrated space-group and crystal-structure determination. Acta Crystallogr A 71:3–8. https://doi.org/10.1107/s2053273314026370
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr C 71:3–8. https://doi.org/10.1107/S2053229614024218
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) OLEX2: a complete structure solution, refinement, and analysis program. J Appl Crystallogr 42:339–341. https://doi.org/10.1107/S0021889808042726
Fujihara K, Shimoyama T, Kawazu R et al (2020) Amyloid β aggregation inhibitory activity of triterpene saponins from the cactus Stenocereus pruinosus. J Nat Med 75:284–298. https://doi.org/10.1007/s11418-020-01463-0
Acknowledgements
The authors thank Dr. Hiroyasu Sato and Mr. Keigo Takahira (Rigaku Corporation, Tokyo, Japan) for their advice and support in this MicroED measurement.
Funding
This work was supported in part by a grant from the Dementia Drug Resource Development Center Project (DRC), the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan (S1511016).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Otsuka, H., Nakai, K., Shimizu, E. et al. Photoreaction products of extract from the fruiting bodies of Polyozellus multiplex. J Nat Med (2024). https://doi.org/10.1007/s11418-024-01790-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11418-024-01790-6