Abstract
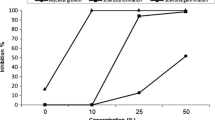
Secondary metabolites of plants are used to protect plants against fungal and bacterial diseases. This research evaluated the effect of 10 plant extracts prepared with either ethanol solvent or methanol solvent on the control of Sclerotium rolfsii, the causal agent of peanut white stem rot disease, under in vitro and greenhouse conditions. The plant extracts were extracted from Trachyspermum copticum, Ocimum basilicum, Eugenia caryophillata, Eucalyptus camaldulensis, Thymus pubescens, Mentha aquatical, Urtica dioica, Rosmarinus officinalis, Artemisia dracunculus, and Viola odorata. The in vitro trials were conducted on both the ethanolic and methanolic extracts at different rates of 0, 0.5, 0.75, 0.125, and 1.0 in a factorial experiment based on a completely randomized design in three replications. The greenhouse trials examined the effects of 10 net plant extracts on the morphological traits and peanut crown infection percentage. According to the in vitro trials, the highest inhibitory activity was observed in the ethanolic and methanolic extracts of E. caryophillata so that it fully inhibited the mycelial growth of the pathogen irrespective of its rate. In the greenhouse trials, E. camaldulensis among the ethanolic extracts and R. officinalis among the methanolic extracts were most effective in reducing disease severity. All studied plant extracts improved the vegetative traits of the peanuts. Based on the results, the extracts of E. caryophillata, E. camaldulensis, and R. officinalis can be used to biologically control peanut white stem rot disease. How plant extracts act has practical relevance for suppressing pathogenic fungi because they can provide useful information for preparing appropriate specific formulations.






Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Abdi Garmestani, S. M. (2017). Antifungal effects of some plant extracts on the growth of Sclerotinia sclerotiorum. Proceedings of the 23th Plant Protection Congress, 5–8 September, Gorgan, Iran.
Abdolmaleki, M., Bahraminejad, S., & Abbasi, S. (2011). Antifungal activity of some plant crude extracts on four phytopathogenic fungi. Journal of Medicinal Plants, 10(8), 148–155.
Agrios, G. (2005). Plant pathology, (5th edn, p. 922). Academic Press.
Ayoughi, F., Barzegar, M., Sahari, M. A., & Naghdibadi, H. (2011). Chemical compositions of essential oils of Artemisia dracunculus L. and endemic Matricaria chamomilla L. and an evaluation of their antioxidative effects. Journal of Agricultural Science and Technology, 13(1), 79–87.
Bertrand, P. F., & Gottwald, T. R. (1997). Evaluation of fungicides for pecan disease control. In K. D. Hickey (Ed.), Methods for evaluating pesticides for control of plant pathogens (pp. 179–181). Oxford and IHB Publisher.
Campo, J. D., Amort, M. J., & Nguyen, C. (2000). Antimicrobial effect of rosemary extract. Journal of Food Protection, 63, 1359–1368. https://doi.org/10.4315/0362-028X-3.10.1359
Cardoso, J. E., Santos, A. A., Rossetti, A. G., & Vidal, J. C. (2004). Relationship between incidence and severity of cashew gummosis in semiarid north-eastern Brazil. Plant Pathology, 53(3), 363–367. https://doi.org/10.1111/j.0032-0862.2004.01007.x
Choi, G. J. A., Jang, K. S., Kim, J. S., Lee, S. W., Cho, J. Y., Cho, K. Y., & Kim, J. C. (2004). In vivo antifungal activities of 57 plant extracts against six plant pathogenic fungi. Plant Pathology, 20, 184–191. https://doi.org/10.5423/PPJ.2004.20.3.184
Cooper, W. E. (1961). Strains of Sclerotium rolfsii resistance to antagonists. Phytopathology, 51, 113–116.
Da Nóbrega, L. P., da Silva França, K. R., Lima, T. S., de Figueredo Alves, F. M., Ugulino, A. L. N., da Silva, A. M., & de Mendonça Júnior, A. F. (2019). In vitro fungitoxic potential of copaiba and eucalyptus essential oils on phytopathogens. Journal of Experimental Agriculture International, 29(3), 1–10. https://doi.org/10.9734/JEAI/2019/46083
Dethoup, T., Songkumarn, P., Rueangrit, S., Suesa-ard, S., & Kaewkrajay, C. (2018). Fungicidal activity of Thai medicinal plant extracts against Alternaria brassicicola causing black spot of Chinese kale. European Journal of Plant Pathology, 152(1), 157–167. https://doi.org/10.1007/s10658-018-1460-5
Ghasemi, S., Abbasi, S., Bahraminejad, S., & Harighi, B. (2012). Inhibitory effect of some crude extracts against damping off agents. Australasian Plant Pathology Journal, 41, 331–338. https://doi.org/10.1007/s13313-012-0129-3
Ghezelbash, N., Abdollahi, M., & Shahriari, D. (2013). Evaluation of antifungal activity of Shirazi thyme and Chavil extracts on Fusarium oxysporum f.sp. lycopersici, the causal agent of tomato wilt under laboratory and greenhouse conditions. Plant Protection Journal, 36(4), 53–65.
Giordani, C., Simonetti, G., Natsagdorj, D., Choijamts, G., Ghirga, F., Calcaterra, A., & Pasqua, G. (2020). Antifungal activity of Mongolian medicinal plant extracts. Natural Product Research, 34(4), 449–455. https://doi.org/10.1080/14786419.2019.1610960
Habibian Dehkordi, S., Sadeghi, H., Rahimi, R., & Ebrahimi, A. (2017). The evaluation of antifungal effects of Althaea officinalis and Syzygium aromaticum aqueous extracts against Penicillium spp and Aspergillus spp isolates. Veterinary Researches and Biological Product, 30(2), 147–152.
Hamad, Y. K., Abobakr, Y., Salem, M. Z., Ali, H. M., Al-Sarar, A. S., & Al-Zabib, A. A. (2019). Activity of plant extracts/essential oils against three plant pathogenic fungi and mosquito larvae: GC/MS analysis of bioactive compounds. Bioresources, 14(2), 4489–4511. https://doi.org/10.15376/biores.14.2.4489-4511
Hammons, R. O., Herman, D., & Stalker, H. T. (2016). Origin and early history of the peanut. In Stalker, H. T., & Wilson, R. F., (Eds.), Peanuts. AOCS Press, pp. 1–26.
Hasheminejad, N., Khodaiyan, F., & Safari, M. (2019). Improving the antifungal activity of clove essential oil encapsulated by chitosan nanoparticles. Food Chemistry, 275, 113–122. https://doi.org/10.1016/j.foodchem.2018.09.085
Huang, Y., Zhao, J., Zhou, L., Wang, J., Gong, Y., Chen, X., & Jiang, W. (2010). Antifungal activity of the essential oil of Illicium verum fruit and its main component trans-anethole. Molecules, 15(11), 7558–7569. https://doi.org/10.3390/molecules15117558
Hyldgaard, M., Mygind, T., & Meyer, R. L. (2012). Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Frontiers in Microbiology, 3, 1–12. https://doi.org/10.3389/fmicb.2012.00012
Katooli, N., Maghsodlo, R., & Razavi, S. E. (2017). Evaluation of eucalyptus essential oil against some plant pathogenic fungi. Journal of Plant Breeding and Crop Science, 3(2), 41–43.
Kordali, S., Cakir, A., Akcin, T. A., Mete, E., Akcin, A., Aydin, T., & Kilic, H. (2013). Antifungal and herbicidal properties of essential oils and n-hexane extracts of Achillea gypsicola Hub-Mor. and Achillea biebersteinii Afan (Asteraceae). Industrial Crops and Products, 29(2), 562–570. https://doi.org/10.1016/j.indcrop.2008.11.002
Kottearachchi, N. S., Sammani, A., Kelaniyangoda, D. B., & Samarasekara, R. (2012). Anti-fungal activity of essential oils of Ceylon Eucalyptus species for the control of Fusarium solani and Sclerotium rolfsii. Archives of Phytopathology and Plant Protection, 45(17), 2026–2035. https://doi.org/10.1080/03235408.2012.720469
Le, C. N., Kruijt, M., & Raaijmakers, J. M. (2012). Involvement of phenazines and lipopeptides in interactions between Pseudomonas species and Sclerotium rolfsii, causal agent of stem rot disease on groundnut. Journal of Applied Microbiology, 112, 390–403. https://doi.org/10.1111/j.1365-2672.2011.05205.x
Liu, Q., Meng, X., Li, Y., Zhao, C. N., Tang, G. Y., & Li, H. B. (2017). Antibacterial and antifungal activities of spices. International Journal of Molecular Sciences, 18(6), 1283–1291. https://doi.org/10.3390/ijms18061283
Mahadevakumar, S., Yadav, V., Tejaswini, G. S., & Janardhana, G. R. (2015). Morphological and molecular characterization of Sclerotium rolfsii associated with fruit rot of Cucurbita maxima. European Journal of Plant Pathology. https://doi.org/10.1007/s10658-015-0818-1
Maskouki, A. M., & Mortazavi, A. (2004). Inhibitory effects of thyme and ajowan oils on growth of Aspergilus parasiticus on pear during cold storage. Journal of Water and Soil Science, 8(2), 207–215.
Mehri, Z., Khodaparast, S. A., & Mosanejad, S. (2013). Genetic diversity in Sclerotium rolfsii population based on mycelial compatibility groups in Guilan province. Iranian Journal of Plant Pathology, 49(3), 317–324.
Mirzabagheri, D., Abbaszadeh, M., Derijani, S., & Sadradini, S. (2014). Inhibition effects of Zataria multiflora, Eucalyptus camaldulensis and Myrtus communis essential oil on mycelial growth of green mold of orange. International Journal of Advanced Biological and Biomedical Research, 2(1), 86–99.
Mohammadkhani, N., & Yekta, A. (2019). Antifungal activity of Cuminum cyminum and Rosmarinus officinalis essential oils on wheat infected by Bipolaris sorokiniana. Journal of Agricultural Science and Sustainable Production, 29(3), 181–196.
Montanari, R. M., Barbosa, L. C., Demuner, A. J., Silva, C. J., Carvalho, L. S., & Andrade, N. J. (2011). Chemical composition and antibacterial activity of essential oils from Verbenaceae species: Alternative sources of (E)-caryophyllene and germacrene-D. Química Nova, 34(9), 1550–1555. https://doi.org/10.1590/S0100-40422011000900013
Nikoliš, M., Jovanoviš, K. K., Markoviš, T., Markoviš, D., Gligorijeviš, N., Raduloviš, S., & Sokoviš, M. (2014). Chemical composition, antimicrobial, and cytotoxic properties of five Lamiaceae essential oils. Industrial Crops and Products, 61, 225–232. https://doi.org/10.1016/j.indcrop.2014.07.011
Ojagian, M. R., Chen, Y., Chen, S., Cui, Z. Q., Xie, G. L., & Zhang, J. (2014). Antifungal and enzymatic evaluation of plant crude extracts derived from cinnamon and rosemary against Sclerotinia carrot rot. Annals of Applied Biology, 164(3), 415–429. https://doi.org/10.1111/aab.12111
Okabe, I., & Matsumoto, N. (2000). Population structure of Sclerotium rolfsii in peanut fields. Mycoscience, 41, 145–148. https://doi.org/10.1007/BF02464323
Okoh, O. O., Sadimenko, A. P., & Afolayan, A. J. (2010). Comparative evaluation of the antibacterial activities of the essential oils of Rosmarinus officinalis L. obtained by hydrodistillation and solvent free microwave extraction methods. Food Chemistry, 120(1), 308–312. https://doi.org/10.1016/j.foodchem.2009.09.084
Punja, Z. K., & Grogan, R. G. (1983). Basidiocarp induction, nuclear condition variability and heterokaryon incompatibility in Athelia (Sclerotium) rolfsii. Phytopathology, 73, 1273–1278. https://doi.org/10.1094/Phyto-73-1273
Purdy, L. H. (1979). Sclerotinia sclerotiorum: history, disease and symptomatology, host range, geographic distribution, and impact. Phytopathology, 69, 875–880. https://doi.org/10.1094/Phyto-69-875
Saccardo, P. A. (1981). Notae Mycologicae Series XIII. Annales Mycologici, 9(3), 249–257.
Sajjadi, S. F., & Asemi, H. (2014). Study of antifungal activity of plant extracts of catmint, tobacco and thyme on tobacco pathogens fungal. Biological Control of Pests and Plant Diseases, 3(1), 41–52.
Salek Meraji, H., Salek Naghdi, R., & Tafreshi, S. (2015). Inhibitory effect of rosemary and fennel extract on Fusarium oxysporum. Journal of Plant Disease Research, 3(2), 57–68.
Sennoi, R., Singkham, N., Jogloy, S., Boonlue, S., Saksirirat, W., Kesmala, T., & Patanothai, A. (2013). Biological control of southern stem rot by Sclerotium rolfsii using by Trichoderma harzianum and arbuscular mycorrhizal fungi on Jerusalem artichoke (Helianthus tuberosus L.). Plant Protection, 54, 148–153. https://doi.org/10.1016/j.cropro.2013.08.011
Seraji, A., Mahfouzi, N., Safaei Chaeikar, S., & Yahyavi Azad, A. (2017). The effect of hydroalcoholic extracts of Mentha piperita L., Eucalyptus camaldulensis and Allium sativum on biological inhibition of Pratylenchus loosi in vitro. Applied Plant Protection, 6(2), 135–146.
Sharma, A., Rajendran, S., Srivastava, A., Sharma, S., & Kundu, B. (2017). Antifungal activities of selected essential oils against Fusarium oxysporum f. sp. lycopersici 1322, with emphasis on Syzygium aromaticum essential oil. Journal of Bioscience and Bioengineering, 123(3), 308–313. https://doi.org/10.1016/j.jbiosc.2016.09.011
Shitole, A. V., Gade, R. M., & Wavare, S. H. (2017). Evaluation of plant extracts against Aspergillus niger and Sclerotium rolfsii causing collar rot and seed rot in chickpea. Journal of Plant Disease Sciences, 12(1), 101–107.
Suleiman, M. N., & Emua, S. A. (2009). Efficacy of four plant extracts in the control of root rot disease of cowpea (Vigna unguiculata [L.] Walp). African Journal of Biotechnology, 8(16), 3806–3808.
Talibi, I., Boubaker, H., Boudyach, E. H., & Ait Ben Aoumar, A. (2014). Alternative methods for the control of postharvest citrus diseases. Journal of Applied Microbiology, 117(1), 1–17. https://doi.org/10.1111/jam.12495
Tamura, K., Stecher, G., Peterson, D., Filipski, A., & Kumar, S. (2013). MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30, 2725–2729. https://doi.org/10.1093/molbev/mst197
Vesaltalab, Z., & Gholami, M. (2012). The Effect of clove buds and rosemary extracts and essences on control of Botrytis cinerea growth. Plant Production Technology, 3(2), 1–11.
White, T., Bruns, T., Lee, S., & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In Innis, M. A., Gelfand, D. H., Sninsky, J. J., & White, T. J. (Eds.), PCR Protocols, a Guide to Methods and Applications (pp. 315–322). New York: Academic Press.
Yahya-abadi, S., Zeabanejad, E., & Doudi, M. (2011). Effect of plant extracts on growth of Aspergillus fungi. Journal of Herbal Drugs, 2(1), 69–81.
Yaqub, F., & Shahzad, S. (2005). Pathogenicity of Sclerotium rolfsii on different crops and effect of inoculums density on colonization of mungbea and sunflower roots. Pakistan Journal of Botany, 37(1), 175–180.
Zammouri, S., Kalai-Grami, L., & Mnari-Hattab, M. (2018). Optimization of simple DNA extraction method suitable for diverse microorganisms. Tunisian Journal of Plant Protection, 13(2), 315–329.
Zhang, Y. P., Uyemoto, & Kirkpatrick, B. C. (1998). A small scale procedure for extracting nucleic acids from woody plants infected with various phytopathogens for PCR assay. Journal of Virological Methods, 71, 45–50. https://doi.org/10.1016/S0166-0934(97)00190
Acknowledgements
This experiment was supported by the Islamic Azad University, Rasht Branch, Iran.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MRSM and LE designed research; MRSM analyzed data; MRSM and LE contributed materials and preliminary imaging method development; MRSM wrote the article; MRSM edited the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
All authors read and approved the manuscript.
Consent for publication
All authors read and approved the manuscript.
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Motlagh, M.R.S., Ebrahimi, L. The antifungal effects of some plant extracts on Sclerotium rolfsii, the causal agent of peanut white stem rot disease. Eur J Plant Pathol (2024). https://doi.org/10.1007/s10658-024-02848-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s10658-024-02848-7