Abstract
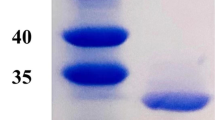
The L-asparaginase (ASPN) enzyme has received recognition in various applications including acrylamide degradation in the food industry. The synthesis and application of thermostable ASPN enzymes is required for its use in the food sector, where thermostable enzymes can withstand high temperatures. To achieve this goal, the bacterium Bacillus subtilis was isolated from the hot springs of Tapovan for screening the production of thermostable ASPN enzyme. Thus, ASPN with a maximal specific enzymatic activity of 0.896 U/mg and a molecular weight of 66 kDa was produced from the isolated bacteria. The kinetic study of the enzyme yielded a Km value of 1.579 mM and a Vmax of 5.009 µM/min with thermostability up to 100 min at 75 °C. This may have had a positive indication for employing the enzyme to stop polyacrylamide from being produced. The current study has also been extended to investigate the interaction of native and mutated ASPN enzymes with acrylamide. This concluded that the M10 (with 10 mutations) has the highest protein and thermal stability compared to the wild-type ASPN protein sequence. Therefore, in comparison to a normal ASPN and all other mutant ASPNs, M10 is the most favorable mutation. This research has also demonstrated the usage of ASPN in food industrial applications.
Graphical Abstract









Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Agrawal S, Jana UK, Kango N (2021) Heterologous expression and molecular modelling of L-asparaginase from Bacillus subtilis ETMC-2. Int J Biol Macromol 192:28–37. https://doi.org/10.1016/j.ijbiomac.2021.09.186
Alam S, Nagpal T, Singhal R, Khare SK (2021) Immobilization of L-asparaginase on magnetic nanoparticles: kinetics and functional characterization and applications. Bioresour Technol 339:125599. https://doi.org/10.1016/j.biortech.2021.125599
Aly N, El-Ahwany A, Ataya FS, Saeed H (2020) Bacillus sonorensis L. asparaginase: cloning, expression in E. coli and characterization. Protein J 39:717–729. https://doi.org/10.1007/s10930-020-09932-x
Badoei-Dalfard A (2016) L-asparaginase production in the Pseudomonas pseudoalcaligenes strain JHS-71 isolated from Jooshan Hot-spring. Mol Biol Res Commun Mar 5(1):1
Bateman A, Martin M, O’donovan C, Magrane M, Apweiler R, Alpi E. 440 Bingley M (2015) UniProt: a hub for protein information. Nucleic Acids Res 43:D204-441
Buchholz F, Sonntag J, RojoRomanos T, Schneider PM, Schmitt LT, Paszkowski-Rogacz M, Lansing F (2020) A heterodimer of evolved designer-recombinases precisely excises a human genomic DNA locus. Nucleic Acids Res 48(1):472–485. https://doi.org/10.1093/nar/gkz1078
Chauhan PS, Tripathi SP, Sangamwar AT, Puri N, Sharma P, Gupta N (2015) Cloning, molecular modeling, and docking analysis of alkali-thermostable β-mannanase from Bacillus nealsonii PN-11. Appl Microbiol Biotechnol 99:8917–25. https://doi.org/10.1007/s00253-015-6613-2
Chi H, Wang Y, Xia B, Zhou Y, Lu Z, Lu F, Zhu P (2022) Enhanced thermostability and molecular insights for L-asparaginase from Bacillus licheniformis via structure-and computation-based rational design. J Agric Food Chem 70(45):14499–509. https://doi.org/10.1021/acs.jafc.2c05712
Chi H, Zhu X, Shen J, Lu Z, Lu F, Lyu Y, Zhu P (2023) Thermostability enhancement and insight of L-asparaginase from Mycobacterium sp. via consensus-guided engineering. App Microb Biotech 107(7–8):2321–33. https://doi.org/10.1007/s00253-023-12443-1
Chi H, Chen M, Jiao L, Lu Z, Bie X, Zhao H, Lu F (2021) Characterization of a novel L-asparaginase from Mycobacterium gordonae with acrylamide mitigation potential. Foods 10(11). 10.3390/foods10112819
Chohan SM, Rashid N, Sajed M, Imanaka T (2019) Pcal_0970: an extremely thermostable L-asparaginase from Pyrobaculum calidifontis with no detectable glutaminase activity. FM 64:313–2. https://doi.org/10.1007/s12223-018-0656-6
Chohan SM, Rashid N (2013) TK1656, a thermostable L-asparaginase from Thermococcus kodakaraensis, exhibiting highest ever reported enzyme activity. J Biosci Bioeng 116(4):438–443. https://doi.org/10.1016/j.jbiosc.2013.04.005
Correa CLO, Penha ED, dos Anjos MR, Pacheco S, Freitas-Silva O, Luna AS, Gottschalk LMF (2021) Use of asparaginase for acrylamide mitigation in coffee and its influence on the content of caffeine, chlorogenic acid, and caffeic acid. Food Chem 338. https://doi.org/10.1016/j.foodchem.2020.128045
Duda-Chodak A, Tarko T, Sroka P, Satora P (2016) A review of the interactions between acrylamide, microorganisms and food components. Food Funct 7(3):1282–1295. https://doi.org/10.1039/C5FO01294E
Dumina M, Zhgun A, Pokrovskaya M, Aleksandrova S, Zhdanov D, Sokolov N, El’darov M (2021) Highly active thermophilic L-asparaginase from Melioribacter roseus represents a novel large group of type II bacterial L-asparaginases from Chlorobi-ignavibacteriae-bacteroidetes clade. Int J Mol Sci 22(24):13632. https://doi.org/10.3390/ijms222413632
Dzun NJ, Intan B, Azlin SA, Zaki Z (2015) Isolation and characterization of thermophilic bacteria producing L-asparaginase from Malaysia hotspring and enzyme activity using different carbon and nitrogen sources. Appl Agric Sci 10(5 Special):69–77
El-Bessoumy AA, Sarhan M, Mansour J (2004) Production, isolation, and purification of L-asparaginase from Pseudomonas aeruginosa 50071 using solid-state fermentation. BMB Rep 37(4):387–393. https://doi.org/10.5483/bmbrep.2004.37.4.387
El-Naggar NE, El-Shweihy NM (2020) Bioprocess development for L-asparaginase production by Streptomyces rochei, purification and in-vitro efficacy against various human carcinoma cell lines. Sci Rep 10(1):7942. https://doi.org/10.1038/s41598-020-64052-x
El-Naggar NEA, Deraz SF, Soliman HM, El-Deeb NM, El-Ewasy SM (2016) Purification, characterization, cytotoxicity and anticancer activities of ASPN, anti-colon cancer protein, from the newly isolated alkaliphilic Streptomyces fradiae NEAE-82. Sci Rep 6(1):32926. https://doi.org/10.1038/srep32926
Feng Y, Liu S, Jiao Y, Gao H, Wang M, Du G, Chen J (2017) Enhanced extracellular production of L-asparaginase from Bacillus subtilis 168 by B. subtilis WB600 through a combined strategy. Appl Microbiol Biotechnol 101:1509–20. https://doi.org/10.1007/s00253-016-7816-x
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47(7):1739–49. https://doi.org/10.1021/jm0306430
Gasteiger E, Hoogland C, Gattiker A, Duvaud SE, Wilkins MR, Appel RD, Bairoch A (2005) Protein identification and analysis tools on the ExPASy server. Humana Press. https://doi.org/10.1385/1-59259-890-0:571
Grahame DS, Dupuis JH, Bryksa BC, Tanaka T, Yada RY (2020) Comparative bioinformatic and structural analyses of pepsin and renin. Enzyme Microb Technol 141:109632. https://doi.org/10.1016/j.enzmictec.2020.109632
Guo J, Coker AR, Wood SP, Cooper JB, Chohan SM, Rashid N, Akhtar M (2017) Structure and function of the thermostable L-asparaginase from Thermococcus kodakarensis. Acta Crystallogr D 73(11):889–895. https://doi.org/10.1107/S2059798317014711
Hendriksen HV, Kornbrust BA, Ostergaard PR, Stringer MA (2009) Evaluating the potential for enzymatic acrylamide mitigation in a range of food products using an asparaginase from Aspergillus oryzae. J Agric Food Chem 57(10):4168–4176. https://doi.org/10.1021/jf900174q
Homaei A, Izadpanah Qeshmi F (2022) Purification and characterization of a robust thermostable protease isolated from Bacillus subtilis strain HR02 as an extremozyme. J Appl Microbiol 133(5):2779–2789. https://doi.org/10.1111/jam.15725
Jia MM, Xu MJ, He BB, Rao ZM (2013) Cloning, expression, and characterization of L-asparaginase from a newly isolated Bacillus subtilis B11–06. J Agric Food Chem 61(39):9428–9434. https://doi.org/10.1021/jf402636w
Joo YC, Oh DK (2012) Lipoxygenases: potential starting biocatalysts for the synthesis of signaling compounds. Biotechnol Adv 30:1524–1532. https://doi.org/10.1016/j.biotechadv.2012.04.004
Kahled E, Fouad MF, Badawi MH, El-Rahim A, Mohamed W, Shawky H, Moawad H (2022) Thermostable protease, amylase and lipase enzymes of thermophilic bacteria isolated from Egyptian hot springs. Egypt J Chem 65(10):225–38. https://doi.org/10.21608/ejchem.2022.113659.5190
Kukurova K, Morales FJ, Bednarikova A, Ciesarova Z (2009) Effect of L-asparaginase on acrylamide mitigation in a fried-dough pastry model. Mol Nutr Food Res 53(12):1532–1539. https://doi.org/10.1002/mnfr.200800600
Kumar NV, Rani ME, Gunaseeli R, Kannan ND, Sridhar J (2012) Modeling and structural analysis of cellulases using Clostridium thermocellum as template. Bioinformation 8(22):1105. https://doi.org/10.6026/2F97320630081105
Lambré C, Barat Baviera JM, Bolognesi C, Cocconcelli PS, Crebelli R, Gott DM, Grob K, Lampi E, Mengelers M, Mortensen A, Rivière G, Steffensen IL, Tlustos C, Van Loveren H, Vernis L, Zorn H, Roos Y, Andryszkiewicz M, Kovalkovicova N, Liu Y, Lunardi S, Chesson A (2023) Safety evaluation of the food enzyme asparaginase from the genetically modified Bacillus subtilis strain NZYM-CK. EFSA J. https://doi.org/10.2903/j.efsa.2023.7908
Lansing F, Mukhametzyanova L, Rojo-Romanos T, Iwasawa K, Kimura M, Paszkowski-Rogacz M, Karpinski J, Grass T, Sonntag J, Schneider P M, Günes C, Hoersten J, Schmitt L T, Rodriguez-Muela N, Knöfler R, Takebe T, Buchholz F (2022) Correction of a factor VIII genomic inversion with designer-recombinases. Nat Commun 13(1). https://doi.org/10.1038/s41467-022-28080-7
Li X, Zhang X, Xu S, Xu M, Yang T, Wang L, Zhang H, Fang H, Osire T, Rao Z (2019) Insight into the thermostability of thermophilic L-asparaginase and non-thermophilic L-asparaginase II through bioinformatics and structural analysis. App Microb Biotech 103:7055–7070. https://doi.org/10.1007/s00253-019-09967-w
Lineback DR, Coughlin JR, Stadler RH (2012) Acrylamide in foods: a review of the science and future considerations. Annu Rev Food Sci T 3:15–35. https://doi.org/10.1146/annurev-food-022811-101114
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56(3):658–666. https://doi.org/10.1021/ja01318a036
Liu K, Xu Z, Zhao Z, Chen Y, Chai Y, Ma L, Li S (2022) A dual fluorescence assay enables high-throughput screening for pet hydrolases. ChemSusChem e202202019. https://doi.org/10.1002/cssc.202202019
Long S, Zhang X, Rao Z, Chen K, Xu M, Yang T, Yang S (2016) Amino acid residues adjacent to the catalytic cavity of tetramer L-asparaginase II contribute significantly to its catalytic efficiency and thermostability. Enzyme Microb Technol 82:15–22. https://doi.org/10.1016/j.enzmictec.2015.08.009
Lowry O, Rosebrough N, Farr AL, Randall R (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Lu X, Chen J, Jiao L, Zhong L, Lu Z, Zhang C, Lu F (2019) Improvement of the activity of L-asparaginase I improvement of the catalytic activity of L-asparaginase I from Bacillus megaterium H-1 by in vitro directed evolution. J Biosci Bioeng 128(6):683–689. https://doi.org/10.1016/j.jbiosc.2019.06.001
Mahajan RV, Kumar V, Rajendran V, Saran S, Ghosh PC, Saxena RK (2014) Purification and characterization of a novel and robust L-asparaginase having low-glutaminase activity from Bacillus licheniformis: in vitro evaluation of anti-cancerous properties. PLoS One 9(6):e99037. https://doi.org/10.1371/journal.pone.0099037
Maqsood B, Basit A, Khurshid M, Bashir Q (2020) Characterization of a thermostable, allosteric L-asparaginase from Anoxybacillus flavithermus. Int J Biol Macromol 152:584–592. https://doi.org/10.1016/j.ijbiomac.2020.02.246
Meena B, Anburajan L, Dheenan PS, Begum M, Vinithkumar NV, Dharani G, Kirubagaran R (2015) Novel glutaminase free L-asparaginase from Nocardiopsis alba NIOT-VKMA08: production, optimization, functional and molecular characterization. Bioprocess Biosyst Eng 38:373–388. https://doi.org/10.1007/s00449-014-1277-3
Mohammad BT, Al Daghistani HI, Jaouani A, Abdel-Latif S, Kennes C (2017) Isolation and characterization of thermophilic bacteria from Jordanian hot springs: Bacillus licheniformis and Thermomonas hydrothermalis isolates as potential producers of thermostable enzymes. Int. J Microbiol https://doi.org/10.1155/2017/6943952
Morgenthaler AB, Kinney WR, Ebmeier CC, Walsh CM, Snyder DJ, Cooper VS, Old WM, Copley SD (2019) Mutations that improve efficiency of a weak-link enzyme are rare compared to adaptive mutations elsewhere in the genome. eLife 8:e53535. https://doi.org/10.7554/eLife.53535
Mostafa Y, Alrumman S, Alamri S, Hashem M, Al-izran K, Alfaifi M, Elbehairi SE, Taha T (2019) Enhanced production of glutaminase-free L-asparaginase by marine Bacillus velezensis and cytotoxic activity against breast cancer cell lines. Electron J Biotechnol 42:6–15. https://doi.org/10.1016/j.ejbt.2019.10.001
Mukherjee R, Bera D (2022) Biochemical characterization and thermodynamic principles of purified L-asparaginase from novel Brevibacillus borstelensis ML12. Biocatal Agric Biotechnol 39:102260. https://doi.org/10.1016/j.bcab.2021.102260
Onwubolu GC, Kumar S (2006) Response surface methodology-based approach to CNC drilling operations. J Mater Process Technol 171(1):41–47. https://doi.org/10.1016/j.jmatprotec.2005.06.064
Pfleger C, Rathi P C, Klein D L, Radestock S, Gohlke H (2013) Constraint network analysis (CNA): a Python software package for efficiently linking biomacromolecular structure, flexibility, (thermo-) stability, and function. https://doi.org/10.1021/ci400044m
Pola M, Rajulapati SB, Durthi CP, Erva RR, Bhatia M (2018) In silico modelling and molecular dynamics simulation studies on L-asparaginase isolated from bacterial endophyte of Ocimum tenuiflorum. Enzyme Microb Technol 117:32–40. https://doi.org/10.1016/j.enzmictec.2018.06.005
Prakash P, Singh HR, Jha SK (2020) Indicator dye-based screening of glutaminase free L-asparaginase producer and kinetic evaluation of enzyme production process. Prep Biochem Biotechnol 50(8):803–813. https://doi.org/10.1080/10826068.2020.1737942
Rahimzadeh M, Poodat M, Javadpour S, Qeshmi FI, Shamsipour F (2016) Purification, characterization and comparison between two new L-asparaginases from Bacillus PG03 and Bacillus PG04. Open Biochem J 10:35. https://doi.org/10.2174/2F1874091X01610010035
Roy MP, Das V, Patra A (2019) Isolation, purification and characterization of an extracellular L-ASPN produced by a newly isolated Bacillus megaterium strain MG1 from the water bodies of Moraghat forest, Jalpaiguri. India J Gen Appl Microbiol 65(3):137–144. https://doi.org/10.2323/jgam.2018.07.004
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Sánchez-Moguel I, Costa-Silva TA, Pillaca-Pullo OS, Flores-Santos JC, Freire RK, Carretero G, da Luz BJ, Camacho-Cordova DI, Santos JH, Sette LD, Pessoa-Jr A (2023) Antarctic yeasts as a source of L-asparaginase: characterization of a glutaminase-activity free L-asparaginase from psychrotolerant yeast Leucosporidium scottii L115. Process Biochem 129:121–132. https://doi.org/10.1016/j.procbio.2023.03.003
Sanghvi G, Bhimani K, Vaishnav D, Oza T, Dave G, Kunjadia P, Sheth N (2016) Mitigation of acrylamide by l-asparaginase from Bacillus subtilis KDPS1 and analysis of degradation products by HPLC and HPTLC. SpringerPlus 5:1–11
Santana CA, Izidoro SC, de Melo-Minardi RC, Tyzack JD, Ribeiro AJ, Pires DE, Thornton JM, de A. Silveira S (2022) GRaSP-web: a machine learning strategy to predict binding sites based on residue neighborhood graphs. Nucleic Acids Res 50(W1):W392-7. https://doi.org/10.1093/nar/gkac323
Saranya R, Jayapriya JT (2018) Purification, characterization, molecular modeling and docking study of fish waste protease. Int J Biol Macromol 118:569–583. https://doi.org/10.1016/j.ijbiomac.2018.06.119
Sarion C, Codina G G, Dabija A (2021) Acrylamide in bakery products: a review on health risks, legal regulations and strategies to reduce its formation. Int J Env Res Pub He 18(8) .https://doi.org/10.3390/ijerph18084332
Schmitt LT, Paszkowski-Rogacz M, Jug F, Buchholz F (2022) Prediction of designer-recombinases for DNA editing with generative deep learning. Nat Commun 13(1):7966. https://doi.org/10.1038/s41467-022-35614-6
Shakambari G, Birendranarayan AK, Lincy MJ, Rai SK, Ahamed QT, Ashokkumar B, Saravanan M, Mahesh A, Varalakshmi P (2016) Hemocompatible glutaminase free L-asparaginase from marine Bacillus tequilensis PV9W with anticancer potential modulating p53 expression. RSC Adv 6(31):25943–25951. https://doi.org/10.1039/C6RA00727A
Shakambari G, Sameer Kumar R, Ashokkumar B, Varalakshmi P (2017) Agro waste utilization for cost-effective production of L-asparaginase by Pseudomonas plecoglossicida RS1 with anticancer and acrylamide mitigation potential. ACS Omega 2(11):8108–8117. https://doi.org/10.1021/acsomega.7b01429
Shi R, Liu Y, Mu Q, Jiang Z, Yang S (2017) Biochemical characterization of a novel L-asparaginase from Paenibacillus barengoltzii being suitable for acrylamide reduction in potato chips and mooncakes. Int J Biol Macromol 96:93–99. https://doi.org/10.1016/j.ijbiomac.2016.11.115
Shifrin S, Parrott C (1974) In vitro assembly of L-asparaginase subunits. J Biol Chem 249(13):4175–4180. https://doi.org/10.1016/S0021-9258(19)42499-X
Sindhu R, Manonmani HK (2018) Expression and characterization of recombinant l-asparaginase from Pseudomonas fluorescens. Protein Expr Purif 143:83–91. https://doi.org/10.1016/j.pep.2017.09.009
Singh Y, Srivastava SK (2014) Performance improvement of Bacillus aryabhattai ITBHU02 for high-throughput production of a tumor-inhibitory L-asparaginase using a kinetic model-based approach. J Chem Technol Biotechnol 89(1):117–127. https://doi.org/10.1002/jctb.4121
Stadler RH, Blank I, Varga N, Robert F, Hau J, Guy PA, Robert MC, Riediker S (2002) Acrylamide from Maillard reaction products. Nature 419(6906):449–450. https://doi.org/10.1038/419449a
Stothard P (2000) The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. Biotechniques 28(6):1102–1104. https://doi.org/10.2144/00286ir01
Tian W, Chen C, Lei X, Zhao J, Liang J (2018) CASTp 3.0: computed atlas of surface topography of proteins. Nucleic Acids Res 46(W1):W363–7. https://doi.org/10.1093/nar/gky473
Vidya J, Ushasree MV, Pandey A (2014) Effect of surface charge alteration on stability of l-asparaginase II from Escherichia sp. Enzyme Microb Technol 56:15–9. https://doi.org/10.1016/j.enzmictec.2013.12.012
Vimal A, Jha A, Kumar A (2018) Eugenol derivatives prospectively inhibit l-asparaginase: a heady target protein of Salmonella typhimurium. Microb Pathog 114:8–16. https://doi.org/10.1016/j.micpath.2017.11.009
Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TA, Rempfer C, Bordoli L, Lepore R (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46(W1):W296-303. https://doi.org/10.1093/nar/gky427
Weist S, Süssmuth RD (2005) Mutational biosynthesis—a tool for the generation of structural diversity in the biosynthesis of antibiotics. Appl Microbiol Biotechnol 68:141–150. https://doi.org/10.1007/s00253-005-1891-8
Xu F, Oruna-Concha MJ, Elmore JS (2016) The use of asparaginase to reduce acrylamide levels in cooked food. Food Chem 210:163–171. https://doi.org/10.1016/j.foodchem.2016.04.105
Yim S, Kim M (2019) Purification and characterization of thermostable l-asparaginase from Bacillus amyloliquefaciens MKSE in Korean soybean paste. LWT 109:415–421. https://doi.org/10.1016/j.lwt.2019.04.050
Yousafi Q, Ali HA, Rashid H, Khan MS (2019) In silico comparative proteomic analysis of enzymes involved in fatty acid biosynthesis in castor bean (Ricinus communis) and soybean (Glycine max). Turk J Bot 43(1):1–26. https://doi.org/10.3906/bot-1708-48
Zhang YQ, Tao ML, Shen WD, Zhou YZ, Ding Y, Ma Y, Zhou WL (2004) Immobilization of L-asparaginase on the microparticles of the natural silk sericin protein and its characters. Biomaterials 25(17):3751–3759. https://doi.org/10.1016/j.biomaterials.2003.10.019
Zhou Y, Jiao L, Shen J, Chi H, Lu Z, Liu H, Lu F, Zhu P (2022) Enhancing the catalytic activity of type II L-asparaginase from Bacillus licheniformis through semi-rational design. nt. J Mol Sci 23(17):9663. https://doi.org/10.3390/ijms23179663
Zuo S, Xue D, Zhang T, Jiang B, Mu W (2014) Biochemical characterization of an extremely thermostable L-asparaginase from Thermococcus gammatolerans EJ3. J Mol Catal B Enzym 109:122–129. https://doi.org/10.1016/j.molcatb.2014.08.021
Acknowledgements
AGS is grateful to SASTRA Deemed to be University for the Ph.D. fellowship. NVK is grateful to UGC for start-up research grants (30–561/2021(BSR)).
Funding
This was funded by UGC, India, through start-up research grants (30–561/2021(BSR)).
Author information
Authors and Affiliations
Contributions
All authors contributed to the manuscript writing. Srivarshan Shanmuga Sundaram and Aravind Kannan conceived and designed the research. Pratham Gour Chintaluri contributed to the molecular docking studies. Aparna Ganapathy Vilasam Sreekala supervised and contributed to the drafting of the manuscript. Vinod Kumar Nathan supervised and contributed to the editing of the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• Highly thermostable L-asparaginase obtained from Bacillus subtilis.
• Optimized factors for L-asparaginase production and thermostability checked.
• In silico mutation has been done to boost the thermal stability of the wild-type ASPN and to improve the interaction of acrylamide for its depolymerization ability.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sundaram, S.S., Kannan, A., Chintaluri, P.G. et al. Thermostable bacterial L-asparaginase for polyacrylamide inhibition and in silico mutational analysis. Int Microbiol (2024). https://doi.org/10.1007/s10123-024-00493-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10123-024-00493-y