Abstract
The study provides a detailed investigation into several representatives of La Tène jewellery. Primarily, it deals with non-metallic decorative inlays from the Late La Tène period, particularly with their application on a metal base. Unique artefacts have been selected for the study, all coming from the Czech Republic and Slovakia. A prime representative of the finds is a torc coming from a La Tène burial site in Prague (Czech Republic, Central Europe). In this geographical context, such finds are rather exceptional. The torc examined is remarkable both in terms of its origin and the production technology utilised, namely the application of decorative inlays made of red opaque glass. Available literature classifies this decorative element as an enamel technique. The highly specific type of red soda high-lead glass applied there was examined using LA-ICP-MS. Based on the trace elements detected, it can be established that the glass is most likely related to contemporary Egyptian production. Other items of the artefact set consist of two Münsingen brooches from Moravia and an exceptional brooch from Slovakia. The latter find was found to be decorated with coral (as confirmed by Raman spectroscopy). Besides identifying the materials of the decorations, we were also able to characterize the binder between individual decorative inlays and their metal base. The analysis of the binder has revealed the presence of birch tar (determined by FTIR and GC/MS). The results obtained expand the knowledge about the production technologies applied to the jewellery from the La Tène period.
Similar content being viewed by others
Introduction
The application of non-metallic decorative inlays from the La Tène period has been evidenced in a number of artefacts – weapons, armoury, jewellery and even utility items (Challet 1992, Sankot 1998). Our paper primarily focuses on two types of jewellery – brooch and torc. The selection of the objects was conditioned by their geographical origin (Bohemia, Moravia, and Slovakia), the chronological period of the 4th to 3rd century BC, and the variability of the material applied. The first section of the paper presented deals with decorative inlays made of red opaque glass and coming from a unique torc uncovered at a La Tène burial site in Prague (Czech Republic, Central Europe). Other parts of the text discuss red coral (also used as a decorative element in the jewel investigated) and the binder used to fix decorative inlays.
Opaque red glass was used by the Celts as early as the 4th century BC. For this period, two types of enamel techniques can be distinguished. The first technique consists in glass coatings fused to metal substrates (i.e. enamel in the literal sense of the word) and the second approach involves the use of decorative, shaped glass pieces applied to metal artefacts (also, rivets were frequently used to fix decorations onto the metal surface). In the case of the torc studied, pieces of glass were pre-shaped as desired by cold or hot forming (pressing or moulding). This technique was often used to decorate the earliest artefacts from the Early La Tène period, and such cold inlays can typically be seen in torcs and brooches (Brun and Pernot 1992).
The La Tène enamels of continental Europe are almost exclusively red. Opaque red glass/enamel appears to be a Celtic speciality, whereas just a few other enamelled artefacts are known from the Mediterranean area. A unique group of Celtic enamelling workshops was identified at an oppidum in Mont-Beuvray. Various (Challet 1992) hypotheses exist regarding the origin of opaque red glass: (1) it was imported from a centre of production located outside the La Tène (Celtic) Europe; (2) it was produced locally in or near enamelling workshops. Also, two production options are likely: (a) glass produced from basic raw materials (sand, natronFootnote 1, etc.) or (b) raw materials containing copper and lead added to the glass characteristic of the given period (i.e. soda natron type). The presence of lead is known to have assisted in dissolving copper to form a homogeneous melt (Hughes 1972).
Given the crafting skills of the Celts, who are known to have been proficient in metalworking (specifically copper and lead-based), it seems likely that they were also able to produce opaque red glass by adding metal compounds to imported soda glass (Brun and Pernot 1992). The import of raw soda glass into La Tène Europe from primary Mediterranean workshops (primary glassmaking centres may have been in both Egypt and the Ancient Near East) is also mentioned in Rolland and Venclová (2021). The authors discuss several local secondary workshops (e.g. Němčice and Staré Hradisko, Moravia), where objects typical of the region (glass bracelets, beads) were produced from imported glass. Given the prevalence of certain goods in continental Europe, these objects were definitely made by local craftsmen. This supports the hypothesis that red glass was indeed produced in La Tène Europe.
While analyses of European La Tène glass are typically processed well (Rolland 2021), analyses of La Tène enamels are rather scarce and lack data regarding the content of trace elements (Brun and Pernot 1992). However, it is often the content of trace elements that can help determine the primary workshops of glass production (Shortland et al. 2007).
Another material applied to produce decorative inlays in the period of our interest (the La Tène period) is coral (Corallium rubrum type). Coral was primarily used in bronze, and more rarely in iron and gold jewellery, where, just as familiar glass discs, it was set in pre-prepared grooves, possibly fixed with a central rivet (Challet 1992). Its popularity considerably increased as early as the late Hallstatt period, i.e. in the 6th century BC, which can be linked to the intensification of trade and cultural ties between Transalpine Europe and the Mediterranean. Trading with red coral as a luxurious commodity can be related to the existence of social elites as consumers of this precious commodity. Coral artefacts prevalently come from rich tombs of the contemporary nobility, but several examples of coral were also uncovered in common settlements, e.g. two fibulae with a decorative foot and coral inlays (found in a mound near Rovná, southern Bohemia; dated to the turn of the 6th/5th century BC). The use of coral as an ornamental inlay increased in the 5th and 4th centuries BC (Fürst 2014). Surprisingly, finds of raw coral are documented in the Transalpine region tooFootnote 2.
As mentioned earlier, the fixation of non-metallic inlays onto their base was often realised with rivets. However, a significant finding lies in the discovery of preserved organic matter found between glass inlays and a metal base. A similar brown-black mass is described in (Challet 1992). The author believes that the material was intended to help fix the inlay to the metal object (using rivets passing through the hole), but also to provide an elastic connection between the fragile glass and the metal base. The author suggests that the substance used as a binder could actually be birch bark tar - this fact was also noted in a La Tène torc from grave 23 situated in the Gäufelden-Nebringen area in Germany (Müller 1989). However, the cited paper does not state what method was used for the characterization of the mass, nor does it present specific results. The work merely states that the binder applied consists of birch tar.
The use of birch tar as a binder is noted as early as the Middle and Younger Palaeolithic. (Niekus et al. 2019). Birch tar was used throughout European prehistory. For the Neolithic period, polished stone tools and chipped tools were identified as containing residues of tar, but the substance was also applied to ceramic vessels (Prokeš et al. 2014). In the Hallstatt period, tar also appeared on bronze vessels (Chytráček et al. 2019; Egg and Kramer 2016). Also, the literature recorded a case where tar was used as a binder to join two bronze hemispheres forming a pendant of a necklace dated to the Hallstatt D1 period (625-550 BCE; Courel et al. 2018).
The primary aim of the presented work was to put the objects studied in a European context typologically and to examine the materials (red opaque glass and coral) represented in the artefact. For the first time, we publish the data on the composition of red opaque glass used in cold inlays including the content of trace elements (using LA-ICP-MS). This creates a possibility to identify the primary glass workshops. Also, the scope of the study was extended to include the characterisation of the binder applied (using GC/MS) as a fixation medium, which further completes the information concerning the jewellery production of the given period.
Artefacts examined and archaeological context
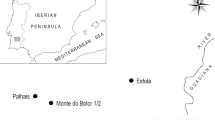
The artefacts analysed in this research study are shown in Fig. 1, including the geographical location of the finds.
Jewellery Examined and Map of Sites 1. Disc Torc (detail); Inv. No. H1 - 52559, Praha-Žižkov (Site Coordinates: 50°05'11.2"N 14°27'25.3"E); 2. (a): Bronze Pin, Inv. No. 107.242, Pustiměřské Prusy; 2. (b): Bronze Brooch, Inv. No. 107.243, Pustiměřské Prusy (49°18'00.4"N 17°01'25.7"E); 3. Bronze Brooch, Inv. No. - I 2733, Chotín (47°48'42.6"N 18°12'11.6"E), (Komárno Region, Slovakia). Detailed information on the inlays and dimensions can be found in the Catalogue of Presented Artefacts (S1)
The torc examined comes from an inhumation burial site located in Prague-Žižkov – Komenský Square (Fig. 1, No. 1), which was accidentally discovered during construction work in 1872. Finds from this burial site were made public as early as 1874 (Beneš 1874). The author of the paper pointed out the specific decoration of the torc our study examines as being in the form of a “small, enamelled disc”. Interestingly, the study (Beneš 1874) even analysed the chemical composition of the decorative inlays found on the torc, and the major components of the decorative glass such as SiO2, PbO and CuO were identified (including the relation between copper oxide and the red colour of the glass). Based on the burial inventory, the burial site is dated to the phase of LT B, i.e. from the 4th until the first part of the 3rd century BC. Generally, the torc (Fig. 2) does not fit in the domestic funeral inventory from the La Tène period. It is an opulent piece of jewellery, a so-called disc-shaped torc (Scheibenhalsringe), decorated with inlays made of opaque red glass fixed on bronze disc-shaped beds.
Distribution Map of Disc-Shaped Torcs – Type Scheibenhalsringe F (Müller 1989, Hlava 2017): 1 – Lovasberény; 2: Stetten; 3: Attenschwiller; 4: Diepflingen; 5: Eguisheim; 6: Andelfingen; 7: Bruchsal – Untergrombach; 8: Prague – Žižkov – the upper right corner shows a drawing of the artefact studied: two-part bronze torc with specific pointed closure
According to Müller (1989), the torc from Prague-Žižkov can be classified as group Scheibenhalsringe F (leichte Scheibenhalsringe mit gegossenem Dekor), characterized by a thinner hoop with fine cast decoration. This type of jewellery is most represented along the Upper Rhine River section in the area between the Vosges and Lake Constance (Müller 1989). In the context of this area, the local production of such torcs can be considered. Still, it is a rather exceptional find in the region of Central Europe (and it is the only known representative of this type in the Czech Republic). A similar torc was found in Hungary (Lovasberény), and a disc-shaped torc is also known from southwestern Slovakia (probably Lehota), but the original provenance of the Slovak torc is not entirely clear (Müller 1989). Based on the typological analysis, the torc from Prague - Žižkov is dated to LT B1 (390/380 – 330/320 BC). For phase LT B2 (320 – 260/250 BC), similar torcs are represented minimally, and they are typical with their robust types and cast decoration (Müller 1989; Westhausen 2017). The closest parallel to the Žižkov torc comes from a grave in Andelfingen, which has been dated to LT B2 (Pierrevelcin 2012). It can be stated that the occurrence of Scheibenhalsringe F torcs is centred around phase LT B1, but they have also been documented for later phase B2. The most striking elements of the Žižkov jewel are three disc-shaped beds decorated with red opaque glass. This decorative technique was widely used in the environment of the La Tène culture. Generally, such glass applications can be seen in ring ornaments - for instance, in the already mentioned torcs or different types of bracelets (Challet 1992).
Various inlays made of red glass or organic materials (also discussed below) were also applied to decorate so-called Münsingen brooches. There, the inlays were inserted into a pre-prepared bed and fixed with a central rivet. Subsequently, a form of an adhesive binder was applied to fix the inlays – the binder has sometimes remained preserved on certain pieces of jewellery in the form of a dark/black substance (Challet 1992).
To study this “substance”, two so-called Münsingen broochesFootnote 3 were selected from a burial site in Pustiměřské Prusy (Fig. 1; No. 2 a, 2b). As mentioned earlier, at phase LT B1 (beginning of the 4th century BC), Münsingen brooches started to appear in our territory, often decorated with various inlays, such as red glass, bone, and coral. The brooches of this type mostly come from grave contexts, but a smaller number of settlement finds have been documented as well. Münsingen brooches are characteristic of their loose disc-shaped base, and they can be found almost in the entire La Tène Europe, mainly concentrated around Switzerland and the Upper Rhine. They form a rather variable group differing in both the shape of the foot and the bow (Bujna 1998). The Swiss burial site of Münsingen contained brooches with bows decorated in the so-called "Waldalgesheim style" (dated to LT B1). However, bows can also be smooth, decorated with knobs (Pustiměřské Prusy), or massive and richly profiled, which is typical of later specimens (Hlava 2017; Müller 1998). The occurrence of Münsingen brooches is associated with phases LT B1a-LT B2a and exceptionally LT B2b (400/390 - 1st half of the 3rd century BC) (Bujna 1998). From the region of Bohemia, we can mention a fragment of a brooch coming from the site of Prague-Dejvice (Hlava 2017) and four brooches of the identical type from Prague-ĎábliceFootnote 4. Other known finds of brooches with presumed coral inlays come from Moravia, e.g. from Blučina, Brankovice, Brno and other localities (for a complete inventory see Bedáň 2018).
The last object evaluated in our set is a brooch of exceptional size (length 18 cm, see Fig. 1, No. 3) from Chotín (Slovakia). The massive brooch is decorated with a massive rosette, originally identified as made of shells (Zachar 1987). The object was uncovered during the excavation of 47 graves in the Chotín necropolis in 1971-72. The burial site dates to LTB2-C1 (320/310 –180 BC) (Ratimorská 1981). From the site, four finds of jewellery are known to contain organic inlay decorations (a bracelet and bronze brooches). The brooch examined (Fig. 1, No. 3) comes from female inhumation grave No. 22/72. It represents a later type of the Early La Tène brooch scheme with a disc foot, which can be classified as group BF-D4, type 6 and dated to LTB2 - LTB2b (Bujna 2003). In the context of the Slovak region, it represents a rather unique find as no other parallel objects have been identified in this area yet (so far, we do not even know any comparable analogy from abroad). Two brooches of a similar type were uncovered at the burial sites of Palarikovo and Veľká Maňa (Zachar 1987), but they were markedly smaller (3.5-4.5 cm). Also, Early La Tène brooches with a disc foot from group BF D1 – D4 were found at burial sites in Kamenín, Michal nad Žitavou and Dubník. However, those finds lack decorative coral inlays (Bujna 2003).
Analytical methods
An Olympus SZX9 stereomicroscope was used to obtain the basic documentation of the torc (Fig. 1 No. 1). Also, images of crystals in glass were acquired by scanning electron microscopy using a backscattered electron detector (SEM Hitachi S4700). Subsequently, the chemical composition of the metal base was determined by micro-XRF (EDAX-Orbis; S2) under an accelerating voltage of 50 kV. It was not necessary to extract a sample for analysis as the chamber of the instrument allows to measure even larger samples (the analysed site was cleaned in advance both from corrosion products and secondary contamination). LA-ICP-MS was used to determine the chemical composition of the glass, including the content of trace elements, and the sample was measured in the form of a polished resin puck (a small fragment of the glass was embedded in an epoxy resin block, ground and polished).
Laser ablation was performed with an excimer laser operating at a wavelength of 193 nm (Analyte Excite, Teledyne Cetac). Each circular spot was 50 μm in diameter. At a repetition rate of 10 Hz, the average fluence was 4.24 J/cm2. Elemental analysis was conducted using a high-resolution double-focusing sector field ICP-MS (Element 2, Thermo Fisher). The results are represented as an average of seven ablated spots in the sample. The measurement process of each spot lasted 30 s, followed by the washing time of 25 s. Before the start of the sample measurement, a 15 s blank measurement was performed to identify the background signal of each element. As an external calibrant, NIST SRM 610 synthetic glass (Jochum 2011) was utilised. The accuracy and precision of the analysis were verified by applying the measurement on NIST SRM 612 glass (S2), and Corning Archaeological Reference Glasses B and C (Adlington 2017). Isotope 29Si was applied as an internal standard for both resolutions. The time-resolved signal data obtained were processed using the Glitter software (van Achterbergh et al. 2001) to select signal areas free of any other mineral/fluid inclusions and inhomogeneities, to subtract the background signal and to recalculate intensities to concentrations using the external and internal calibration.
The Raman spectroscopy method was applied to study opaque glass particles (again, the sample was measured in the form of a polished resin puck). Raman spectroscopy was also utilised to study another important material, coral (the sample in the form of a fragment, without further preparation). Raman spectra were measured with a Thermo Scientific Raman dispersive spectrometer - model DXR Microscope, equipped with an Olympus confocal microscope. The excitation source was a 532 nm Nd:YAG laser with an input power of 10 mW (7 mW for coral samples), and a grid of 900 notches/mm was utilised. As a detector, a multichannel thermoelectrically cooled CCD camera was used. The samples were measured at 50x magnification with a measurement footprint of approx. 1 µm through the aperture of a 50 µm pinhole. Omnic 9 (Thermo Scientific) was employed to process the spectra obtained.
The organic binder identified on the reverse side of the inlays was measured using a Thermo Scientific Nicolet iN10 micro FTIR spectrometer with an MCT-A detector equipped with a germanium microATR attachment. The samples were measured at a magnification of 15x with a measurement trace of approx. 35x35 µm. The spectra were measured in the spectral range 4000 – 675 cm-1 at a resolution of 4 cm-1, with 128 spectral accumulations, and an aperture of 150 µm. Again, Omnic 9 (Thermo Scientific) was employed to process the spectra acquired.
GC/MS (gas chromatography-mass spectrometry) analyses were performed with an Agilent Technologies 8890 instrument connected to an FID and a TOF/MS from LECO. The separation of compounds was carried out in a Restek Rxi-5MS column (diameters 30 m x 250 μm x 0.25 μm. As a carrier gas, helium was utilised at a flow rate of 1.4 ml. The sampled material was crushed to fine powder. After the addition of the internal standard (5α-cholestane; 0.1% solution), the material (3 to 15 mg) was extracted with 6 ml chloroform/methanol mixture (2:3; v/v) at a lab temperature using an ultrasonic bath for 20 min. The extract was subsequently centrifuged, and the supernatant was taken for further analysis. The excess of the solvent was then evaporated under the stream of inert gas. For GC analyses, an aliquot was taken from the sample extracts, dried under the stream of inert gas and derivatized using a derivatization reagent BSTFA (N-O-(bis)trifluoroacetamide) at 70 °C for 1 h (after Correa-Ascencio and Evershed 2014). The excess of the derivatization reagent was then evaporated under a stream of inert gas, dissolved in a defined amount of n-hexane (HPLC quality), and injected into the column in GC. The identification of the compounds present was carried out using ChromaTOF software (LECO corp.), the spectra available in the literature and NIST MS library 2.4.
Results and discussion
Characterisation of glass inlay
The preservation state of the glass inlay in the torc analysed (Fig. 1 No. 1) can be seen in Fig. 3a. It is evident that the surface of the inlay is altered due to the corrosive action of the environment just as the underlying metalFootnote 5 showing a patina of greenish colour. The colour of the glass is determined by red crystals dispersed in the material (Fig. 3b). In the following picture (Fig. 3c), a brownish substance is visible between the glass inlays and the metal, the detail is shown in Fig. 3d.
Description of the Torc: (a) Glass inlay, machined in circles, with a drilled hole in the centre, production marks of red glass inlays reported by Challet (1992); (b) Sample taken as a polished resin puck, red crystals in colourless glass; (c) Residues of brownish substance between the inlay and the underlying metal; (d) Image of dark residue on the metal bed (the section after removing the inlay)
The chemical composition of the glass can be characterized as soda glass of the natron type (according to the contents of K2O and MgO reaching up to 1.5%; see Fig. 4) with a relatively high content of lead (33.2% PbO) and copper (7.8% CuO; respectively 7.05% Cu2O).
Scatter plot of K2O and MgO with glass inlay (from this study) compared to glasses of similar dating. Natron glasses are highlighted in the bottom left section. For comparison, older glasses of the “plant ash” type are shown as well (blue dots in the right section). For “glass inlay”, a so-called “reduced” composition was utilised; for comparative purposes, PbO and CuO (opacifier) were excluded, and the ‘reduced’ composition re – cast to 100 per cent was used; e.g. Davis and Freestone 2018
The results (Table 1 and Fig. 5) correspond well with the literature (Brun and Pernot 1992), where 5-10% Cu2O and 20-50% PbO are reported for La Tène enamels. Higher Fe2O3 contents are also mentioned there, often exceeding 1.5% (in our sample examined, 1.3% Fe2O3 was detected).
For glass in general, the iron content is usually related to the sand introduced. However, the content identified in our analysis does not correspond with the data available for colourless glass from the same period. For instance, Oikonomou et al. 2023 mention roughly 0.3% Fe2O3 for Hellenistic glass from Thebes (specifically in samples TH27 to TH29). Also, Oikinomou et al. (2018) state that a typical value of Fe2O3 detected in 1st Millennium BCE glass and mainly Roman glass is around 0.5 wt.%. Values above this mean could suggest the deliberate addition of Fe2O3.
Therefore, to modify melting conditions, the probable introduction of additional raw material can be assumed, particularly to provide a reducing environment that positively affects the formation of dendritic Cu2O crystals (Fig. 6). Cu2O crystals have a dual function in glass - they colour glass red and at the same time obscure it/make it opaque (as confirmed by Raman spectroscopy, see Fig. 7). The presence of cuprite (Cu2O) particles is often associated with a group of high-copper high-lead glass, also referred to as a "sealing wax" red variety (Freestone 2003).
Raman spectrum of crystals in red glass; with significant band for Cu2O at 216 cm-1 corresponding with literature (Gedzevičiūtė et al. 2009)
The possibility of comparing the chemical composition of enamel inlays with similar types of objects is rather limited, but certain results for a set of objects including the inlays are provided by Brun (1991). However, only major elements are listed there.
By comparing the composition of the glass inlays in Brun (1991) with our object, it can be concluded that they are quite similar. PbO values in the work discussed range from 34 to 39.2% PbO (compared to 33.2% detected in our torc). In contrast to conventional soda natron glass, glass inlays generally display lower contents of Na2O (ca. 9.8%) and CaO (values ca. 2 to 3.4%; adopted from Brun 1991). However, this mostly results from the increased contents of CuO and especially PbO. If we recalculate the values of our sample concerning PbO and CuO (normalizing the values to 100% without these two components), we obtain 14.5% Na2O and 6.1% CaO. The representation of the majority elements in natron La Tène glass is mentioned by Rolland-Venclová (2021), namely: 15-20% Na2O and about 8% CaO. It is evident that the values for the red glass examined are rather lower even after recalculation. In the mentioned work, La Tène glasses were divided into 5 separate groups (Rolland and Venclová 2021- Table 3), but our sample cannot be classified as any of them.
Given the results from LA-ICP-MS, our assessment can be shifted to focus on the possible area of glass production or the differentiation/use of sands from various geographical areas. As 181 ppm Sr and 29 ppm Zr were detected in the glass sample obtained, these levels suggest the use of sands from Egypt (see Fig. 8; more Shortland et al 2007). Another criterion may be the content of Y2O3 and ZrO2, which were used to identify glass from the 7th-1st centuries BC (Oikonomou et al. 2023). Comparing these data again suggests an Egyptian production area. Finally, La and Cr contents used by Walton et al (2009) can be mentioned. The higher La values (2.9 ppm) and lower Cr contents (6 ppm) detected in our object again correspond to the values measured in Egyptian glass (ratio of Cr/La presented in Fig. 9).
Scatter plot of Zr and Sr for glass inlay (in this study) compared with Egyptian and Mesopotamian Glasses (Shortland et al 2007)
Scatter plot of 1000*Zr/Ti and La/Cr for glass inlay (in this study) compared with Egyptian and Mesopotamian Glasses (Shortland et al 2007)
Characterisation of red coral
Figure 1 shows that the artefacts (2a, 2b and 3) are not decorated with an enamel inlay. Owing to their white colouring, visual determination alone can be ambiguous. For example, in connection with the brooch from Chotín (Fig. 1, sample 3), the text (Zachar 1987) states that the material used consists of shell fragments. Therefore, Raman spectroscopy was used to provide further characterization (Fig. 10).
Raman spectroscopy; sample obtained from brooch rosette from Chotín, Slovakia. Material identified as coral. White colouring occurs due to the ageing of the material, during which the original red colour and lustre are lost (Fürst et al. 2016)
As a result, the material was identified as coral. The bands found at 1518 cm-1 and 1130 cm-1 (Fig. 10) refer to red colouring. Similar values are presented in a study dealing with the assessment of the coral type Corallium rubrum by Raman spectroscopy (Fürst et al. 2016). The work describes two main spectroscopy bands as follows: stretching modes of C=C double bonds (ν1) at around 1520 cm-1 and C-C single bonds (ν2) at around 1130±15 cm-1 (bands representing unmethylated polyene pigments). The band around 1087 cm-1 belongs to the carbonate anion. Here, specifically, it is calcite to which the bands at 713 cm-1 and 285 cm-1 correspond as well. During the Iron Age (particularly between 600 and 100 BC), precious red corals (Corallium rubrum) became very popular in many regions of Central Europe, often associated with so-called (early) Celts (Fürst et al. 2016). Moreover, we cannot help but notice the appearance similar to red glass.
Coral inlays of the La Tène period are not documented just for brooches. They are also known from ring ornaments. This is, for instance, the case of the decoration of an exceptional saddle-shaped bracelet originating from the Moravian site of Pustiměřské Prusy (Čižmářová 2013). A La Tène period double grave (LT B1) from a site in Brno-Horní Heršpice contained a necklace rather unique for our territory consisting of glass beads and pierced branches of red coral (Meduna 1970)Footnote 6. Coral inlays were also applied to decorate swords and shields (Fürst 2014). For the region of the Czech Republic, we can mention the finds of shield fittings from Letki and Sulejovice (Moucha 1969)Footnote 7 and Brno-Maloměřice (Čižmářová 2005). An extraordinary example of a helmet entirely decorated with coral inlays comes from Agris (Lourdaux-Jurietti 2003). In the LT B2 stage and the Middle La Tène period, the occurrence of jewellery with coral gradually began to decline. It is unclear whether that happened due to the depletion of raw coral resources, the change of trade routes or a general change in taste (Moucha 1980; Fürst 2014).
The trade and processing of precious red coral is undoubtedly crucial to understanding its importance in the La Tène society. Nowadays, C. rubrum can primarily be found in the Mediterranean area, specifically near the coasts of North Africa, Spain, Portugal, France (Marseille and Corsica) and Italy (Sardinia, Sicily, Tyrrhenian Sea). It is also known to occur in the Red Sea and Atlantic Ocean between the Algarve and Cape Verde (Schrickel et al. 2013, Fürst et al. 2016). Coral was typically brought to Central Europe from the Mediterranean via Greek emporia and Etruscan settlements in northern Italy. Another important source of red coral (Corallium rubrum) lay in Phoenician/Punic settlements, which specialized in the gathering and processing of this material. Three main trade routes can be assumed here: through southern France along the Rhone and both the eastern and western Alps, most likely using the Great St. Bernard Pass (Fürst 2014; Moucha 1980).
Characterisation of organic binder
A binder of dark colour was detected in all objects examined. The following section deals with its detailed characterisation. In the first step, all dark-coloured samples were measured by infrared spectroscopy (a sample of the binder from the torc can be seen in Fig. 3d and the FTIR spectrum is provided in Fig. 11). This spectrum as detected (Fig. 11) corresponds to organic matter. The bands at 2926 and 2854 cm-1 and also the bands at 1456, 1377 and 729 cm-1 belong to alkyl groups of esters and acids (C-H bonds of aliphatic hydrocarbons), while bands occurring at 883 and 822 cm-1 indicate the presence of aromatic hydrocarbon bonds. Also, two bands at 1732 and 1711cm-1 are visible in the spectrum, being typical of the carbonyl group (C=O). Based on the available literature (Chen et al. 2022), the spectrum measured best matches the spectrum of birch tar. Significant bands were found in the range of 3000-2500 cm-1 and 1800-1500 cm-1. Generally, pine tar displays triple C-H stretching bands at 2954-2866 cm-1, but the spectrum of birch tar only shows two bands, which is consistent with our data. The above discussed doublet with maxima at 1732 and 1711 cm-1 is also characteristic of birch tar.
µATR-FTIR Spectrum of dark-coloured binder (sample No. 1); significant bands visible in the range of 3000-2500 cm-1 and 1800-1500 cm-1; Comparable to Chen et al. (2022). The spectra of all samples were identical, their representative is shown here
Natural birch tar is usually a mixture of several organic substances. Therefore, merely based on infrared spectra, it cannot be completely ruled out that those are, for instance, natural resins or other similar condensed substances, being a mix of substances with similar functional groups (mainly carboxylic, aliphatic, and aromatic hydrocarbon compounds). GC/TOF-MS method was thus chosen for more accurate determination.
The samples were examined for the presence of extractable organic compounds, especially pentacyclic triterpenes such as betulin or lupeol and their degradation products, thus confirming the presence of birch resin and tar residues (Regert et al. 2003; Regert et al. 2006, Rageot et al. 2019 or Stacey et al. 2020). The identification of triterpene compounds of birch resin and tar was carried out using mass spectrometry scanning the peaks characteristic of lupane (m/z 189 and 203) and oleanane families (m/z 190) (Rageot et al. 2019). The determination of triterpene compounds in sample No. 1, the torc, was hindered due to the very low weight of the available sample (4 mg of scraped material for extraction). However, several birch bark tar markers such as lup-20(29)-ene, lupenone, lupeol and betulin were detected owing to their characteristic peaks at m/z 189 and 203 (Fig. 12a). The analysis of samples No. 2a and 2b (Fig. 12b) has identified lupeol, betulin and their degraded markers lupenone, lup-20(29)-ene, and betulin heating degradative marker 3-oxoallobetulane confirming the presence of birch bark tar in extracted samples. Furthermore, samples No. 2a and 2b contained free fatty acids dominated by palmitic (C16:0), stearic (C18:0) and oleic (C18:1) acids, long-chain fatty acids dominated by behenic acid (C22:0) and also odd and even diacids. The presence of aliphatic odd and even n-alkanes and long linear fatty alcohols was also confirmed. Very long fatty acids and diacids, together with n-alkanes and fatty alcohols, probably originate from the degradation of the suberic polymer of birch bark during birch tar manufacturing (Rageot et al. 2019; Stacey et al. 2020). The presence of saturated long fatty acids C16:0 and C18:0 and mono palmitoyl glycerol may also suggest the mixing of birch tar with animal fat for further refinement to modify birch tar properties (Stacey et al. 2020; Rageot et al. 2019; Pietrzak 2012). It is important to note that all samples displayed some contamination (especially phthalates), possibly coming from modern glues and plastic packaging.
a Partial Ion Chromatogram of Sample Extracted from Torc (No. 1) at m/z 189 (Upper Peaks) and m/z 203 (Lower Peaks). b Total Ion Chromatogram of Sample No. 2b; Key: FA - Fatty Acids, DCA - Dicarboxylic Acids, MAG – Monoacylglycerol, ALK - Alkanes x - Contamination, TMS - Trimethyl Silyl Group, IS - Internal Standard, the symbol of asterisk designates other triterpenoid compounds from birch resin and tar
In sum, our results from both FTIR and GC-MS TOF measurements add significantly to the information regarding the use of birch tar as a historical binder. The authors of a more recent study (Courel et al. 2018) also identified birch bark tar as a binder in jewellery (due to the predominance of triterpenoids from the lupane series; determined by GC-MS).
The technique of gluing decorative inlays, made of red glass or other material, using tar is well documented as early as phase LT B1. For instance, birch tar was detected on an Amfreville helmet based on the determination of betulin (Lourdaux-Jurietti 2003). We can also mention the torc from Gäufelden-Nebringen (Müller 1989), which has already been discussed above. In the La Tène era, tar was not used in jewellery making only. We know that tar coatings were also applied to pottery, where they are often referred to as pitch coatings (Venclová eds. 2008), but the question remains whether they played a functional or just an aesthetic role.
Conclusions
The article primarily presents a complex research study into four exceptional artefacts dated to the Iron Age. Namely, it examines a bronze torc with disc inlays made of red opaque glass and three brooches. In the region of the Czech Republic, the torc represents a unique find most likely coming from the area located between the Vosges mountains and Lake Constance. Based on its typological examination, the torc may be dated to the phase of LT B1 (390/380 – 330/320 BC), or possibly to the beginning of LT B2 (320 – 260/250 BC).
The article also provides data related to the red glass used in the earliest enamel decorative techniques (in the form of inlays). Based on its major elements, the glass can be identified as a soda (natron) type, which is characteristic of the La Tène period. Furthermore, our research has also detected the presence of trace elements, which have been determined in this type of glass for the very first time. Besides the composition of the “basic” glass used, other elements have also been identified in higher amounts such as Pb, Cu, and Fe. These elements or raw materials containing them affected the technology of contemporary glass production and its resulting properties.
Another material applied to produce decorative inlays during the Early La Tène period is coral (Corallium rubrum type), and our study has indeed identified this material in the decorative rosette of the unique brooch from Chotín (originally assumed to be made of shells).
A key finding of the study lies in detecting pentacyclic triterpenoids (such as epilupeol, betulin, lupenone, lup-20(29)-ene and 3-oxoallobetulane in the material applied between the red glass inlay and the base metal of the torc, which contributes to the understanding of contemporary production technologies. The presence of these chemical substances confirms the application of birch tar. Used as a fixation medium, birch tar was also identified in other objects and different materials – specifically, in our study set, it was applied to bind a coral rosette to a base metal. The results obtained in our study expand the scope of knowledge on the use of birch tar in jewellery techniques in the La Tène period. The research also illustrates the occurrence of such unique finds in the region of Central Europe and provides an analogy for the objects examined.
Data Availability
No datasets were generated or analysed during the current study.
Notes
Natron is a combination of sodium salts and occurs as a natural evaporite on the shores of desert lakes such as Wadi Natrun in Egypt (Shortland et al. 2006). Used as a flux, natron was necessary to lower the melting point of silica (in the form of sand) to a level where molten glass could be produced. The third essential ingredient to produce stable glass is calcium, which acts as a stabilizer and improves glass chemical resistance. To produce glass, sand from coastal areas was applied containing shell residues that were probably the source of the calcium required.
For instance, we can mention Late Hallstatt to Early La Tène period hillforts of Heuneburg and Mont Lassois, or a slightly younger settlement of Bourguignonès-Morey. In Bohemia, partially worked coral branches were found at the Minice hillfort near Kralupy nad Vltavou (Trefný - Slabina 2015) and the settlement of Kounice (Doubek 2021), while raw red coral was uncovered in a Late Hallstatt settlement near Poříčany (Čtverák 1986; Chytráček 2002) and a settlement at Zvoleněves dating to the 3rd century BC (Moucha 1980; Sankot 2000).
In this case, only the extraction of the binder applied was possible. We were not able to extract a sample of the non-metallic inlays as it could destroy them due to the state of the material. However, the inlays there were not made of red glass.
Other sites can also be mentioned where brooches with similar decoration were uncovered, such as Prague-Veleslavín (Hlava 2017); and Kutná Hora-Karlova (Valentová - Sankot 2011). Finds of Münsingen brooches with preserved decorative inlays are documented from burial sites in Radovesice and Lovosice (Zápotocký 1973), and in a La Tène skeletal grave from Čelákovice (Špaček 1979).
The metal matrix of the torc was determined to be bronze with a low lead content (determined to be 88% copper, 9% tin and 3% Pb). The work (Challet 1992; Davis 2014) dealing with enamel objects from the La Tène period notes that this mixture is a typical metal alloy associated with this type of objects.
A similar artefact comes from Dürrnberg, Austria (Moucha 1980). An extraordinary find of a necklace consisting of glass beads and 90 drilled coral branches was reported from Dubník (Slovakia) by (Bujna 1989). Further evidence confirming the use of drilled coral branches comes from the La Tène period graves of Hatvan-Boldog and Kósd in Hungary (Meduna 1970).
Here, however, it is not certain whether it is Corallum rubrum or shells. In fact, several other materials used for jewellery decoration, such as bone, ivory, limestone, and shells, are also noted for the La Tène period (Fürst 2014).
References
Adlington LW (2017) The Corning Archaeological Reference Glasses: New Values for “Old” Compositions. Papers Institute Archaeol 27:1–8. https://doi.org/10.5334/pia-515
Arletti L, Rivi D, Ferrari G, Vezzalini, (2011) The Mediterranean Group II: analyses of vessels from Etruscan contexts in northern Italy. J Archaeol Sci 38:2094–2100. https://doi.org/10.1016/j.jas.2010.10.028
Bedáň L (2018) Využití nekovových materiálů ve výzdobě kovových předmětů v mladší době železné na Moravě. Bachelor thesis, Masaryk University
Beneš FX (1874) Starožitnosti nalezené v Žižkově. Památky archeologické 10:67–78
Brun N, Pernot M (1992) The opaque red glass of Celtic enamels from Continental Europe. Archaeometry 34:235–252. https://doi.org/10.1111/j.1475-4754.1992.tb00495.x
Brun N (1991) Etude de verres opaques celtiques et gallo-romains. Dissertation, Universite de Paris-Sud
Bujna J (1989) Das La Tène zeitliche Gräberfeld bei Dubník I. Slovak Archaeology 37:254–354
Bujna J (2003) Spony z keltských hrobov bez výzbroje z územia Slovenska. Typovo-chronologické triedenie LTB- a C1-spôn. Slovenská archeológia 51:39–108
Bujna J (1998) Münsingen-Rain und die keltischen Gräberfelder im mittleren Donaugebiet. Kontakte im Spiegel des frühlatènezeitlichen Fundmaterials. In: Müller F (ed.) Münsingen-Rain, ein Markstein der keltischen Archäeologie. Funde, Befunde und Methoden im Vergleich. Schriften des Bernischen Historischen Museums, Band 2. Bern. 171-203
Challet V (1992) Les Celtes et l`émail, C.T.H.S.
Chen S, Vahur S, Teearu A, Juus T, Zhilin M, Savchenko S, Oshibkina S, Asheichyk V, Vashanau A, Lychagina E, Kashina E, German K, Dubovtseva E, Kriiska A, Leito I, Oras E (2022) Classification of archaeological adhesives from Eastern Europe and Urals by ATR-FT-IR spectroscopy and chemometric analysis. Archaeometry 64:227–244. https://doi.org/10.1111/arcm.12686
Chytráček M, Chvojka O, Egg M, John J, Michálek J, Cícha J, Hladil J, Koník P, Kozáková R, Křivánek R, Kyselý R, Majer A, Novák J, Navelka J, Rašková Zelinková M, Stránská P, Světlík I, Šálková T (2019) Interdisciplinární výzkum knížecí mohyly doby halštatské v Rovné u Strakonic. Reprezentace sociální identity a symbolika uměleckého projevu elit starší doby železné. Památky Archeologické 110:59–172
Chytráček M (2002) Südwestböhmen im überregionalen Verkehrsnetz der Hallstatt- und Frühlatènezeit. In: Chytráček M et al. (eds) Archäologische Arbeitsgemeinschaft Ostbayern / West- und Südböhmen. 11. Treffen. 121-142
Čižmářová J (2005) Keltské pohřebiště v Brně-Maloměřicích. Ústav archeologické památkové péče. Brno
Čižmářová J (2013) Keltská pohřebiště na Moravě. Okresy Blansko a Vyškov. Brno
Correa-Ascencio M, Evershed RP (2014) High throughput screening of organic residues in archaeological potsherds using direct acidified methanol extraction. Analytical Methods 6:1330–1340. https://doi.org/10.1039/C3AY41678J
Courel B, Schaeffer P, Féliu C, Thomas Y, Adam P (2018) Birch bark tar and jewellery: The case study of a necklace from the Iron Age (Eckwersheim, NE France). J Archaeol Sci: Rep 20:72–79. https://doi.org/10.1016/j.jasrep.2018.04.016
Čtverák V (1986) A fortified settlement of the Late Hallstatt period at Poříčany (central Bohemia). In: Pleiner R, Hrala J (eds) Archaeology in Bohemia, 1981–1985. Prague. 109-114
Davis M, Freestone IC (2018) Trading North: Things That Travelled, 107–133. https://doi.org/10.2307/J.CTT21C4TB3.12
Davis M (2014) Technology at the transition: relationships between culture, style and function in the Late Iron Age determined through the analysis of artefacts. Dissertation, Cardiff University
Doubek F (2021) 22. Kounice, okr. Nymburk. In: Lutovský M et al. (eds.) Terénní výzkumy Ústavu archeologické památkové péče středních Čech v roce 2020. Archeologie ve středních Čechách. Ústav archeologické památkové péče středních Čech 25/2. Praha. 738
Egg M, Kramer D (2016) Die hallstattzeitlichen Fürstengräber von Kleinklein in der Steiermark: Die beiden Hartnermichelkogel und der Pommerkogel. Monographien des Römisch-Germanischen Zentralmuseums 125. Mainz
Sankot P (1998) „Münsinger Fibeln” aus den Gräberfeldern Böhmens. In: Müller F. (ed.): Münsingen/Rain, ein Markstein der keltischen Archäologie: Funde, Befunde und Methoden im Vergleich: Akten des Internationalen Kolloquiums "Das keltische Gräberfeld von Münsingen-Rain 1906–1996", Münsingen/Bern, 9.–12. Oktober 1996, 205–212
Fürst S, Müller K, Gianni L, Paris C, Bellot-Gurlet L, Pare CFE, Reiche I (2016) Raman Investigations to Identify Corallium rubrum in Iron Age Jewelry and Ornaments. Minerals 6:56. https://www.mdpi.com/2075-163X/6/2/56
Fürst S (2014) Korallen am Übergang zur Früh La Tène zeit - zum wissenschaftlichen Potenzial eines problematischen Schmuckmaterials. In: Hornung S (ed) Produktion Distribution-Ökonomie: Siedlungs- und Wirtschaftsmuster der Latene zeit. Akten des Internationalen Kolloquiums in Otzenhausen. Bonn,. 41-66
Gedzevičiūtė V, Welter N, Schüssler U et al (2009) Chemical composition and colouring agents of Roman mosaic and millefiori glass, studied by electron microprobe analysis and Raman microspectroscopy. Archaeol Anthropol Sci 1:15–29. https://doi.org/10.1007/s12520-009-0005-4
Hlava M (2017) Laténská pohřebiště v Praze: nálezy do roku 1981. Muzeum hlavního města Prahy, Praha
Hughes MJ (1972) A technical study of opaque red glass of the Iron Age in Britain. Proc Prehistoric Soc 38:98–107. https://doi.org/10.1017/S0079497X00012068
Freestone Ian, Stapleton C, Rigby V (2003) The production of red glass and enamel in the Later Iron Age, Roman and Byzantine periods. In: Entwistle C (ed) Through a Glass Brightly - Studies in Byzantine and Medieval Art and Archaeology Presented to David Buckton. Oxbow. 142-154
Jochum KP, Weis U, Stoll B, Kuzmin D, Yang Q, Raczek I, Jacob DE, Stracke A, Birbaum K, Frick DA, Günther D, Enzweiler J (2011) Determination of Reference Values for NIST SRM 610–617 Glasses Following ISO Guidelines. Geostandards Geoanalytical Res 35:397–429. https://doi.org/10.1111/j.1751-908X.2011.00120.x
Lourdaux-Jurietti S (2003) L´utilisation du corail sur le casque de la grotte des Perrats a Agris (Charente). In: Buchsenschutz O, Bulard A, Chardenoux MB, Ginoux N (eds): Décors, images et signes de l´age du Fer européen, XXVIe colloque de l´AFEAF, théme spécialisé. Tours. 113-120
Meduna J (1970) Laténské pohřebiště v Brně-Horních Heršpicích. Památky archeologické LXI:225-235
Moucha V (1969) Latenezeitliche Gräber aus Sulejovic)e in Nordwestböhmen. Archaeological Research XXI:596-617
Moucha V (1980) Corallium Rubrum (L.) in a latent pit in Zvoleněves (o. Kladno). Archaeological Research XXXII:512-520
Müller F (1989) Die frühlatènezeitlichen Scheibenhalsringe. Verlag Philipp von Zabern, Mainz
Müller F (1998) Die Entwicklung des Waldalgesheimstils in Münsingen-Rain. In: Müller F (ed) Münsingen-Rain, ein Markstein der keltischen Archäeologie. Funde, Befunde und Methoden im Vergleich. Schriften des Bernischen Historischen Museums, Band 2. Bern, pp 71-83
Niekus MJLT, Kozowyk PRB, Langejans GHJ, Ngan-Tillard D, van Keulen H, van der Plicht J, Cohen KM, van Wingerden W, van Os B, Smit BI, Amkreutz LWSW, Johansen L, Verbaas A, Dusseldorp GL (2019) Middle Paleolithic complex technology and a Neandertal tar-backed tool from the Dutch North Sea. Proc National Acad Sci. 116:22081–22087. https://doi.org/10.1073/pnas.1907828116
Oikonomou A, Triantafyllidis P (2018) a) An archaeometric study of Archaic glass from Rhodes, Greece: Technological and provenance issues. J Archaeol Sci: Rep 22:493–505. https://doi.org/10.1016/j.jasrep.2018.08.004
Oikonomou A, Henderson J, Gnade M et al (2018) b) An archaeometric study of Hellenistic glass vessels: evidence for multiple sources. Archaeol Anthropol Sci 10:97–110. https://doi.org/10.1007/s12520-016-0336-x
Oikonomou A, Kaparou M, Šelih VS, van Elteren JT, Zacharias N, Chenery S, Henderson J (2023) Theban Glass Traditions in the 1st Millennium BCE, Greece: New LA-ICP-MS Data and Their Archaeological Implications. Heritage 6:705–723. https://doi.org/10.3390/heritage6010038
Pierrevelcin G (2012) Les relations entre la Bohême et la Gaule du IVe au Ier siècle avant J.-C. In: Klápště J, Měřínský Z. (eds.) Dissertationes archaeologicae Brunenses/Pragensesque, Charles University in Prague, Prague
Pietrzak S. (2012) Wood Tars in the Dnieper and Elbe Communities: 6th – 2nd Millenium BC. Baltic-Pontic-Studies 17. Uniwersytet im. Adama Mickiewicza, Poznań
Prokeš L, Procházková M, Kuča M, Parma D, Fojtík P, Humpola D (2014) Identifikace tmavých smolných hmot z neolitických nálezů na Moravě. Sborník prací Filosofické fakulty brněnské university. Řada Archeologická 58–59:113–130
Rageot M, Théry-Parisot I, Beyries S, Lepère C, Carré A, Mazuy A, Filippi J-J, Fernández X, Binder D, Regert M (2019) Birch Bark Tar Production: Experimental and Biomolecular Approaches to the Study of a Common and Widely Used Prehistoric Adhesive. J Archaeol Method Theory 26:276–312. https://doi.org/10.1007/s10816-018-9372-4
Ratimorská P (1981) Keltské pohrebisko v Chotíne I. Západné Slovensko 8:15–79
Regert M, Garnier N, Decavallas O, Cren-olivé C, Rolando C (2003) Structural characterization of lipid constituents from natural substances preserved in archaeological environments. Measure Sci Technol 14:1620–1630. https://doi.org/10.1088/0957-0233/14/9/313
Regert M, Alexandre V, Thomas N, Lattuati-Derieux A (2006) Molecular characterisation of birch bark tar by headspace solid-phase microextraction gas chromatography–mass spectrometry: A new way for identifying archaeological glues. J Chromatog A 1101:245–253. https://doi.org/10.1016/j.chroma.2005.09.070
Rolland J, Venclová N (2021) Iron Age glass-working in Moravia, Central Europe: new archaeometric research on raw glass and waste - 3rd-first century BC. Archaeological Anthropological Sci 13:124. https://doi.org/10.1007/s12520-021-01374-5
Rolland J (2021) Le verre de l'Europe celtique : Approches archéométriques, technologiques et sociales d'un artisanat du prestige au second âge du Fer. Sidestone Press
Sankot P (2000) Ein Beitrag zu den Wegen und dem kulturellen Austausch zwischen Ostbayern, Österreich nd Böhmen in der Stufe LT A. In: Chytráček M et al. (eds.) Archäologische Arbeitsgemeinschaft Ostbayern / West- und Südböhmen. 11. Treffen. 143-149
Schrickel M, Bente K, Fleischer F, Franz A (2013) Importation ou imitation du corail à la fin de l'âge du Fer? Première approche par analyses du matériau. In: Colin A, Verdin F (eds) L'âge du Fer en Aquitaine et sur ses marges. Mobilité des hommes, diffusion des idées, circulation des biens dans l'espace européen à l'âge du Fer. Bordeaux. 753-759
Shortland A, Schachner L, Freestone I, Tite M (2006) Natron as a flux in the early vitreous materials industry: sources, beginnings, and reasons for decline. J Archaeol Sci 33:521–530. https://doi.org/10.1016/j.jas.2005.09.011
Shortland A, Rogers N, Eremin K (2007) Trace element discriminants between Egyptian and Mesopotamian Late Bronze Age glasses. J Archaeol Sci 34:781–789. https://doi.org/10.1016/j.jas.2006.08.004
Špaček J (1979) Další laténský hrob z Čelákovic. Archeologické rozhledy 31:295–298
Stacey RJ, Dunne J, Brunning S, Devièse T, Mortimer R, Ladd S, Parfitt K, Evershed RP, Bull ID (2020) Birch bark tar in early Medieval England – Continuity of tradition or technological revival? Journal of Archaeological Science. Reports 29:102118. https://doi.org/10.1016/j.jasrep.2019.102118
Trefný M, Slabina M (2015) K nejdůležitějším aspektům architektury, hmotné kultury a k významu halštatského hradiště v Minicích (Kralupy nad Vltavou, okr. Mělník). Archeologické rozhledy 67:45–78
Valentová J, Sankot P (2011) Das latènezeitliches Gräberfeld in Kutná Hora-Karlov (okr. Kutná Hora), Mittelböhmen, Tschechische Republik. Eine Rettungsgrabung aus den Jahren 1988–1989. Jahrbuch des Römisch-germanisches Zentralmuseum, Mainz 58:279–401
Van Achterbergh E, Ryan CG, Jackson SE, Griffin WL (2001) Data reduction software for LA-ICP-MS. In: Sylvester PJ (ed) Laser ablation-ICP-mass spectrometry in the Earth Sciences: Principles and applications. Mineralogical Association of Canada, Ottawa, pp 239–243
Venclová N et al. (2008) Archeologie pravěkých Čech 7. Doba laténská. Praha
Walton MS, Shortland A, Kirk S, Degryse P (2009) Evidence for the trade of Mesopotamian and Egyptian glass to Mycenaean Greece. J Archaeolog Sci 36:1496–1503. https://doi.org/10.1016/j.jas.2009.02.012
Westhausen I (2017) Ein Ring sie ewig zu binden... - Halsringe der späten Hallstatt- und frühen Latènezeit aus Baden-Württemberg, dem Elsass und dem Schweizer Mittelland. In: Wilczek J (ed) Interdisciplinarité et nouvelles approches dans les recherches sur l’âge du Fer. Masarykova univerzita, Brno, pp 161–165
Zachar L (1987) Keltské umenie na Slovensku. Tatran, Bratislava
Zápotocký M (1973) Keltská pohřebiště na Litoměřicku. Archeologické rozhledy 25:139–184
Acknowledgements
The research was supported by Czech Science Foundation Grant No. 22-00828S Enamelled Archaeological Objects - Archaeometry Contribution to the Knowledge of Production Technologies. LA-ICP-MS measurements could be performed thanks to the institutional project RVO 67985831 at the Institute of Geology of the Czech Academy of Sciences.
Funding
Open access publishing supported by the National Technical Library in Prague. This research was funded by the Czech Science Foundation (GACR) – Project No. 22-00828S Enamelled Archaeological Objects - Archaeometry Contribution to the Knowledge of Production Technologies.
Author information
Authors and Affiliations
Contributions
ZZC wrote the main manuscript and performed SEM/EDS analysis including data evaluation. VČ analyzed archaeological data and interpreted them in the context of chemical analyses. VB performed GC/MS analysis including data evaluation. LL performed FTIR and Raman spectroscopy analysis including data evaluation. GB and HČ cooperate in preparing the archaeological part of the text. TK performed analyses of metal. ŠM performed LA-ICP-MS analyses including data evaluation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zlámalová Cílová, Z., Čisťakova, V., Brychová, V. et al. Non-metallic decorative inlays in La Tène jewellery - contribution of archaeometry to the understanding of production technologies. Archaeol Anthropol Sci 16, 60 (2024). https://doi.org/10.1007/s12520-024-01963-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12520-024-01963-0