Abstract
Invasive plants often alter ecosystem function and processes, especially soil N cycling. In eastern United States forests, the shrub Rosa multiflora (“rose”) is a dominant invader, yet potential effects on N cycling are poorly understood. Moreover, invasive plant management can impact soil N cycling by decreasing plant N uptake and disturbing the soil. The objectives of this study were to evaluate N cycling along a gradient of rose invasion (observational) and investigate potential changes to N cycling (manipulative) under four different management strategies: (1) do nothing (the control), (2) invasive plant removal, (3) removal followed by native seed mix addition, (4) removal, native seed mix, and chipped rose stem addition. We selected three forest sites experiencing a Low, Medium, or High amount of shrub invasion, and measured N cycling in the early (June) and late (September) growing seasons. We found N was immobilized in June and mineralized in September. One year after experimental management, removal alone had no effect on N cycling compared to control plots, but addition of native seed mix and chipped stems reduced early-season nitrification in our Medium invasion site. Our findings suggest that rose invasion may increase N cycling rates when soils are dry, which may occur more frequently with future climate change. In addition, N cycling responds differentially to management in the year following invasive plant removal, but most noticeably under moderate rose invasion.
Similar content being viewed by others
Introduction
Non-native invasive plants (hereafter “invasive”) pose a significant threat to biodiversity worldwide (e.g., Vitousek et al. 1996, 1997a), and the eastern U.S. is one of the most vulnerable regions (Early et al. 2016). In addition to negative effects on native biodiversity, invasive plants are often associated with alterations to ecosystem function and processes, such as decomposition (e.g., Ashton et al. 2005; Heneghan et al. 2007; Trammell et al. 2012), plant productivity (Ladwig and Meiners 2009; León et al. 2018), and biogeochemical cycling of nutrients (Vitousek et al. 1997c; Liao et al. 2008; Pellegrini et al. 2021). Changes in carbon (C) and nitrogen (N) cycling are cause for concern, as these cycles are inextricably linked. Nitrogen cycling has been greatly perturbed in the last 150 years (Vitousek et al. 1997b; Galloway et al. 2008; Fowler et al. 2013; Kanakidou et al. 2016), which can have cascading effects that ultimately impacts other ecosystems via eutrophication and atmospheric N deposition (Vitousek et al. 1997a; Galloway et al. 2003, 2004; Clark et al. 2017).
Since 1900, atmospheric N deposition has more than doubled (Sutton et al. 2011; Fowler et al. 2013) leading to N saturation in many terrestrial ecosystems (Vitousek et al. 1997b; Aber et al. 1998). Global reactive N emissions now far exceed what is naturally produced (Fowler et al. 2013; Kanakidou et al. 2016), beyond what may be considered a “safe operating space” (Rockström et al. 2009; Steffen et al. 2015). Excess N in terrestrial ecosystems can have detrimental effects, leading to declines in native plant diversity (e.g., Stevens et al. 2010; Boscutti et al. 2020), increased invasive plant abundance (e.g., Corbin and D’Antonio 2004), shifts in species composition and/or ecosystem type (Gilliam 2006; Bobbink et al. 2010), direct plant toxicity (Bobbink et al. 2010; Pardo et al. 2011), increased susceptibility of plants to extreme weather events (Grulke et al. 1998; Caporn et al. 2000; Friedrich et al. 2012), soil acidification (Stevens et al. 2010, 2018), loss of soil cations and reduced cation uptake efficiency (Pardo et al. 2011), and eutrophication caused by nitrate (NO3−) leaching (Stevens et al. 2018). While some of these environmental impacts have been addressed by enacting policies (Fowler et al. 2015; Gilliam et al. 2016; Reid and Aherne 2016) and regulations to control emissions (e.g., Clean Air Act), the effects of invasive plants have largely not been addressed and are often localized (e.g., Barney and Tekiela 2020; Pyšek et al. 2020). Therefore, there is a great need to understand how N cycling is altered in the presence and spread of invasive plants, and how restoration and management efforts can mitigate their effect.
In forests of the northeastern United States, a dominant invasive plant is Rosa multiflora (multiflora rose), a shrub native to eastern Asia (Kurtz and Hansen 2013; Schulz and Gray 2013; Huebner et al. 2014; Trammell et al. 2020). While current Forest Inventory and Analysis (FIA) data and results of large-scale studies show this shrub as the most abundant, other invasive plants (e.g., Berberis thunbergii, Microstegium vimineum, Lonicera japonica, Celastrus orbiculatus) dominate these forests and are increasing as well. Previous studies have found invasive shrubs are associated with changes in soil N cycling (e.g., Ehrenfeld et al. 2001; Ehrenfeld 2003) due to various plant traits such as higher leaf litter quality and quantity (Jo et al. 2015, 2017; Lee et al. 2017), accelerated decomposition rates (Hawkes et al. 2005; Trammell et al. 2012), or shifts in microbial communities (Kourtev et al. 2002; Elgersma and Ehrenfeld 2011; McLeod et al. 2016). However, most studies have focused on invasion in a single site, or comparisons between invaded and uninvaded habitats, and it remains uncertain how soil N cycling is altered along a gradient of invasion. Here, we focus on R. multiflora due to its dominance in small forests across our region. Additionally, R. multiflora has one of the highest leaf N concentrations (Jo et al. 2016), and its litter decomposes faster and releases N quicker than other native and invasive plants (Ashton et al. 2005), which may contribute to increased nutrient cycling in forest soils. Thus, studying soil N cycling along a gradient of invasive plant abundance, and specifically R. multiflora invasion, can identify thresholds of density that influence soil N cycling rates, and assist in prioritizing forests for management interventions.
While studies have examined vegetation responses following forest restoration (e.g., Hartman and McCarthy 2004; Johnson and Handel 2016; Hopfensperger et al. 2019), to our knowledge it remains unclear how soil N cycling in forest ecosystems may respond to invasive shrub removal, and specifically removal of R. multiflora. Moreover, additional management and restoration strategies after invasive shrub removal may lead to further changes in N cycling. For example, Clark et al. (2019) found that C addition was the only remediation approach that decreased soil N availability, while prescribed burning, thinning, and liming had no effect. Similarly, other studies have shown C additions such as wood chips or mulch may stimulate microbial N immobilization by increasing C:N in soils (e.g., Homyak et al. 2008; Perry et al. 2010). Ultimately, we hope to understand whether soil N cycling can be altered to prevent or limit the spread of invasive shrubs in susceptible forests.
The overarching goal of this research was to study the abundance-impact of Rosa multiflora (hereafter “rose”) on soil N cycling using an observational (fine-scale) and manipulative experimental approach in three forests with varying invasion densities. In this study, we assessed soil N cycling rates (i.e., potential net N mineralization and net nitrification) and soil C:N under a gradient of rose cover, and we conducted a rose removal experiment to understand how short-term soil N processes respond to invasive plant removal. Specifically, we asked the following questions: (1) What are the fine-scale patterns (i.e., within forest sites) in potential mineralization rates under varying rose density, the density of other woody and herbaceous plants (i.e., non-rose stem density), and soil water content?, (2) How will rose removal influence mineralization rates?, (3) How will N mineralization rates respond to additional management strategies (i.e., native seed mix addition and C-rich soil amendment) within 1 year?, and (4) How does seasonality (early compared to late growing season) influence soil N cycling before and after management?
We expected soil N cycling rates to increase with increasing rose stem density, and in forest sites with greater rose invasion. Following invasive plant removal, we expected larger reductions in N cycling where invasion density was greatest, with smaller reductions as invasion density decreased due to fewer shrubs affecting soil N cycling. Furthermore, we expected the addition of seed mix would lead to increased plant N uptake (from new grass and sedge germinants; Moore et al. 2023), thereby reducing nitrification via lower soil ammonium (NH4+) pools. We expected the application of C-rich rose stems (as mulch) would directly increase soil C:N, slowing N cycling rates, and the net change in %C (and thus molar C:N) would be positive and greater than the control group, while net change in %N should decrease. We expected to see differences in soil N cycling rates between peak growing season (warm, wet) and late growing season (warm, dry), specifically if soil moisture regimes influence rose impacts on soil N cycling rates.
Methods
Study area and design
We chose three forest sites that are part of a long-term urban forest network called the FRAME (FoRests Among Managed Ecosystems; https://sites.udel.edu/frame/) for soil analyses and invasive plant removal. The selected sites were located in the piedmont region of northern Delaware and extreme southeast Pennsylvania within 13 km of one another. For the region, mean annual temperature is 13.2 °C and mean annual precipitation is 119.3 cm year−1 (NOAA 2020). Due to their proximity, differences in precipitation among sites were minimal or non-existent; thus, differences in soil moisture likely reflect different biotic (vegetation, microbial community) or abiotic conditions. Soils in this Piedmont region are mostly ultisols, and our sites were Fallsington loam, Brinklow channery loam, and Glenelg silt loam soil series.
In 2015, vegetation was sampled in all 38 FRAME sites, and sites were classified along an invasion gradient (Trammell et al. 2020) based on the number of non-native woody stems ha−1, of which 75% were rose stems. Sites were then categorized as experiencing Low invasion (0.2–25 non-native stems ha−1), Medium invasion (26–80.5 non-native stems ha−1), or High invasion (> 80.5 non-native stems ha−1) intensity. We selected one forest site from the Low, Medium, and High invasion categories to assess initial woody stem density and soil C and N content, to be followed by an invasive plant removal experiment.
In March of 2017, we surveyed each study forest site (i.e., Low, Medium, and High invasion) for invasive plant management locations (the “management zone”). Within each management zone, a 400 m2 patch of invasive shrubs (the “removal area”) was selected for invasive plant removal, to be carried out the following year. Though each forest site differed in invasion intensity, we focused on where the rose invasion was greatest in each site; thus, the size (400 m2) and amount of non-native shrub cover within the management zone was consistent across sites (Fig. 1a). At each forest site, the management zone had to meet the following criteria: (1) invasive shrub cover, particularly rose, must be dense enough to establish 12 plots per site (9 treatment plots within the removal area and 3 rose-invaded control plots beyond its perimeter; see below for further details on the treatments), (2) plot size must be sufficiently small (4 m2) to accurately sample stem densities, and (3) each plot must be at least 1 m away from any other plots and/or trees (Fig. 1b).
Vegetation sampling
In 2017, prior to invasive plant removal, we collected abundance data for all trees, shrubs, and herbaceous vegetation in each control (n = 3 site−1) and treatment plot (n = 9 site−1) at each forest site (n = 3 sites). All plots were 4 m2 (2 m × 2 m). We revisited each plot for post-removal vegetation sampling in 2018 and 2019 to assess plant abundance following invasive plant removal (Moore et al. 2023). All tree, shrub, and herbaceous stems were counted across all individuals of a given species during peak growing season, beginning in July. For multi-stemmed shrubs, rooting at the nodes when in contact with the ground (“layering”) was not observed in plots. Thus, each tree, shrub, and herb stem visibly emerging from the ground was defined as a single stem and counted. If identification to species was not possible, plants were identified to genus. Data were divided into rose stem density and non-rose stem density as potential drivers of soil N cycling rates.
Experimental removal
Our experimental design incorporated a control and three manipulative treatments (hereafter “management strategies”). The goal of the manipulations was to conduct “easy-to-implement” and “cost-effective” strategies for landowners, managers, and restoration professionals. Due to the constraints of the management zone and number of management strategies, sample sizes at each forest site were small (n = 3 per management strategy). Within each site, 12 plots were established for a total of 36 plots across the three forest sites. Treatment strategies were additive, such that each subsequent treatment augmented the methods of the previous strategy. They were:
-
(1)
Control—no invasive plant removal,
-
(2)
Removal—hand pulling or cutting invasive plants, followed by stump treatment with the herbicide glyphosate,
-
(3)
Seed mix—removal strategy with native plant seed mix amendment, and
-
(4)
Mulch—removal and seed mix strategy with the addition of the cut/pulled rose stems, chipped and applied as mulch.
Implementation of management strategies
In February and March 2018, all invasive plants (i.e., trees/shrubs, vines, and herbs) within the 400 m2 removal zone were removed at each of our forest sites. Small plants were hand-pulled, while larger plants were cut at approximately 10 cm above ground. Herbicide (50.2% glyphosate solution) was applied to the resulting stump immediately afterward. Invasive stems were collected and allowed to air-dry at the study site for at least 6 weeks prior to chipping and mulching. In general, the resulting native plant community within each 400 m2 removal area (i.e., the removal, seed mix, and mulched treatment plots, plus surrounding space between them; see Fig. 1b) consisted of the shrubs Lindera benzoin, Viburnum dentatum, and Rubus spp.; seedlings and saplings of Acer rubrum, Liriodendron tulipifera, Liquidambar styraciflua, and Prunus serotina; woody and herbaceous vines; and numerous spring ephemerals, ferns, and other herbaceous plants (Moore et al. 2023).
In March 2018, a native seed mix was applied to plots in management strategies (3) and (4). Due to seasonal and local unavailability, we relied on seed collected from the midwestern U.S. (Prairie Moon Nursery, Winona, MN 55987). The seed mix was customized to include common, shade-tolerant native forest herbs and graminoids (grasses and sedges) present in or near our forest sites, and excluded species known to be palatable to deer (e.g., Trillium spp.) The seed mix consisted of 29 herbaceous and 7 graminoid (grass and sedge) species in quantities based upon seed size (Moore et al. 2023). Treatment plots (6 site−1) were hand sown and overseeded with 26.96 g of seed (6.74 g/m2).
In May 2018, R. multiflora (rose) stems were separated from other invasive stems that were cut during removal and chipped on-site using a Tazz K32 Chipper Shredder. Due to the large number of entangled stems, other woody invasive species may have unintentionally been chipped (e.g., Japanese barberry [Berberis thunbergii]), though care was taken to exclude them to the best of our ability. The resulting mass of chipped rose stems was similar at each site (Low = 10.55 kg, Medium = 10.65 kg, High = 10.55 kg). Within 3 days of mulching, 3.5 kg of chipped stems were applied by hand to each plot in the mulch treatment group (n = 3 site−1), forming a layer approximately ¼ inch thick on the soil surface.
Soil sampling and in vitro laboratory incubation
Soils were collected in June and September of 2017 (pre-removal year) and 2018 (first season post-removal) at each forest site. Our initial goal was to sample soils in 2019 (two seasons post-removal) as well, but due to building renovations and lab relocation in 2019, and the COVID-19 pandemic in 2020, we were unable to sample more than 1-year post-removal. In each plot (n = 12 site−1), leaf litter was removed prior to sampling, and a soil push probe was used to collect 2-cm diameter cores from the top 10 cm of soil. Three composite samples were taken per plot, bagged and homogenized. Soils were transported to the lab on ice and processed either upon arrival, or stored at 4 °C in a refrigerator and processed within 48 h after collection. Soils were sieved (2 mm) to remove rocks, roots, leaves, and other debris. Three 10 g (± 0.1 g) subsamples were weighed and used for determining soil moisture content, initial nitrate (NO3−) and ammonium (NH4+) content, and potential net nitrification and net N mineralization rates following a 30-day in vitro lab incubation. Incubations determine the net (not gross) amount for both nitrification and N mineralization, and these are referred to herein simply as Nitrification and N mineralization.
For gravimetric determination of soil moisture content, soils were weighed before being oven dried at 105 °C for 72 h, then removed and placed in a desiccator to allow cooling before re-weighing. Oven-dried soil mass was used to calculate the fresh-weight-to-dry-weight conversion factor and gravimetric soil moisture content [i.e., soil water content (SWC)], which exerts control over N cycling and is important in interpreting potential differences among sites. For the 30-day incubation, 10 g (± 0.1 g) of field-moist soil were placed into a tared, 250 mL Erlenmeyer flask, then sealed with parafilm. Parafilm was punctured with a small syringe needle to allow gas exchange. Flasks were placed in an incubator and kept at 25 °C for the duration of the incubation. Soils were incubated at field-level SWC values for the entire incubation period, thus exerting site-specific soil moisture on N cycling rates. To ensure that soil moisture was maintained at field moisture content values, flasks were weighed at least once per week. If the weight decreased by more than 1 g, an equivalent amount of DI water was injected into the flask through the parafilm using a small syringe. After approximately 30 days, soils were removed from the incubator and prepared for extraction.
Determination of nitrate, ammonium, and nitrogen mineralization rates
Prior to analysis, NO3− and NH4+ were extracted from soil using a 2 M KCl solution. For the soil samples collected in 2017, the extract was analyzed for NO3− and NH4+ by colorimetric spectrophotometry using a SEAL AQ2 Discrete Analyzer (SEAL Analytical, Mequon, Wisconsin 53092). Due to instrument down time, the SEAL AQ2 was used only for NH4+ quantification for June 2018 soil samples. For NO3− analyses on the June 2018 soil samples, a spectro::lyser V2 (s::can Messtechnik GmbH, Vienna, Austria) was used to measure NO3− via UV–Vis spectrometry. The September 2018 soil samples were analyzed for NH4+ and NO3− by colorimetric spectrophotometry using a SEAL Analytical AutoAnalyzer 3 flow injection analyzer at the University of Delaware Soil Testing Lab.
To ensure that NO3− concentration results were comparable across the analytical instruments, a randomly chosen subsample of soil extracts (n = 14) previously analyzed on the AQ2 in 2017 were analyzed on the other two instruments as well. Results of one-way ANOVA indicated no significant difference (P = 0.875) in the measured NO3− concentrations among these three instruments.
Potential nitrification was calculated as the net change in soil NO3−–N between initial soil collection and incubated extract following 30-day incubation. Potential N mineralization was calculated as the net change in soil NO3−–N plus NH4+–N between initial soil content and incubated extract.
Soil C and N
To determine total soil C and N, all soils from September 2017 and the control and mulch treatment group from September 2018 were ground using a ball mill (Mixer Mill MM200, Retsch, Haan, Germany). Soil C (%), N (%), and C:N were measured using an elemental combustion system (4010 CHNSO analyzer, Costech Analytical, Costech Valencia, CA, USA) interfaced with a Thermo Delta V Ratio Mass Spectrometer (Thermo, Bremen, Germany) at the University of Maryland Center for Environmental Science’s Appalachian Laboratory (Frostburg, MD). Soil C:N ratio was expressed on a molar basis.
Statistical data analysis
All data analyses were performed in R version 4.1.0 (R Core Team 2021) using the rstatix (Kassambara 2021) and relaimpo (Grömping 2006) packages. Significance was considered at the α = 0.05 level, but in some cases the α ≤ 0.10 critical values are reported to identify potential trends. For all analyses, data for June and September were analyzed separately since we were not directly testing for differences between growing seasons. After checking for normality and homoscedasticity of the data, we performed one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc multiple comparisons test to determine potential differences between forest sites in soil N cycling metrics (i.e., nitrification and N mineralization rates) and soil C and N metrics (i.e., soil %C, %N, and molar C:N) prior to management. To determine N cycling patterns across all sites, we first used the ‘calc.relimp’ function of relaimpo to assess relative importance of explanatory variables (rose stem density, non-rose stem density, and soil water content [SWC]), which considers the contribution of each variable in explaining variance (i.e., partitioned R2) in multiple linear regression models, and then determined R2 of the model for each month (June and September) by N cycling metric (nitrification and N mineralization rates) combination. For models that explained a considerable amount of variance (i.e., with R2 above 0.3 threshold), we report ‘lmg’ (i.e., partitioned R2, hereafter “Variable Effect Size”) for each explanatory variable and then perform simple linear regressions between explanatory variables and soil N cycling rates. Non-rose stem density was log-transformed (ln) to improve normality, and we report results of those analyses with transformed data.
To assess differences in soil N cycling among management strategies following invasive plant removal, we calculated the net change between pre- and post-removal (2017 to 2018) nitrification and N mineralization rates. Then, we performed one-way ANOVA followed by Tukey’s post-hoc test to determine significant differences among management strategies, analyzing each site and month of sampling separately. We also calculated net change in soil water content (SWC) and analyzed the change between years with a paired t-test for June and September separately. To determine potential differences in soil C and N metrics between the control and mulched treatment (i.e., C amendment) groups, we calculated the net change between the pre- and post-removal (2017 to 2018) soil %C, %N, and molar C:N, then used Welch’s t test due to small sample sizes (n = 3 plots strategy−1; 6 plots site−1) and differences (> threefold) in standard deviations between groups.
Results
Initial Soil N cycling and C:N
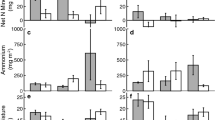
Prior to invasive plant removal and management, we analyzed differences in soil N cycling rates across forests (Fig. 2; Table 1 for means and Table S1 for results of one-way ANOVA and post-hoc analyses). In June, the Low invasion site had significantly lower nitrification and N mineralization compared to the Medium and High invasion sites (P < 0.01; Fig. 2a, b). In September, there was a shift from N immobilization to N mineralization, and nitrification was higher in the Low invasion site compared to the Medium (P < 0.001) and High invasion sites (P = 0.021; Fig. 2c). Furthermore, N mineralization was lower in the Medium invasion site compared to the Low invasion site in September (P = 0.060), though neither differed from the High invasion site (Fig. 2d).
Means (± 1 SE) of potential net nitrification and net N mineralization in the early (June) (a, b) and late (September) (c, d) growing season of 2017. Letters indicate significant* differences (P < 0.05) between sites. Note the different y-axes scales. *Indicates a trend toward significant differences (P < 0.10) between sites
Prior to experimental manipulation, we found significant differences in soil %C (F2,33 = 36.52, P < 0.001), %N (F2,33 = 25.18, P < 0.001), and molar C:N (F2,33 = 84.09, P < 0.001) across the sites. Soil C (%), N (%), and molar C:N were significantly highest in the Low invasion site, followed by the Medium invasion site, and lowest in the High invasion site (Table 2).
Soil N cycling along a gradient of invasion
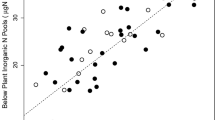
To understand how N cycling related to rose invasion, we sampled the plant community and determined species abundance (i.e., initial stem densities) prior to management. While many invasive shrubs were present, only two nonnative herbs (Alliaria petiolata, Cardamine impatiens) and one nonnative grass (Microstegium vimineum) were present; however, grasses were estimated as percent cover and were not included in stem counts (Moore et al. 2023). Relative importance analysis indicated that the models (i.e., rose stem density, non-rose stem density, and SWC) explained a considerable amount of variance (i.e., R2 > 0.30) for nitrification (R2 = 0.630) and N mineralization (R2 = 0.651) in June, and for nitrification (R2 = 0.349) in September (Fig. 3a–c). However, for N mineralization in September, the proportion of variance explained by the model was considerably low (i.e., R2 < 0.18; Fig. 3d). In June, soil water content (SWC) was most important for explaining N process rates (Variable Effect Size; nitrification = 0.38, N mineralization = 0.51), while rose stem density was most important for explaining nitrification in September (Variable Effect Size = 0.15).
For models with R2 > 0.3, relationships between rose stem density, SWC, non-rose stem density, and soil N cycling rates are reported. In the early growing season (June), potential nitrification (R2 = 0.271, P = 0.001) and N mineralization (R2 = 0.116, P = 0.042) decreased as rose density increased (Fig. 4a, b). Similarly, nitrification (R2 = 0.451, P < 0.001) and N mineralization (R2 = 0.566, P < 0.001) decreased with increasing SWC (Fig. 5a, b). The relationships between non-rose stem density and soil N cycling were opposite to the relationships observed with rose density; nitrification (R2 = 0.451, P < 0.001) and N mineralization increased (R2 = 0.374, P < 0.001) as non-rose stem density increased (Fig. 6a, b). Patterns in the late growing season (September) were mostly opposite those found in the early growing season. Potential nitrification increased with increasing rose density (R2 = 0.284, P < 0.001; Fig. 4c) and SWC (R2 = 0.146, P = 0.022; Fig. 5c), yet decreased as non-rose stem density increased (R2 = 0.295, P < 0.001; Fig. 6c).
Potential net nitrification and net N mineralization as a function of R. multiflora (rose) stems in the early (June) (a, b) and late (September) (c, d) growing season across all three forest sites. Equation, R2, P value, and trendline are shown for models with Variable Effect Size (Partitioned R2) > 0.30, as indicated by the R package ‘relaimpo’. Note the different y-axes scales
Potential net nitrification and net N mineralization as a function of Soil Water Content (SWC) in the early (June) (a, b) and late (September) (c, d) growing season across all three forest sites. Equation, R2, P value, and trendline are shown for models with Variable Effect Size (Partitioned R2) > 0.30, as indicated by the R package ‘relaimpo’. Note the different axes scales
Potential net nitrification and net N mineralization as a function of non-rose stem density (all herbs, shrubs, and trees except R. multiflora) in the early (June) (a, b) and late (September) (c, d) growing season across all three forest sites. Graphs show raw data, but R2 and P values are based on log-transformed (ln) non-rose stem density, and are shown for models with Variable Effect Size (Partitioned R2) > 0.30, as indicated by the R package ‘relaimpo’. Note the different y-axes scales
The proportion of N mineralization due to ammonification differed among our sites prior to management. In June, ammonification accounted for 22% of N mineralization at the Medium invasion site, yet less than 10% of N mineralization was from ammonification at the Low and High invasion sites. In September, ammonification accounted for more than 62% of N mineralization at the Medium invasion site, but 26% at the Low invasion site and 44% at the High invasion site was from ammonification (Table S2).
Soil N responses to invasive plant management
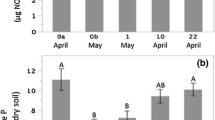
Within each of our forest sites, we found that removing invasive plants (i.e., strategy #2) had no effect on soil N cycling (i.e., nitrification and N mineralization) when compared to control plots (i.e., strategy #1; P > 0.10; Figs. 7, S1, S2; Table S3) after 1 year. However, post-removal treatments (i.e., addition of native seed mix [strategy #3] and invasive stems as mulch [strategy #4]) resulted in greater soil N cycling responses in the Medium invasion site in June (Fig. 7). Net change in nitrification rate was positive under all management strategies, though the increase was smaller in the seed mix (P = 0.067) and mulched (P = 0.061) treatment groups compared to the control group (Fig. 5a). There were no differences in N cycling metrics among management strategies at the Low and High invasion sites in either the early or late growing seasons (P > 0.10; Figs. S1, S2).
Mean net change (± 1 SE) in potential net nitrification and net N mineralization rates during early (June) (a, b) and late (September) (c, d) growing season in plots at the Medium Invasion site following management in 2018. Letters indicate trends toward significant differences (P < 0.10) between groups. Note the different y-axes scales
Within each forest site, amending the soil with carbon-rich, chipped rose stems resulted in site-specific changes in soil C and soil N metrics (%C, %N, and molar C:N) when compared to the control strategy (Fig. 8). In the Low invasion site, there were trends toward significant net decreases in mean soil C (%) and N (%) in the mulched treatment group (%C = -0.66 ± 0.19%; %N = -0.01 ± 0.02%) compared to the control group (%C = 0.22 ± 0.32%, P = 0.091; %N = + 0.05 ± 0.02%, P = 0.073; Fig. 8a, b). In the High invasion site, there were trends toward a significant net increase in mean soil N (%) and net decrease in mean soil C:N in the mulched treatment group (%N = 0.08 ± 0.03%; C:N = − 1.029 ± 0.423) compared to the control group (%N = -0.003 ± 0.012, P = 0.074; C:N = 0.392 ± 0.262, P = 0.057; Fig. 8h, i). There were no differences in soil C and N metrics in the Medium invasion site (P > 0.10).
Mean net change (± 1 SE) in soil % C, % N, and molar C:N in control and mulched treatment plots at the Low (a, b, c), Medium (d, e, f), and High (g, h, i) invasion sites following invasive plant removal in 2018. Letters indicate trends toward significant differences (P < 0.10) between groups. Note the different y-axes scales
Discussion
In general, we found differences in N cycling across forests along a gradient of rose density (Fig. 4), soil water content (SWC; Fig. 5), and non-rose stem density (Fig. 6) as expected. Assessing N cycling during early and late growing seasons also revealed differences in nitrification and N mineralization rates, and the importance of SWC and rose stem density in explaining N cycling varied by month (Fig. 3). Across sites, rose density was a better predictor of N cycling in September (Fig. 4), while SWC was a better predictor in June (Fig. 5). N cycling responses following invasive plant removal varied by site and among management strategies (Figs. 7, S1, S2). The addition of the seed mix, both with and without mulch, resulted in decreases in the June nitrification rate at the Medium invasion site compared to control plots (Fig. 7). Variation in N cycling responses to different management strategies emphasizes the importance of studying forests that differ along a gradient of invasion and understanding N cycling within invaded forests prior to management.
N Cycling within and across forests
Across our forest sites, N cycling differed by invasion intensity (Fig. 2) and was significantly related to rose invasion (i.e., rose stem density) during both seasons prior to invasive plant removal (Fig. 4). Previous studies have found increased rates of nitrification and N mineralization in the presence of invasive shrubs (e.g., Ehrenfeld et al. 2001; Ehrenfeld 2003; Jo et al. 2015), though we found this relationship was only true in September, and potential nitrification and N mineralization decreased with increasing rose stem density in June. In contrast, patterns between N cycling and non-rose stem density were opposite (Fig. 6) the patterns observed with rose stem density (Fig. 4). Thus, rose invasion influences soil N cycling rates in an opposite direction than all other plants in the same location. While other invasive shrubs have been shown to affect N cycling, rose is the most abundant invader in our study system (i.e., 83% of non-native stems prior to management), and we expected the effects of other invasive species to be minimal. These findings provide evidence that rose is driving changes in N cycling since the relationship between rose and N cycling rates are not a consequence of plant abundance, but the presence of rose shrubs specifically.
In the early growing season (June), soil water content was more important (i.e., had higher Variable Effect Size) than rose density and non-rose density in explaining variation in N cycling (Fig. 3a, b). However, the combined plant effects still explained considerable variation, and both rose density (P < 0.05) and non-rose density (P < 0.01) had clear impacts on N cycling (Fig. 4). All three sites had low C:N values (< 17:1; Table 2) indicative of microbial C-limitation and N mineralization prior to invasive plant removal and management. Therefore, organic matter quality may not explain the patterns we observed in N immobilization in the early growing season. Water quantity may be an important mechanism, and high SWC suggests gaseous N loss (i.e., denitrification, nitrous oxide [N2O] emission) may be occurring (Myers et al. 1982; Bateman and Baggs 2005; Robertson and Groffman 2015; De Neve 2017). Additionally, nitrification and N mineralization decreased as SWC increased (Fig. 5a, b) within our forest sites, suggesting that denitrification may play a prominent role in N cycling in the early growing season, when soils are wetter and plant dormancy has recently ended (Groffman and Tiedje 1989, Groffmann et al. 1993), in our system. Moreover, soil N pools of NH4+ and NO3− significantly increased in June, yet ammonification and nitrification significantly decreased with increasing SWC at these sites (Moore 2022), which may suggest an elevated amount of N2O is being produced during nitrification when soils are wetter (Bateman and Baggs 2005) and warmer (Inatomi et al. 2019). Thus, more study is needed to understand the relationship between rose and soil moisture across spatial and temporal scales. Other factors which we did not consider in this study, such as soil pH, texture and microbial community composition, may promote N immobilization under lab conditions, and future studies could investigate these potential relationships.
N cycling responses to management strategies
Overall, responses to management strategies varied within our forest sites, and did not support our hypothesis that removing invasive plants would decrease N cycling rates in the most invaded site within the time period of the experiment. Furthermore, unmanipulated control plots exhibited unexpected changes in N cycling across sites. For example, in 2018, all sites experienced a net increase in potential N mineralization in June, but a net decrease in September (Figs. 7, S1, S2). Changes in soil water availability between the 2 years may be responsible for these patterns. In 2017, soils were wetter in June and drier in September, but in 2018, soils were wetter in September and drier in June (Fig. S3). The year-to-year variation in SWC is thus an important driver of N cycling rates in this system, and may override plant influences, especially since rose had a stronger influence when soils were drier.
Post-removal treatments (i.e., seed mix addition, seed mix plus mulched stem amendment) at the medium invasion site led to a smaller net increase in nitrification rate compared to the control group (Fig. 5a). The vast majority of germinants from the seed mix in 2018 were sedges and grasses, though 5 of 29 herb species germinated in low abundances (Moore et al. 2023). This suggests that newly germinating plants were assimilating N from soils. Moreover, in 2019, we observed 24 of 29 herb species were present, many with relatively high abundance (Moore et al. 2023). Thus, a single year of management may not be sufficient to observe changes in N cycling among the various strategies in the Low and High invasion sites, and continued management over subsequent years may be necessary to observe differences in N cycling. Over time, net changes in plant communities (Moore et al. 2023) and soil amendments may increasingly influence N cycling post invasive plant removal.
Amending the soil with C-rich mulched stems (plus hand-sowing the native seed mix) after invasive plant removal resulted in changes to soil C and N metrics, yet the observed changes differed by site and did not follow our expectations (Fig. 8). In the Low invasion site, mulch amendment reduced soil N (%) as expected, yet also reduced soil C (%) unexpectedly (P < 0.10). The low invasion site primarily consisted of small rose shrubs that were hand-pulled (Moore et al. 2023), which could have caused the decreased soil C via belowground root removal. We cannot rule out the possibility there was not enough time for decomposition of mulch amendments to increase soil C content within 1 year. However, in the Medium invasion site, soil C increased and C:N decreased in the mulch treatment, though these patterns were not significant due to high variation and low sample size. In the high invasion site, soil C had a non-significant increase with the mulch addition and soil N (%) significantly increased (P < 0.10) leading to decreased C:N (P < 0.10) compared to control plots in the first year following management. Iannone et al. (2013) found that mulch from the invasive shrub Rhamnus cathartica (European buckthorn), with similar C:N (72:1) compared to our rose mulch (77:1), increased soil available N. Microbial access to mulch may be impeded initially due to the low surface area:volume of mulch (Cornwell et al. 2009), thereby limiting N immobilization and negating short-term effectiveness of mulch. However, rose stem mulch was more N-rich in the High invasion site compared to the other sites, which may have caused these observed changes in soil N (%) and C:N following the soil amendment management technique. This further suggests a positive feedback loop with N, as rose litter and stem quality may have had a long-term influence on soil conditions (higher available N) in the high invasion site with legacies of rose invasion. Thus, differences in C:N of the rose mulch between invasion densities suggests a possible threshold for management.
In this manipulated experiment, we found that addition of mulched stems reduced invasive shrubs in our highly invaded forest site (Moore et al. 2023), but we caution land managers to consider potential adverse effects on soil C and N if employing this strategy short-term, especially since it did not result in significant changes to N cycling that we expected to observe after 1 year of management in the high invasion site. Moreover, C:N declined under both control and mulched management strategies in the low and medium invasion sites suggesting that C-rich mulched stem addition following invasive plant removal is likely not enough to offset potential changes in soil C and N cycling due to other processes, such as decomposition, microbial respiration, or changes in soil moisture due to climate change. Thus, more time may be needed to incorporate this C-rich organic matter into soil C and N pools.
Conclusion
We found N cycling was related to rose invasion and SWC within sites, but these relationships varied by season. Rose density and SWC were unrelated in June 2017 (when soils were very wet), and SWC was the most important predictor of N cycling rates. However, SWC increased with increasing rose density in September 2017 when soils were drier, and rose density had the greatest effect on N cycling. Therefore, rose has a greater effect on nitrification, and thus N mineralization, when soils are less saturated, and invasion may have a more pronounced effect on soil N cycling in climates predicted to receive more moderate rainfall. Amending the soil with C-rich rose stems (chipped and applied as mulch) did not increase soil C:N within our sites, and is likely not a viable short-term management strategy, if the goal is to reduce nitrification and losses of N from the system quickly, given the additional cost and labor. While this study examined potential N cycling responses only in the year following invasive plant removal, it is possible that these changes take more time to occur. Our goal was to sample soils in 2019 as well, but we were unable to sample more than 1-year post-removal. However, in our assessment of the plant community in 2019 (Moore et al. 2023), we found greater differences 2 years post removal. Thus, long-term research could uncover meaningful patterns that may be associated with different management strategies. Continued research in forests along a gradient of invasion and evaluation of management strategies will be necessary to further comprehend the relationships between invasive shrubs and soil N cycling.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon request, and will be made publicly available on EDI post-review.
References
Aber J, McDowell W, Nadelhoffer K, Magill A, Berntson G, Kamakea M, McNulty S, Currie W, Rustad L, Fernandez I (1998) Nitrogen saturation in temperate forest ecosystems. Bioscience 48(11):921–934. https://doi.org/10.2307/1313296
Ashton IW, Hyatt LA, Howe KM, Gurevitch J, Lerdau MT (2005) Invasive species accelerate decomposition and litter nitrogen loss in a mixed deciduous forest. Ecol Appl 15(4):1263–1272. https://doi.org/10.1890/04-0741
Barney JN, Tekiela DR (2020) Framing the concept of invasive species “impact” within a management context. Invasive Plant Sci Manag 13(2):37–40. https://doi.org/10.1017/inp.2020.8
Bateman EJ, Baggs EM (2005) Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol Fertil Soils 41(6):379–388. https://doi.org/10.1007/s00374-005-0858-3
Bobbink R, Hicks K, Galloway J, Spranger T, Alkemade R, Ashmore M, Bustamante M, Cinderby S, Davidson E, Dentener F, Emmett B, Erisman J-W, Fenn M, Gilliam F, Nordin A, Pardo L, Vries WD (2010) Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecol Appl 20(1):30–59. https://doi.org/10.1890/08-1140.1
Boscutti F, Pellegrini E, Casolo V, de Nobili M, Buccheri M, Alberti G (2020) Cascading effects from plant to soil elucidate how the invasive Amorpha fruticosa L. impacts dry grasslands. J Vegetat Sci 31(4):667–677. https://doi.org/10.1111/jvs.12879
Caporn SJM, Ashenden TW, Lee JA (2000) The effect of exposure to NO2 and SO2 on frost hardiness in Calluna vulgaris. Environ Exp Bot 43(2):111–119. https://doi.org/10.1016/S0098-8472(99)00050-7
Clark CM, Bell MD, Boyd JW, Compton JE, Davidson EA, Davis C, Fenn ME, Geiser L, Jones L, Blett TF (2017) Nitrogen‐induced terrestrial eutrophication: cascading effects and impacts on ecosystem services. Ecosphere 8(7):e01877. https://doi.org/10.1002/ecs2.1877
Clark CM, Richkus J, Jones PW, Phelan J, Burns DA, de Vries W, Du E, Fenn ME, Jones L, Watmough SA (2019) A synthesis of ecosystem management strategies for forests in the face of chronic nitrogen deposition. Environ Pollut 248:1046–1058. https://doi.org/10.1016/j.envpol.2019.02.006
Corbin JD, D’Antonio CM (2004) Effects of exotic species on soil nitrogen cycling: implications for restoration. Weed Technol 18(sp1):1464–1467. https://doi.org/10.1614/0890-037X(2004)018[1464:EOESOS]2.0.CO;2
Cornwell WK, Cornelissen JHC, Allison SD, Bauhus J, Eggleton P, Preston CM, Scarff F, Weedon JT, Wirth C, Zanne AE (2009) Plant traits and wood fates across the globe: rotted, burned, or consumed? Glob Change Biol 15(10):2431–2449. https://doi.org/10.1111/j.1365-2486.2009.01916.x
De Neve S (2017) Organic matter mineralization as a source of nitrogen. In: Tei F, Nicola S, Benincasa P (eds) Advances in research on fertilization management of vegetable crops. Springer, New York, pp 65–83. https://doi.org/10.1007/978-3-319-53626-2_3
Early R, Bradley BA, Dukes JS, Lawler JJ, Olden JD, Blumenthal DM, Gonzalez P, Grosholz ED, Ibañez I, Miller LP, Sorte CJB, Tatem AJ (2016) Global threats from invasive alien species in the twenty-first century and national response capacities. Nature Commun 7, 12485. https://doi.org/10.1038/ncomms12485
Ehrenfeld JG (2003) Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 6(6):503–523. https://doi.org/10.1007/s10021-002-0151-3
Ehrenfeld JG, Kourtev P, Huang W (2001) Changes in soil functions following invasions of exotic understory plants in deciduous forests. Ecol Appl 11(5):1287–1300. https://doi.org/10.1890/1051-0761(2001)011[1287:CISFFI]2.0.CO;2
Elgersma KJ, Ehrenfeld JG (2011) Linear and non-linear impacts of a non-native plant invasion on soil microbial community structure and function. Biol Invasions 13(3):757–768. https://doi.org/10.1007/s10530-010-9866-9
Fowler D, Coyle M, Skiba U, Sutton MA, Cape JN, Reis S, Sheppard LJ, Jenkins A, Grizzetti B, Galloway JN, Vitousek P, Leach A, Bouwman AF, Butterbach-Bahl K, Dentener F, Stevenson D, Amann M, Voss M (2013) The global nitrogen cycle in the twenty-first century. Philos Trans R Soc b 368(1621):20130164. https://doi.org/10.1098/rstb.2013.0164
Fowler D, Steadman CE, Stevenson D, Coyle M, Rees RM, Skiba UM, Sutton MA, Cape JN, Dore AJ, Vieno M, Simpson D, Zaehle S, Stocker BD, Rinaldi M, Facchini MC, Flechard CR, Nemitz E, Twigg M, Erisman JW, Galloway JN (2015) Effects of global change during the 21st century on the nitrogen cycle. Atmos Chem Phys 15(24):13849–13893. https://doi.org/10.5194/acp-15-13849-2015
Friedrich U, von Oheimb G, Kriebitzsch W-U, Schleßelmann K, Weber MS, Härdtle W (2012) Nitrogen deposition increases susceptibility to drought—experimental evidence with the perennial grass Molinia caerulea (L.) Moench. Plant Soil 353(1):59–71. https://doi.org/10.1007/s11104-011-1008-3
Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB, Cosby BJ (2003) The nitrogen cascade. Bioscience 53(4):341–356. https://doi.org/10.1641/0006-3568(2003)053[0341:TNC]2.0.CO;2
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM, Michaels AF, Porter JH, Townsend AR, Vörösmarty CJ (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70(2):153–226. https://doi.org/10.1007/s10533-004-0370-0
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320(5878):889–892. https://doi.org/10.1126/science.1136674
Gilliam FS (2006) Response of the herbaceous layer of forest ecosystems to excess nitrogen deposition. J Ecol 94(6):1176–1191. https://doi.org/10.1111/j.1365-2745.2006.01155.x
Gilliam FS, Welch NT, Phillips AH, Billmyer JH, Peterjohn WT, Fowler ZK, Walter CA, Burnham MB, May JD, Adams MB (2016) Twenty-five-year response of the herbaceous layer of a temperate hardwood forest to elevated nitrogen deposition. Ecosphere 7(4):e01250. https://doi.org/10.1002/ecs2.1250
Groffman PM, Tiedje JM (1989) Denitrification in north temperate forest soils: spatial and temporal patterns at the landscape and seasonal scales. Soil Biol Biochem 21(5):613–620. https://doi.org/10.1016/0038-0717(89)90053-9
Groffmann PM, Zak DR, Christensen S, Mosier A, Tiedje JM (1993) Early spring nitrogen dynamics in a temperate forest landscape. Ecology 74(5):1579–1585. https://doi.org/10.2307/1940085
Grömping U (2006) Relative importance for linear regression in R: the package relaimpo. J Stat Softw 17(1):1–27. https://doi.org/10.18637/jss.v017.i01
Grulke NE, Andersen CP, Fenn ME, Miller PR (1998) Ozone exposure and nitrogen deposition lowers root biomass of ponderosa pine in the San Bernardino Mountains, California. Environ Pollut 103(1):63–73. https://doi.org/10.1016/S0269-7491(98)00130-4
Hartman KM, McCarthy BC (2004) Restoration of a forest understory after the removal of an invasive shrub, amur honeysuckle (Lonicera maackii). Restor Ecol 12(2):154–165. https://doi.org/10.1111/j.1061-2971.2004.00368.x
Hawkes CV, Wren IF, Herman DJ, Firestone MK (2005) Plant invasion alters nitrogen cycling by modifying the soil nitrifying community. Ecol Lett 8(9):976–985. https://doi.org/10.1111/j.1461-0248.2005.00802.x
Heneghan L, Steffen J, Fagen K (2007) Interactions of an introduced shrub and introduced earthworms in an Illinois urban woodland: impact on leaf litter decomposition. Pedobiologia 50(6):543–551. https://doi.org/10.1016/j.pedobi.2006.10.002
Homyak PM, Yanai RD, Burns DA, Briggs RD, Germain RH (2008) Nitrogen immobilization by wood-chip application: protecting water quality in a northern hardwood forest. For Ecol Manag 255(7):2589–2601. https://doi.org/10.1016/j.foreco.2008.01.018
Hopfensperger KN, Boyce RL, Schenk D (2019) Potential reinvasion of Lonicera maackii after urban Riparian Forest restoration. Ecol Restor 37(1):25–33. https://doi.org/10.3368/er.37.1.25
Huebner CD, Steinman J, Hutchinson TF, Ristau TE, Royo AA (2014) The distribution of a non-native (Rosa multiflora) and native (Kalmia latifolia) shrub in mature closed-canopy forests across soil fertility gradients. Plant Soil 377(1):259–276. https://doi.org/10.1007/s11104-013-2000-x
Iannone BV, Umek LG, Heneghan L, Wise DH (2013) Amending soil with Mulched European Buckthorn (Rhamnus cathartica) does not reduce reinvasion. Ecol Restor 31(3):264–273. https://doi.org/10.3368/er.31.3.264
Inatomi M, Hajima T, Ito A (2019) Fraction of nitrous oxide production in nitrification and its effect on total soil emission: a meta-analysis and global-scale sensitivity analysis using a process-based model. PLoS ONE 14(7):e0219159. https://doi.org/10.1371/journal.pone.0219159
Jo I, Fridley JD, Frank DA (2015) Linking above- and belowground resource use strategies for native and invasive species of temperate deciduous forests. Biol Invasions 17(5):1545–1554. https://doi.org/10.1007/s10530-014-0814-y
Jo I, Fridley JD, Frank DA (2016) More of the same? In situ leaf and root decomposition rates do not vary between 80 native and nonnative deciduous forest species. New Phytol 209(1):115–122. https://doi.org/10.1111/nph.13619
Jo I, Fridley JD, Frank DA (2017) Invasive plants accelerate nitrogen cycling: evidence from experimental woody monocultures. J Ecol 105(4):1105–1110. https://doi.org/10.1111/1365-2745.12732
Johnson LR, Handel SN (2016) Restoration treatments in urban park forests drive long-term changes in vegetation trajectories. Ecol Appl 26(3):940–956. https://doi.org/10.1890/14-2063
Kanakidou M, Myriokefalitakis S, Daskalakis N, Fanourgakis G, Nenes A, Baker AR, Tsigaridis K, Mihalopoulos N (2016) Past, present, and future atmospheric nitrogen deposition. J Atmos Sci 73(5):2039–2047. https://doi.org/10.1175/JAS-D-15-0278.1
Kassambara A (2021) rstatix: pipe-friendly framework for basic statistical tests. R package version 0.7.0. https://CRAN.R-project.org/package=rstatix
Kourtev PS, Ehrenfeld JG, Häggblom M (2002) Exotic plant species alter the microbial community structure and function in the soil. Ecology 83(11):3152–3166. https://doi.org/10.1890/0012-9658(2002)083[3152:EPSATM]2.0.CO;2
Kurtz CM, Hansen MH (2013) An assessment of multiflora rose in northern U.S. forests (NRS-RN-182). U.S. Department of Agriculture, Forest Service, Northern Research Station. https://doi.org/10.2737/NRS-RN-182
Ladwig LM, Meiners SJ (2009) Impacts of temperate lianas on tree growth in young deciduous forests. For Ecol Manag 259(2):195–200. https://doi.org/10.1016/j.foreco.2009.10.012
Lee MR, Bernhardt ES, van Bodegom PM, Cornelissen JHC, Kattge J, Laughlin DC, Niinemets Ü, Peñuelas J, Reich PB, Yguel B, Wright JP (2017) Invasive species’ leaf traits and dissimilarity from natives shape their impact on nitrogen cycling: a meta-analysis. New Phytol 213(1):128–139. https://doi.org/10.1111/nph.14115
León MMG, Izquierdo LM, Mello FNA, Powers JS, Schnitzer SA (2018) Lianas reduce community-level canopy tree reproduction in a Panamanian forest. J Ecol 106(2):737–745. https://doi.org/10.1111/1365-2745.12807
Liao C, Peng R, Luo Y, Zhou X, Wu X, Fang C, Chen J, Li B (2008) Altered ecosystem carbon and nitrogen cycles by plant invasion: a meta-analysis. New Phytol 177(3):706–714. https://doi.org/10.1111/j.1469-8137.2007.02290.x
McLeod ML, Cleveland CC, Lekberg Y, Maron JL, Philippot L, Bru D, Callaway RM (2016) Exotic invasive plants increase productivity, abundance of ammonia-oxidizing bacteria and nitrogen availability in intermountain grasslands. J Ecol 104(4):994–1002. https://doi.org/10.1111/1365-2745.12584
Moore ER (2022) Plant invasion in small forests: regeneration and restoration of native understory species [Ph.D. Dissertation, University of Delaware]. https://www.proquest.com/docview/2723556017/abstract/2CA0397E2E38426EPQ/1
Moore E, D’Amico V, Trammell TLE (2023) Plant community dynamics following non-native shrub removal depend on invasion intensity and forest site characteristics. Ecosphere 14(1):e4351. https://doi.org/10.1002/ecs2.4351
Myers RJK, Weier KL, Campbell CA (1982) Quantitative relationship between net nitrogen mineralization and moisture content of soils. Can J Soil Sci 62(1):111–124. https://doi.org/10.4141/cjss82-013
NOAA National Centers for Environmental information, Climate at a Glance: County Time Series, published June 2021 (2020). https://www.ncdc.noaa.gov/cag/. Accessed 15 June 2021
Pardo LH, Robin-Abbott MJ, Driscoll CT (2011) Assessment of Nitrogen deposition effects and empirical critical loads of Nitrogen for ecoregions of the United States. Gen. Tech. Rep. NRS-80. Newtown Square, PA: U.S. Department of Agriculture, Forest Service, Northern Research Station. p 291, 80, 1–291. https://doi.org/10.2737/NRS-GTR-80
Pellegrini E, Boscutti F, Alberti G, Casolo V, Contin M, De Nobili M (2021) Stand age, degree of encroachment and soil characteristics modulate changes of C and N cycles in dry grassland soils invaded by the N2-fixing shrub Amorpha fruticosa. Sci Total Environ 792:148295. https://doi.org/10.1016/j.scitotenv.2021.148295
Perry LG, Blumenthal DM, Monaco TA, Paschke MW, Redente EF (2010) Immobilizing nitrogen to control plant invasion. Oecologia 163(1):13–24. https://doi.org/10.1007/s00442-010-1580-x
Pyšek P, Hulme PE, Simberloff D, Bacher S, Blackburn TM, Carlton JT, Dawson W, Essl F, Foxcroft LC, Genovesi P, Jeschke JM, Kühn I, Liebhold AM, Mandrak NE, Meyerson LA, Pauchard A, Pergl J, Roy HE, Seebens H, Richardson DM (2020) Scientists’ warning on invasive alien species. Biol Rev 95(6):1511–1534. https://doi.org/10.1111/brv.12627
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reid H, Aherne J (2016) Staggering reductions in atmospheric nitrogen dioxide across Canada in response to legislated transportation emissions reductions. Atmos Environ 146:252–260. https://doi.org/10.1016/j.atmosenv.2016.09.032
Robertson GP, Groffman PM (2015) Nitrogen transformations. In: Paul EA (ed) Soil microbiology, ecology and biochemistry, 4th edn. Academic Press, Burlington, pp 421–446
Rockström J, Steffen W, Noone K, Persson Å, Chapin FSI, Lambin E, Lenton TM, Scheffer M, Folke C, Schellnhuber HJ, Nykvist B, de Wit CA, Hughes T, van der Leeuw S, Rodhe H, Sörlin S, Snyder PK, Costanza R, Svedin U, Foley J (2009) Planetary boundaries: exploring the safe operating space for humanity. Ecol Soc 14(2):140232. https://doi.org/10.5751/ES-03180-140232
Schulz BK, Gray AN (2013) The new flora of northeastern USA: quantifying introduced plant species occupancy in forest ecosystems. Environ Monit Assess 185(5):3931–3957. https://doi.org/10.1007/s10661-012-2841-4
Steffen W, Richardson K, Rockström J, Cornell SE, Fetzer I, Bennett EM, Biggs R, Carpenter SR, de Vries W, de Wit CA, Folke C, Gerten D, Heinke J, Mace GM, Persson LM, Ramanathan V, Reyers B, Sörlin S (2015) Planetary boundaries: guiding human development on a changing planet. Science 347(6223):1259855. https://doi.org/10.1126/science.1259855
Stevens CJ, Thompson K, Grime JP, Long CJ, Gowing DJG (2010) Contribution of acidification and eutrophication to declines in species richness of calcifuge grasslands along a gradient of atmospheric nitrogen deposition. Funct Ecol 24(2):478–484. https://doi.org/10.1111/j.1365-2435.2009.01663.x
Stevens CJ, David TI, Storkey J (2018) Atmospheric nitrogen deposition in terrestrial ecosystems: Its impact on plant communities and consequences across trophic levels. Funct Ecol 32(7):1757–1769. https://doi.org/10.1111/1365-2435.13063
Sutton MA, Howard CM, Erisman JW, Billen G, Bleeker A, Grennfelt P, van Grinsven H, Grizzetti B (2011) The European Nitrogen Assessment: sources, effects and policy perspectives. Cambridge University Press, Cambridge. https://doi.org/10.1017/CBO9780511976988
Trammell TLE, Ralston HA, Scroggins SA, Carreiro MM (2012) Foliar production and decomposition rates in urban forests invaded by the exotic invasive shrub, Lonicera maackii. Biol Invasions 14(3):529–545. https://doi.org/10.1007/s10530-011-0093-9
Trammell TLE, D’Amico V, Avolio ML, Mitchell JC, Moore E (2020) Temperate deciduous forests embedded across developed landscapes: younger forests harbour invasive plants and urban forests maintain native plants. J Ecol 108(6):2366–2375. https://doi.org/10.1111/1365-2745.13400
Vitousek PM, D’Antonio CM, Loope LL, Westbrooks R (1996) Biological invasions as global environmental change. Am Sci 84(1):468–478
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman DG (1997a) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7(3):737–750. https://doi.org/10.1890/1051-0761(1997)007[0737:HAOTGN]2.0.CO;2
Vitousek PM, D’Antonio CM, Loope LL, Rejmánek M, Westbrooks R (1997b) Introduced species: a significant component of human-caused global change. N Z J Ecol 21(1):17
Vitousek PM, Mooney HA, Lubchenco J, Melillo JM (1997c) Human domination of earth’s ecosystems. Science 277(5325):494–499. https://doi.org/10.1126/science.277.5325.494
Acknowledgements
We thank Maggie Bayalis, Aubrey Inkster, Covel McDermot, Samantha Nestory, Nathaly Rodriguez, Carl Rosier, Gavin Rosier, and Noah Totsline for field and lab assistance. We thank Meghan Avolio for her contribution toward data analysis approaches and Vince D’Amico for forest site establishment. We acknowledge the University of Delaware Research Foundation and Delaware Environmental Institute for funding. We thank the Delaware Environmental Institute for fellowship awarded to Eric Moore.
Funding
This work was funded by the University of Delaware Research Foundation and Delaware Environmental Institute (DENIN). Author Eric R. Moore was awarded a fellowship and received research support from DENIN.
Author information
Authors and Affiliations
Contributions
Study conception and design, formal analysis, and investigation were performed Eric R. Moore, Tara L. E. Trammell, and Richard V. Pouyat. Methodology was developed by Eric R. Moore and Tara L. E. Trammell. The first draft of the manuscript was written by Eric R. Moore. Tara L. E. Trammell and Richard V. Pouyat assisted with manuscript revisions. Funding, resources, and supervision were provided by Tara L. E. Trammell.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Cynthia Kallenbach
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moore, E.R., Pouyat, R.V. & Trammell, T.L.E. Soil nitrogen cycling in forests invaded by the shrub Rosa multiflora: importance of soil moisture and invasion density. Biogeochemistry 167, 301–319 (2024). https://doi.org/10.1007/s10533-024-01133-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-024-01133-3