Abstract
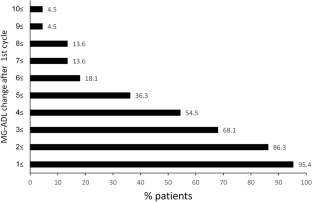
Recommendations for the treatment of myasthenia gravis (MG) have been difficult to develop because of limited evidence from large randomized controlled trials. New drugs and treatment approaches have recently been shown to be effective in phase 3 studies in seropositive generalized (g) MG. One such drug is efgartigimod, a human-Fc-fragment of IgG1, with a high affinity for the endosomal FcRn. We conducted a multicenter study to evaluate the real-world clinical and safety effects of efgartigimod in 22 gMG patients. We evaluated the strategies for the timing of re-treatment with it. The participants received a total of 59 efgartigimod -treatment cycles. The median number of cycles was 2 (range 1–6). Twenty patients (86.3%) improved by at least 2 MG-ADL points after the first treatment cycle. The median MG-ADL score at baseline was 6.5 (range: 3–17) and 2.5 (range: 0–9) post-treatment (p < 0.001). A consistent improvement of at least 2 points in the MG-ADL score after each cycle occurs in 18 patients. The effect duration of the treatment was usually between 4 and 12 weeks. Two major clinical patterns of treatment response were found. Treatment with efgartigimod was also associated with significant reductions of prednisone doses Overall, the treatment was safe and associated with only minor adverse events. The single fatality was apparently due tosevere respiratory failure. We found that efgartigimod is clinically effective, may be used as a steroid sparing agent and is generally safe for gMG patients. We recommend a personalized preventive treatment approach until clinical stabilization, followed by discontinuation and periodic evaluations.




Similar content being viewed by others
Data availability
The research data is available and will be provided after contacting the corresponding author.
References
Vincent A, Newsom-Davis J (1985) Acetylcholine receptor antibody as a diagnostic test for myasthenia gravis: results in 153 validated cases and 2967 diagnostic assays. J Neurol Neurosurg Psychiatry 48(12):1246–1252
Grob D, Brunner N, Namba T, Pagala M (2008) Lifetime course of myasthenia gravis. Muscle Nerve 37(2):141–149
Drachman DB (1994) Myasthenia gravis. N Engl J Med 330(25):1797–1810
Berrih-Aknin S, Le Panse R (2014) Myasthenia gravis: a comprehensive review of immune dysregulation and etiological mechanisms. J Autoimmun 52:90–100
Dresser L, Wlodarski R, Rezania K, Soliven B (2021) Myasthenia gravis: epidemiology, pathophysiology and clinical manifestations. J Clin Med 10(11):2235
McGrogan A, Sneddon S, de Vries CS (2010) The incidence of myasthenia gravis: a systematic literature review. Neuroepidemiology 34(3):171–183
Skeie GO, Apostolski S, Evoli A, Gilhus NE, Illa I, Harms L, Hilton-Jones D, Melms A, Verschuuren J, Horge HW (2010) Guidelines for treatment of autoimmune neuromuscular transmission disorders. Eur J Neurol 17(7):893–902
Sanders DB, Wolfe GI, Benatar M, Evoli A, Gilhus NE, Illa I, Kuntz N, Massey JM, Melms A, Murai H, Nicolle M, Palace J, Richman DP, Verschuuren J, Narayanaswami P (2016) International consensus guidance for management of myasthenia gravis: executive summary. Neurology 87(4):419–425
Narayanaswami P, Sanders DB, Wolfe G, Benatar M, Cea G, Evoli A, Gilhus NE, Illa I, Kuntz NL, Massey J, Melms A, Murai H, Nicolle M, Palace J, Richman D, Verschuuren J (2021) International consensus guidance for management of myasthenia gravis: 2020 update. Neurology 96(3):114–122
Gilhus NE (2016) Myasthenia gravis. N Engl J Med 375(26):2570–2581
Jani-Acsadi A, Lisak RP (2010) Myasthenia gravis. Curr Treat Options Neurol 12(3):231–243
Gilhus NE, Verschuuren JJ (2015) Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol 14(10):1023–1036
Pascuzzi RM, Coslett HB, Johns TR (1984) Long-term corticosteroid treatment of myasthenia gravis: report of 116 patients. Ann Neurol 15(3):291–298
Howard JF Jr, Utsugisawa K, Benatar M, Murai H, Barohn RJ, Illa I, Jacob S, Vissing J, Burns TM, Kissel JT, Muppidi S, Nowak RJ, O’Brien F, Wang JJ, Mantegazza R (2017) Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol 16(12):976–986
Meisel A, Annane D, Vu T, Mantegazza R, Katsuno M, Aguzzi R, Frick G, Gault L, Howard JF (2023) Long-term efficacy and safety of ravulizumab in adults with anti-acetylcholine receptor antibody-positive generalized myasthenia gravis: results from the phase 3 CHAMPION MG open-label extension. J Neurol 270(8):3862–3875
Howard JF Jr, Bresch S, Genge A, Hewamadduma C, Hinton J, Hussain Y, Juntas-Morales R, Kaminski HJ, Maniaol A, Mantegazza R, Masuda M, Sivakumar K, Śmiłowski M, Utsugisawa K, Vu T, Weiss MD, Zajda M, Boroojerdi B, Brock M, de la Borderie G, Duda PW, Lowcock R, Vanderkelen M, Leite MI (2023) Safety and efficacy of zilucoplan in patients with generalised myasthenia gravis (RAISE): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Neurol 22(5):395–406
Howard JF Jr, Bril V, Vu T, Karam C, Peric S, Margania T, Murai H, Bilinska M, Shakarishvili R, Smilowski M, Guglietta A, Ulrichts P, Vangeneugden T, Utsugisawa K, Verschuuren J, Mantegazza R (2021) Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 20(7):526–536
Bril V, Drużdż A, Grosskreutz J, Habib AA, Mantegazza R, Sacconi S, Utsugisawa K, Vissing J, Vu T, Boehnlein M, Bozorg A, Gayfieva M, Greve B, Woltering F, Kaminski HJ (2023) Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol 22(5):383–394
Pyzik M, Sand KMK, Hubbard JJ, Andersen JT, Sandlie I, Blumberg RS (2019) The neonatal Fc receptor (FcRn): a misnomer? Front Immunol 10:1540
Ulrichts P, Guglietta A, Dreier T, van Bragt T, Hanssens V, Hofman E, Vankerckhoven B, Verheesen P, Ongenae N, Lykhopiy V, Enriquez FJ, Cho J, Ober RJ, Ward ES, de Haard H, Leupin N (2018) Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans. J Clin Invest 128(10):4372–4386
Howard JF Jr, Bril V, Burns TM, Mantegazza R, Bilinska M, Szczudlik A, Beydoun S, Garrido FJRR, Piehl F, Rottoli M, Van Damme P, Vu T, Evoli A, Freimer M, Mozaffar T, Ward ES, Dreier T, Ulrichts P, Verschueren K, Guglietta A, de Haard H, Leupin N, Verschuuren JJGM (2019) Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis. Neurology 92(23):e2661–e2673
Riley TR, Douglas JS, Wang C, Bowser KM (2023) An update of the pharmacological treatment options for generalized myasthenia gravis in adults with anti-acetylcholine receptor antibodies. Am J Health Syst Pharm 80(11):652–662
Wolfe GI, Ward ES, de Haard H, Ulrichts P, Mozaffar T, Pasnoor M, Vidarsson G (2021) IgG regulation through FcRn blocking: A novel mechanism for the treatment of myasthenia gravis. J Neurol Sci 430:118074
Heo YA (2022) Efgartigimod: first approval. Drugs 82(3):341–348
VYVGART 2021 (efgartigimod alfa-fcab) injection, for intravenous use. US Food and Drug Administration (FDA) approved product information. Revised December 2021. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761195s000lbl.pdf (Accessed on June 22, 2023).
Nowak RJ, Coffey CS, Goldstein JM, Dimachkie MM, Benatar M, Kissel JT, Wolfe GI, Burns TM, Freimer ML, Nations S, Granit V, Smith AG, Richman DP, Ciafaloni E, Al-Lozi MT, Sams LA, Quan D, Ubogu E, Pearson B, Sharma A, Yankey JW, Uribe L, Shy M, Amato AA, Conwit R, O’Connor KC, Hafler DA, Cudkowicz ME, Barohn RJ (2022) Phase 2 trial of rituximab in acetylcholine receptor antibody-positive generalized myasthenia gravis: the BeatMG study. Neurology 98(4):e376–e389
Lascano AM, Lalive PH (2021) Update in immunosuppressive therapy of myasthenia gravis. Autoimmun Rev 20(1):102712
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors have no conflict interest to declare that are relevant to the content of this article.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fuchs, L., Shelly, S., Vigiser, I. et al. Real-World experience with efgartigimod in patients with myasthenia gravis. J Neurol (2024). https://doi.org/10.1007/s00415-024-12293-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00415-024-12293-5




