Abstract
The considerable changes in lifestyle patterns primarily affect the human gut microbiota and result in obesity, diabetes, dyslipidemia, renal complications, etc. though there are few traditional safeguards such as herbal brews to maintain the ecological stability under intestinal dysbiosis. The present article is designed to collect all the scientific facts in a place to decipher the role of the Indian traditional herbal brews used to balance gut health for centuries. Computerized databases, commercial search engines, research papers, articles, and books were used to search by using different keywords to select the most appropriate published articles from 2000 onward to September 2023. A total of 1907 articles were scrutinized, 46 articles were finally selected from the 254 screened, and targeted information was compiled. Interaction of herbal brews to the gut microflora and resulting metabolites act as prebiotics due to antimicrobial, anti-inflammatory, and antioxidant properties, and modulate the pH of the gut. The effect of brews on gut microbiota has a drastic impact on various gut-related diseases and has gained popularity as an alternative to antibiotics against bacteria, fungi, viruses, parasites, and boosting the immune system and strengthening the intestinal barrier. Berberine, kaempferol, piperine, and quercetin have been found in more than one brew discussed in the present article. Practically, these brews balance the gut microbiota, prevent chronic and degenerative diseases, and reduce organ inflammation, though, there is a knowledge gap on the molecular mechanism to explain their efficacy. Indian traditional herbal brews used to reboot and heal the gut microbiota since centuries-old practice with successful history without toxicity. The systematic consumption of these brews under specific dietary prescriptions has a hope of arrays for a healthy human gut microbiome in the present hasty lifestyle with overall health and well-being.
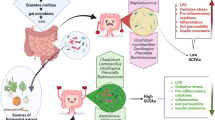
Graphical Abstract





Similar content being viewed by others
References
Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI (2005) Host-bacterial mutualism in the human intestine. Science 307:1915–1920. https://doi.org/10.1126/science.1104816
Sekirov I, Russell SL, Antunes LCM, Finlay BB (2010) Gut microbiota in health and disease. Physiol Rev 90:859–904. https://doi.org/10.1152/physrev.00045.2009
Muegge BD, Kuczynski J, Knights D, Clemente JC, Gonzalez A, Fontana L, Henrissat B, Knight R, Gordon JI (2011) Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science 332:970–974. https://doi.org/10.1126/science.1198719
Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R (2008) Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57:1470–1481. https://doi.org/10.2337/db07-1403
Bortolin RC, Vargas AR, Gasparotto J, Chaves PR, Schnorr CE, Martinello KB, Silveira AK, Rabelo TK, Gelain DP, Moreira JCF (2018) A new animal diet based on human Western diet is a robust diet-induced obesity model: comparison to high-fat and cafeteria diets in term of metabolic and gut microbiota disruption. Int J Obes 42:525–534. https://doi.org/10.1038/ijo.2017.225
Zhu C, Sawrey-Kubicek L, Beals E, Rhodes CH, Houts HE, Sacchi R, Zivkovic AM (2020) Human gut microbiome composition and tryptophan metabolites were changed differently by fast food and Mediterranean diet in 4 days: A pilot study. Nutr Res 77:62–72. https://doi.org/10.1016/j.nutres.2020.03.005
Wu H, Wu X, Huang L, Ruan C, Liu J, Chen X, Liu J, Luo H (2021) Effects of andrographolide on mouse intestinal microflora based on high-throughput sequence analysis. Front Vet Sci 8:702885. https://doi.org/10.3389/fvets.2021.702885
Faith JJ, McNulty NP, Rey FE, Gordon JI (2011) Predicting a human gut microbiota’s response to diet in gnotobiotic mice. Science 333:101–104. https://doi.org/10.1126/science.1206025
Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K, Ishikawa Y, Hara E, Ohtani N (2013) Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499:97–101. https://doi.org/10.1038/nature12347
Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB (2012) Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 487:104–108. https://doi.org/10.1038/nature11225
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505:559–563. https://doi.org/10.1038/nature12820
Singh RP, Halaka DA, Hayouka Z, Tirosh O (2020) High-fat diet induced alteration of mice microbiota and the functional ability to utilize fructo-oligosaccharide for ethanol production. Front Cell Infect Microbiol 10:376. https://doi.org/10.3389/fcimb.2020.00376
Swiątecka D, Arjan N, Karyn RP, Henryk K (2011) The study on the impact of glycated pea proteins on human intestinal bacteria. Int J Food Microbiol 145:267–272. https://doi.org/10.1016/j.ijfoodmicro.2011.01.002
Kim CH, Park J, Kim M (2014) Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw 14:277–288. https://doi.org/10.4110/in.2014.14.6.277
Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, Abrouk M, Farahnik B, Nakamura M, Zhu TH, Bhutani T, Liao W (2017) Influence of diet on the gut microbiome and implications for human health. J Transl Med 15:73. https://doi.org/10.1186/s12967-017-1175-y
Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti J-F, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R (2007) Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56:1761–1772. https://doi.org/10.2337/db06-1491
Kim KA, Gu W, Lee IA, Joh EH, Kim DH (2012) High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoSONE 7:e47713. https://doi.org/10.1371/journal.pone.0047713
Yang J, Yu J (2018) The association of diet, gut microbiota, and colorectal cancer: what we eat may imply what we get. Protein Cell 9:474–487. https://doi.org/10.1007/s13238-018-0543-6
Kopf JC, Suhr MJ, Clarke J, Eyun S, Riethoven JJM, Ramer-Tait AE, Rose DJ (2018) Role of whole grains versus fruits and vegetables in reducing subclinical inflammation and promoting gastrointestinal health in individuals affected by overweight and obesity: A randomized controlled trial. Nutr J 17:72. https://doi.org/10.1186/s12937-018-0381-7
Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto J-M, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, De Vos WM, Brunak S, Doré J, Weissenbach J, Ehrlich SD, Bork P (2011) Enterotypes of the human gut microbiome. Nature 473:174–180. https://doi.org/10.1038/nature09944
De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P (2010) Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 107:14691–14696. https://doi.org/10.1073/pnas.1005963107
Pagliai G, Russo E, Niccolai E, Dinu M, Di Pilato V, Magrini A, Bartolucci G, Baldi S, Menicatti M, Giusti B, Marcucci R, Rossolini GM, Casini A, Sofi F, Amedei A (2020) Influence of a 3-month low-calorie Mediterranean diet compared to the vegetarian diet on human gut microbiota and SCFA: the CARDIVEG Study. Eur J Nutr 59:2011–2024. https://doi.org/10.1007/s00394-019-02050-0
Wallace RK (2020) The microbiome in health and disease from the perspective of modern medicine and Ayurveda. Medicina 56:462. https://doi.org/10.3390/medicina56090462
Khan I, Ullah N, Zha L, Bai Y, Khan A, Zhao T, Che T, Zhang C (2019) Alteration of gut microbiota in inflammatory bowel disease (IBD): cause or consequence? IBD treatment targeting the gut microbiome. Pathogens 8:126. https://doi.org/10.3390/pathogens8030126
Francino MP (2016) Antibiotics and the human gut microbiome: Dysbiosis and accumulation of resistances. Front Microbiol 6. https://doi.org/10.3389/fmicb.2015.01543
Sreenisha SS, Dhanya S, Vineeth PK (2021) An Ayurvedic view on food (Ahara)-a review. Biol Life Sci Forum 6:19. https://doi.org/10.3390/Foods2021-11006
Tamang JP (2022) “Ethno-microbiology” of ethnic Indian fermented foods and alcoholic beverages. J of Appl Microbiol 133:145–161. https://doi.org/10.1111/jam.15382
Ames BN, Shigenaga MK, Hagen TM (1993) Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA 90:7915–7922. https://doi.org/10.1073/pnas.90.17.7915
Sharma PV (2013) Sushruta Samhita, Sutra Sthana, 28th edn. Varanasi, India, Chaukambha Visvabharati
Shim JM (2016) The relationship between the use of complementary and alternative medicine and the use of biomedical services: Evidence from East Asian medical systems. Asia Pac J Public Health 28:51–60. https://doi.org/10.1177/1010539515613411
Verma H, Mahapatra B (2019) Evaluation of an emerging medicinal crop kalmegh [Andrographis paniculata (Burm. f.) Wall. ex. Nees] for commercial cultivation and pharmaceutical & industrial uses: a review. J Pharmacogn Phytochem 8:835–848
Parham S, Kharazi AZ, Bakhsheshi-Rad HR, Nur H, Ismail AF, Sharif S, RamaKrishna S, Berto F (2020) Antioxidant, antimicrobial and antiviral properties of herbal materials. Antioxidants (Basel) 9:1309. https://doi.org/10.3390/antiox9121309
Hills R, Pontefrac B, Mishcon H, Black C, Sutton S, Theberge C (2019) Gut microbiome: profound implications for diet and disease. Nutrients 11:1613. https://doi.org/10.3390/nu11071613
Pandey MM, Rastogi S, Rawat AKS (2013) Indian traditional Ayurvedic system of medicine and nutritional supplementation. Evid based complement altern med 2013:1–12. https://doi.org/10.1155/2013/376327
Subhose V, Srinivas P, Narayana A (2005) Basic principles of pharmaceutical science in Ayurveda. Bull Indian Inst Hist Med Hyderabad 35:83–92
World Health Organization (2013) WHO: traditional medicine strategy: 2014–2023. World Health Organization, Geneva
Peterson CT, Denniston K, Chopra D (2017) Therapeutic uses of Triphala in Ayurvedic medicine. J Altern Complement Med 23:607–614. https://doi.org/10.1089/acm.2017.0083
Lopresti AL (2018) The problem of curcumin and its bioavailability: could its gastrointestinal influence contribute to its overall health-enhancing effects? Adv Nutr 9:41–50. https://doi.org/10.1093/advances/nmx011
Boonrueng P, Wasana PWD, Hasriadi VO, Rojsitthisak P, Towiwat P (2022) Combination of curcumin and piperine synergistically improves pain-like behaviors in mouse models of pain with no potential CNS side effects. Chin Med 17:119. https://doi.org/10.1186/s13020-022-00660-1
Di Meo MG, Crispi P (2019) Curcumin, gut microbiota, and neuroprotection. Nutrients 11:2426. https://doi.org/10.3390/nu11102426
Padhy S, Dash SK (2004) The Soma drinker of ancient India: an ethno-botanical retrospection. J Human Ecology 15:19–26. https://doi.org/10.1080/09709274.2004.11905661
Joshi DD, Deb L, Somkuwar BG, Rana VS (2023) Relevance of Indian traditional tisanes in the management of type 2 diabetes mellitus: a review. Saudi Pharm J 31:626–638. https://doi.org/10.1016/j.jsps.2023.03.003
Chandrasekara A, Shahidi F (2018) Herbal beverages: bioactive compounds and their role in disease risk reduction - a review. J Tradit Complement Med 8:451–458. https://doi.org/10.1016/j.jtcme.2017.08.006
Kala CP, Dhyani PP, Sajwan BS (2006) Developing the medicinal plants sector in northern India: challenges and opportunities. J Ethnobiol Ethnomed 2:32. https://doi.org/10.1186/1746-4269-2-32
McKay DL, Blumberg JB (2007) A review of the bioactivity of South African herbal teas: rooibos (Aspalathus linearis) and honeybush (Cyclopia intermedia). Phytother Res 21:1–16. https://doi.org/10.1002/ptr.1992
Ye L, Wang H, Duncan SE, Eigel WN, O’Keefe SF (2015) Antioxidant activities of vine tea (Ampelopsis grossedentata) extract and its major component dihydromyricetin in soybean oil and cooked ground beef. Food Chem 172:416–422. https://doi.org/10.1016/j.foodchem.2014.09.090
Soni S, Rajani M, Anandjiwala S, Patel G (2008) Validation of different methods of preparation of Adhatoda vasica leaf juice by quantification of total alkaloids and vasicine. Indian J Pharm Sci 70:36. https://doi.org/10.4103/0250-474X.40329
Shoaib A (2022) A systematic ethnobotanical review of Adhatoda vasica (L.). Nees Cell Mol Biol (Noisy-le-grand) 67:248–263. https://doi.org/10.14715/cmb/2021.67.4.28
Lu Q, Gu W, Luo C, Wang L, Hua W, Sun Y, Tang L (2021) Phytochemical characterization and hepatoprotective effect of active fragment from Adhatoda vasica Nees. against tert-butyl hydroperoxide induced oxidative impairment via activating AMPK/p62/Nrf2 pathway. J Ethnopharm 266:113454. https://doi.org/10.1016/j.jep.2020.113454
Chauhan ES, Sharma K, Bist R (2019) Andrographis paniculata: a review of its phytochemistry and pharmacological activities. Rese Jour of Pharm Technol 12:891. https://doi.org/10.5958/0974-360X.2019.00153.7
Xu Y, Tang D, Wang J, Wei H, Gao J (2019) Neuroprotection of andrographolide against microglia-mediated inflammatory injury and oxidative damage in PC12 neurons. Neurochem Res 44:2619–2630. https://doi.org/10.1007/s11064-019-02883-5
Sharma A, Sharma V (2013) A brief review of medicinal properties of Asparagus racemosus (Shatawari). Int J Pure App Biosci 1:48–52
Bishoyi SK, Tripathy UP (2023) Asparagus racemosus: many problems, one solution on its phytochemical and pharmacological potential. J Med Plants Stud 11:3–7
Bopana N, Saxena S (2007) Asparagus racemosus-Ethnopharmacological evaluation and conservation needs. J Ethnopharmacol 110:1–15. https://doi.org/10.1016/j.jep.2007.01.001
Guo Y, Liu Z, Wan Y, Zhang Y, Abdu HI, Yang M, Pei J, Yue T, Zhang X, Hacimuftuoglu A, Abd El-Aty AM (2023) Literature analysis on Asparagus roots and review of its functional characterizations. Front Nutr 9:1024190. https://doi.org/10.3389/fnut.2022.1024190
Folane P, Unhale S, Kale P, Shelke S, Sagrule S, Biyani D (2020) Antidiabetic activity of polyherbal formulation containing Citrullus colocynthis, Piper nigrum, Asparagus racemosus, Cinnamomum tamala (CPAC) in alloxan induced diabetic rats. World J Pharm Pharma Sci 9:1174–1188
Mazumder PM, Das S, Das S, Das MK (2011) Phyto-pharmacology of Berberis aristata DC: a review. J Drug Deliv Ther 1:2
Yu L, Xing ZK, Mi SL, Wu X (2019) Regulatory effect of traditional Chinese medicine on intestinal microbiota. Zhongguo Zhong Yao Za Zhi. 44:34–39
Mukund S (2006) Chemistry and pharmacology of Ayurvedic medicinal plants. Varanasi: Chaukhambha Surabharati Prakashana. https://www.amazon.in/Chemistry-Pharmacology-Ayurvedic-Medical-Plants/dp/B01KXP7L30, as on 23rd Sept 2023
Fatima S, Girdharilal JR (2016) Ethno-therapeutic aspects of four different species of Cassia from Nandurbar district Maharashtra: a review. Epitome Int J Multidiscip Res 2:50–55
Ramchander Pawan J, Middha A (2017) Recent advances on senna as a laxative: a comprehensive review. J Pharm Phytochem 8:349–353
Osman NN, Jambi EJ, Aseri NH (2017) Assessment of antidiabetic and antioxidant activities of Cassia angustifolia and Feoniculum vulgare in diabetic rats. Int J Pharm Res Allied Sci 6:149–162
Srivastava S, Singh P, Mishra G, Jha KK, Khosa RL (2011) Costus speciosus (Keukand): a review. Der Pharma Sinica 2:118–128
Rani AS, Sulakshana G, Patnaik S (2012) Costus speciosus, an antidiabetic plant-review. FS J Pharm Res. 1:52–53
Allaq AA, Sidik NJ, Abdul-Aziz A, Ahmed IA (2020) Cumin (Cuminum cyminum L.): a review of its ethnopharmacology, phytochemistry. Biomed Res Ther 7:4016–4021
Milan KSM, Dholakia H, Tiku PK, Vishveshwaraiah P (2008) Enhancement of digestive enzymatic activity by cumin (Cuminum cyminum L.) and role of spent cumin as a bio nutrient. Food Chem 110:678–683. https://doi.org/10.1016/j.foodchem.2008.02.062
Kirana H, Srinivasan B (2010) Aqueous extract of Garcinia indica Choisy restores glutathione in type 2 diabetic rats. J Young Pharm 2:265–268. https://doi.org/10.4103/0975-1483.66806
Dwivedi RB (2020) Sutra Sthana-Preamble, In: Y.S., D., G, B. (Eds.), Charak Samhita New Edition. Charak Samhita Research, Training and Skill Development Centre (CSRTSDC).pp 2–2 https://doi.org/10.47468/CSNE.2020.e01.s01.002
Nampoothiri L, Sudra P, Dey A, Dhadhal S, Kureshi AA, Kumar S, Dhanani T, Singh R, Kumari P (2021) Fruit juice of Garcinia indica Choisy modulates dyslipidemia and lipid metabolism in cafeteria diet-based rat model. Ann Phytomed Int J 10:78–85
Barve K (2021) Garcinol enriched fraction from the fruit rind of Garcinia indica ameliorates atherosclerotic risk factor in diet induced hyperlipidemic C57BL/6 mice. J Tradit Complement Med 11:95–102. https://doi.org/10.1016/j.jtcme.2019.11.001
Chandran A, Syam RJ, Jerone JJ, SK V (2022) Ethnopharmacological study about Glycyrrhiza glabra L. (Licorice) based on Ayurveda, an Indian system of traditional medicine- A review. Int J Ayurvedic Med 13:587–600
Gupta S (2023) Yashtimadhu (Glycyrrhiza glabra)-uses, benefits and dosage. Institute of applied Food and Allergy URL https://www.iafaforallergy.com/herbs-a-to-z/yastimadhu-glycyrrhiza-glabra/, as on Oct. 6th 2023
Pastorino G, Cornara L, Soares S, Rodrigues F, Oliveira MBPP (2018) Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother Res 32:2323–2339. https://doi.org/10.1002/ptr.6178
Hazarika P, Pandey BK, Tripathi Y (2020) Traditional knowledge for antidiabetic herbs from Majuli Island (Assam), India. Int J Herbal Med 8:47–58
Thakur GS, Sharma R, Sanodiya BS, Pandey M, Prasad G, Bisen PS (2012) Gymnema sylvestre: an alternative therapeutic agent for management of diabetes. J App Pharm Sci 2:1–6. https://doi.org/10.7324/JAPS.2012.21201
Kang MH, Lee MS, Choi MK, Min KS, Shibamoto T (2012) Hypoglycemic activity of Gymnema sylvestre extracts on oxidative stress and antioxidant status in diabetic rats. J Agric Food Chem 60:2517–2524. https://doi.org/10.1021/jf205086b
Arunachalam K, Arun LB, Annamalai SK, Arunachalam AM (2015) Potential anticancer properties of bioactive compounds of Gymnema sylvestre and its biofunctionalized silver nanoparticles. Int J Nanomed 10:31–41. https://doi.org/10.2147/IJN.S71182
Kalita T, Dutta U (2023) Phytochemistry, antioxidant activity and traditional uses of Ipomoea aquatica Forssk among the people of Lower Assam, India. Int J Ayurvedic Med 13:896–904
Toy JYH, Huang J, Song Z, Lin Y, Huang D (2022) Resin glycoside extracts from Ipomoea aquatica retard lipid digestibility of high-fat food in vitro. Food Res Int 159:111552. https://doi.org/10.1016/j.foodres.2022.111552
Sokeng SD, Rokeya B, Hannan JMA, Junaida K, Zitech P, Ali L, Ngounou G, Lontsi D, Kamtchouing P (2007) Inhibitory effect of Ipomoea aquatica extracts on glucose absorption using a perfused rat intestinal preparation. Fitoterapia 78:526–529. https://doi.org/10.1016/j.fitote.2007.06.011
Singh P, Pandey P, Singh PK, Tripathi M, Singh RP, Shukla S, Pathak N, Singh RL (2023) A comprehensive review on phytochemistry, nutritional and pharmacological properties of Momordica charantia. IP Int J Compr Adv Pharm 8:73–79
Bai J, Zhu Y, Dong Y (2016) Response of gut microbiota and inflammatory status to bitter melon (Momordica charantia L.) in high fat diet induced obese rats. J Ethnopharmacol 194:717–726. https://doi.org/10.1016/j.jep.2016.10.043
Joseph B, Jini D (2013) Antidiabetic effects of Momordica charantia (bitter melon) and its medicinal potency. Asian Pac J Trop Dis 3:93–102. https://doi.org/10.1016/S2222-1808(13)60052-3
Chopra RN, Nayar SL, Chopra IC (1956) Glossary of Indian medicinal plants. Council of Scientific and Industrial Research, New Delhi
Luthy N, Martinez-Fortun O (1964) A study of a possible oral hypoglycemic factor in Albahaca Morada (Ocimum Sanctum L.). Ohio J Sci 64:223–224
Hasan MR, Alotaibi BS, Althafar ZM, Mujamammi AH, Jameela J (2023) An update on the therapeutic anticancer potential of Ocimum sanctum L.: Elixir of Life. Molecules 28:1193
Elansary HO, Mahmoud EA (2015) In-vitro antioxidant and antiproliferative activities of six international basil cultivars. Nat Prod Res 29:2149–2154. https://doi.org/10.1080/14786419.2014.995653
Shivananjappa M, Joshi M (2012) Aqueous extract of tulsi (Ocimum sanctum) enhances endogenous antioxidant defenses of human hepatoma cell line (HepG2). J Herbs Spices Med Plants 18:331–348. https://doi.org/10.1080/10496475.2012.712939
Siddique YH, Ara G, Beg T, Afzal M (2007) Anti-genotoxic effect of Ocimum sanctum L. extract against cyproterone acetate induced genotoxic damage in cultured mammalian cells. Acta Biol Hung 58:397–409. https://doi.org/10.1556/ABiol.58.2007.4.7
Narendra K, Swathi J, Sowjanya KM, Krishna Satya A (2012) Phyllanthus niruri: a review on its ethno-botanical, phytochemical, and pharmacological profile. J Pharm Res 5:4681–4691
Kumar A, Saini K, Kumari R (2022) A brief literature review on Piper longum with special references to different Ayurvedic Samhitas. World J Pharm Res 11:683–696
Kumar S, Kamboj J, Sharma S (2011) Overview for various aspects of the health benefits of Piper longum Linn. Fruit. J Acupunct Meridian Stud 4:134–140. https://doi.org/10.1016/S2005-2901(11)60020-4
Tripathi AK, Ray AK, Mishra SK (2022) Molecular and pharmacological aspects of piperine as a potential molecule for disease prevention and management: evidence from clinical trials. Beni-Suef Univ J Basic Appl Sci 11:16. https://doi.org/10.1186/s43088-022-00196-1
Ashokkumar K, Murugan M, Dhanya MK, Pandian A, Warkentin TD (2021) Phytochemistry and therapeutic potential of black pepper (Piper nigrum L.) essential oil and piperine: a review. Clin Phytosci 7:52
Srivastava AK, Singh VK (2017) Biological Action of Piper nigrum - The king of spices. Eur J Bio Res 7:223–233. https://doi.org/10.5281/ZENODO.839039
Tiwari P, Singh D, Singh MM (2008) Anti-trichomonas activity of Sapindus saponins, a candidate for development as microbicidal contraceptive. J Antimicrob Chemother 62:526–534. https://doi.org/10.1093/jac/dkn223
Chitlange S, Payal B, Sanjay D, Nipanikar DN (2016) Development and validation of RPHPLC method for quantification of piperine from single herb formulation containing Piper nigrum extract. Int J Pharm Pharmacol Sci Res 6:16–21
Khare CP (2007) Indian Medicinal Plants- An Illustrated Dictionary. Springer
Sharma N, Kaushik P (2014) Medicinal, biological, and pharmacological aspects of Plumbago zeylanica (Linn.). J Pharm Phytochem 3:117–120
Rajakrishnan R, Lekshmi R, Benil PB, Thomas J, AlFarhan AH, Rakesh V, Khalaf S (2017) Phytochemical evaluation of roots of Plumbago zeylanica L. and assessment of its potential as a nephroprotective agent. Saudi J Bio Sci 24:760–766. https://doi.org/10.1016/j.sjbs.2017.01.001
Kanchana N, Sadiq MA (2011) Hepatoprotective effect of Plumbago zeylanica on paracetamol induced liver toxicity in rats. Int J Pharm Pharm Sci 3:151–154
Kumar M, Tomar M, Amarowicz R, SaurabhV Nair MS, Maheshwari C, Sasi M, Prajapati U, Hasan M, Singh S, Changan S, Prajapat RK, Berwal MK, Satankar V (2021) Guava (Psidium guajava L.) leaves: nutritional composition, phytochemical profile, and health-promoting bioactivities. Foods 10:752
Chu S, Zhang F, Wang H, Xie L, Chen Z, Zeng W, Zhou Z, Hu F (2022) Aqueous extract of guava (Psidium guajava L) leaf ameliorates hyperglycemia by promoting hepatic glycogen synthesis and modulating gut microbiota. Front Pharmacol 13:907702. https://doi.org/10.3389/fphar.2022.907702
Oberoi L, Akiyama T, Lee KH, Liu SJ (2011) The aqueous extract, not organic extracts, of Terminalia arjuna bark exerts cardiotonic effect on adult ventricular myocytes. Phytomedicine 18:259–265. https://doi.org/10.1016/j.phymed.2010.07.006
Amalraj A, Gopi S (2017) Medicinal properties of Terminalia arjuna (Roxb.) Wight & Arn.: a review. J Tradit Complement Med 7:65–78. https://doi.org/10.1016/j.jtcme.2016.02.003
Sultan MT, Anwar MJ, Imran M, Khalil I, Saeed F, Neelum S, Alsagaby SA, Al Abdulmonem W, Abdelgawad MA, Hussain M, El-Ghorab AH, Umar M, Al Jbawi E (2023) Phytochemical profile and pro-healthy properties of Terminalia chebula: a comprehensive review. Int J Food Prop 26:526–551. https://doi.org/10.1080/10942912.2023.2166951
Yoshida T, Amakura Y, Yoshimura M (2010) Structural features and biological properties of ellagitannins in some plant families of the order Myrtales. Int J Mol Sci 11:79–106. https://doi.org/10.3390/ijms11010079
Mishra V, Agrawal M, Onasanwo SA, Madhur G, Rastogi P, Pandey HP, Palit G, Narender T (2013) Anti-secretory and cytoprotective effects of chebulinic acid isolated from the fruits of Terminalia chebula on gastric ulcers. Phytomedicine 20:506–511. https://doi.org/10.1016/j.phymed.2013.01.002
Sharma P, Dwivedee BP, Bisht D, Dash AK, Kumar D (2019) The chemical constituents and diverse pharmacological importance of Tinospora cordifolia. Heliyon 5:e02437. https://doi.org/10.1016/j.heliyon.2019.e02437
Sharma B, Dabur R (2016) Protective effects of Tinospora cordifolia on hepatic and gastrointestinal toxicity induced by chronic and moderate alcoholism. Alcohol Alcohol 51:1–10. https://doi.org/10.1093/alcalc/agv130
Mandal S, DebMandal M (2016) Fenugreek (Trigonella foenum-graecum L.) Oils, in: Essential Oils in Food Preservation Flavor and Safety. Elsevier, pp 421–429. https://doi.org/10.1016/B978-0-12-416641-7.00047-X.
Singh N, Yadav SS, Kumar S, Narashiman B (2022) Ethnopharmacological, phytochemical and clinical studies on Fenugreek (Trigonella foenum-graecum L.). Food Bioscience 46:101546
Snehlata HS, Payal DR (2011) Fenugreek (Trigonella foenum-graecum L.): an overview. Int J Curr Pharm Review Res 2:169–187
Gaurav H, Yadav D, Maurya A, Yadav H, Yadav R, Shukla AC, Sharma M, Gupta VK, Palazon J (2023) Biodiversity, biochemical profiling, and pharmaco-commercial applications of Withania somnifera: A review. Molecules 28:1208. https://doi.org/10.3390/molecules28031208
Saleem S, Muhammad G, Hussain MA, Altaf M, Bukhari SNA (2020) Withania somnifera L. Insights into the phytochemical profile, therapeutic potential, clinical trials and future prospective. Iran J Basic Med Sci. https://doi.org/10.22038/IJBMS.2020.44254.10378
Shalaby EA, Shanab SMM, Hafez RM, El-Ansary AE (2023) Chemical constituents and biological activities of different extracts from ginger plant (Zingiber officinale). Chem Biol Technol Agric 10:14. https://doi.org/10.1186/s40538-023-00385-9
Sadeghi Poor Ranjbar F, Mohammadyari F, Omidvar A, Nikzad F, Doozandeh Nargesi N, Varmazyar M, Dehghankar S, Vosoughian F, Olangian-Tehrani S, Nanbakhsh S, Mansourian T, Deravi N, Tutunchian Z, Salahi M, Poudineh M, Ghayyem H (2022) Zingiber officinale (ginger) as a treatment for inflammatory bowel disease: a review of current literature. Front Drug Discov 2:1043617. https://doi.org/10.3389/fddsv.2022.1043617
Ji M, Gong X, Li X, Wang C, Li M (2020) Advanced research on the antioxidant activity and mechanism of polyphenols from Hippophae species-a review. Molecules 25:917. https://doi.org/10.3390/molecules25040917
Orimadegun BE, Bolajoko EB, Onyeaghala AA, Ademola-Aremu OO (2018) Quantitative analyses of phytochemical and trace elements contents of daily detox, herbal tea consumed in Nigeria. J Med Plants Res 12:289–295. https://doi.org/10.5897/JMPR2018.6578
Liu Y, Guo C, Zang E, Shi R, Liu Q, Zhang M, Zhang K, Li M (2023) Review on herbal tea as a functional food: classification, active compounds, biological activity, and industrial status. J Futur Foods 3:206–219. https://doi.org/10.1016/j.jfutfo.2023.02.002
Postler TS, Ghosh S (2017) Understanding the Holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab 26:110–130. https://doi.org/10.1016/j.cmet.2017.05.008
Feng W, Ao H, Peng C (2018) Gut microbiota, short-chain fatty acids, and herbal medicines. Front Pharm 9:1354. https://doi.org/10.3389/fphar.2018.01354
Blachier F, Mariotti F, Huneau JF, Tomé D (2007) Effects of amino acid-derived luminal metabolites on the colonic epithelium and physio-pathological consequences. Amino Acids 33:547–562. https://doi.org/10.1007/s00726-006-0477-9
Fernandez J, Redondo-Blanco S, Gutiérrez-del-Rio I, Miguelez EM, Villar CJ, Lombo F (2016) Colon microbiota fermentation of dietary prebiotics towards short-chain fatty acids and their roles as anti-inflammatory and antitumour agents: a review. J Funct Foods 25:511–522. https://doi.org/10.1016/j.jff.2016.06.032
Den Besten G, Van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM (2013) The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54:2325–2340. https://doi.org/10.1194/jlr.R036012
Levy M, Thaiss CA, Elinav E (2016) Metabolites: messengers between the microbiota and the immune system. Genes Dev 30:1589–1597. https://doi.org/10.1101/gad.284091.116
Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F (2016) From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 165:1332–1345. https://doi.org/10.1016/j.cell.2016.05.041
Van Der Beek CM, Dejong CHC, Troost FJ, Masclee AAM, Lenaerts K (2017) Role of short-chain fatty acids in colonic inflammation, carcinogenesis, and mucosal protection and healing. Nutr Rev 75:286–305. https://doi.org/10.1093/nutrit/nuw067
El-Gedaily A, Paesold G, Chen CY, Guiney DG, Krause M (1997) Plasmid virulence gene expression induced by short-chain fatty acids in Salmonella dublin: identification of rpoS-dependent and rpo-S-independent mechanisms. J Bacteriol 179:1409–1412. https://doi.org/10.1128/jb.179.4.1409-1412.1997
Lin L, Luo L, Zhong M, Xie T, Liu Y, Li H, Ni J (2019) Gut microbiota: a new angle for traditional herbal medicine research. RSC Adv 9:17457–17472. https://doi.org/10.1039/C9RA01838G
He F, Zuo L (2015) Redox roles of reactive oxygen species in cardiovascular diseases. IJMS 16:27770–27780. https://doi.org/10.3390/ijms161126059
Carabotti M, Scirocco A, Maselli MA, Severi C (2015) The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28:203–209
Ley RE, Peterson DA, Gordon JI (2006) Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124:837–848. https://doi.org/10.1016/j.cell.2006.02.017
Kawata Y, Ma C, Meselhy M, Nakamura N, Wang H (1999) Conversion of aconitine lipoaconitine by human intestinal bacteria and their antinociceptive effects in mice. J Trad Med 16:15–23
Wang Y, Shou JW, Li XY, Zhao ZX, Fu J, He CY, Feng R, Ma C, Wen BY, Guo F, Yang XY, Han YX, Wang LL, Tong Q, You XF, Lin Y, Kong WJ, Si SY, Jiang JD (2017) Berberine-induced bioactive metabolites of the gut microbiota improve energy metabolism. Metabolism 70:72–84. https://doi.org/10.1016/j.metabol.2017.02.003
Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Reddy ND (2015) Role of the normal gut microbiota. World J Gastroenterol 21:8787–8803. https://doi.org/10.3748/wjg.v21.i29.8787
Carbone S, Lavie CJ, Elagizi A, Arena R, Ventura HO (2022) The impact of obesity in heart failure. Cardiol Clin 40:209–218. https://doi.org/10.1016/j.ccl.2021.12.009
Frick A, Ahs F, Engman J, Jonasson M, Alaie I, Bjorkstrand J, Frans O, Faria V, Linnman C, Appel L, Wahlstedt K, Lubberink M, Fredrikson M, Furmark T (2015) Serotonin synthesis and reuptake in social anxiety disorder: a positron emission tomography study. JAMA Psychiat 72:794. https://doi.org/10.1001/jamapsychiatry.2015.0125
Foster JA, Rinaman L, Cryan JF (2017) Stress & the gut-brain axis: regulation by the microbiome. Neurobio Stress 7:124–136. https://doi.org/10.1016/j.ynstr.2017.03.001
Kondapalli NB, Hemalatha R, Uppala S, Yathapu SR, Mohammed S, Surekha VM, Rajendran A, Bharadwaj DK (2022) Ocimum sanctum, Zingiber officinale, and Piper nigrum extracts and their effects on gut microbiota modulations (prebiotic potential), basal inflammatory markers and lipid levels: oral supplementation study in healthy rats. Pharm Biol 60:437–450. https://doi.org/10.1080/13880209.2022.2033797
Narendra Babu K, Hemalatha R, Satyanarayana U, Shujauddin M, Himaja N, Bhaskarachary K, Dinesh Kumar B (2018) Phytochemicals, polyphenols, prebiotic effect of Ocimum sanctum, Zingiber officinale, Piper nigrum extracts. J Herbal Med 13:42–51. https://doi.org/10.1016/j.hermed.2018.05.001
Hsiang CY, Cheng HM, Lo HY, Li CC, Chou PC, Lee YC, Ho TY (2015) Ginger and zingerone ameliorate lipopolysaccharide-induced acute systemic inflammation in mice, assessed by nuclear factor-κB bioluminescent imaging. J Agric Food Chem 63:6051–6058. https://doi.org/10.1021/acs.jafc.5b01801
Kecklund G, Axelsson J (2016) Health consequences of shift work and insufficient sleep. BMJ 355:i5210. https://doi.org/10.1136/bmj.i5210
Zarrinpar A, Chaix A, Panda S (2016) Daily eating patterns and their impact on health and disease. Trends Endocrinol Metab 27:69–83. https://doi.org/10.1016/j.tem.2015.11.007
Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, Abramson L, Katz MN, Korem T, Zmora N, Kuperman Y, Biton I, Gilad S, Harmelin A, Shapiro H, Halpern Z, Segal E, Elinav E (2014) Trans kingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 159:514–529. https://doi.org/10.1016/j.cell.2014.09.048
Smits SA, Leach J, Sonnenburg ED, Gonzalez CG, Lichtman JS, Reid G, Knight R, Manjurano A, Changalucha J, Elias JE, Dominguez-Bello MG, Sonnenburg JL (2017) Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science 357:802–806. https://doi.org/10.1126/science.aan4834
Islam R, Sultana N, Bhakta S, Haque Z, Hasan A, Siddique MP, Islam MR (2023) Modulation of growth performance, gut morphometry, and cecal microbiota in broilers by clove (Syzygium aromaticum) and tulsi (Ocimum sanctum) supplementation. Poult Sci 102:102266. https://doi.org/10.1016/j.psj.2022.102266
Patil R, Jain V (2021) Andrographolide: a review of analytical methods. J Chromatogr Sci 59:191–203. https://doi.org/10.1093/chromsci/bmaa091
Perez RH (2021) Bacteriocin production, optimization, and utilization: facilitating the utilization of bacteriocins through molecular strategies. The Microbiology Consortium of the Philippine Society for Microbiology, Inc. https://www.youtube.com/watch?v=3RFYvZWu0X4, as on 28th Sept 2023
Bussmann RW, Malca-García G, Glenn A, Sharon D, Chait G, Díaz D, Pourmand K, Jonat B, Somogy S, Guardado G, Aguirre C, Chan R, Meyer K, Kuhlman A, Townesmith A, Effio-Carbajal J, Fras-Fernandez F, Benito M (2010) Minimum inhibitory concentrations of medicinal plants used in Northern Peru as antibacterial remedies. J Ethnopharm 132:101–108. https://doi.org/10.1016/j.jep.2010.07.048
Belscak A, Bukovac N, Piljac-Zegarac J (2011) The influence of ascorbic acid and honey addition on the antioxidant properties of fruit tea infusions: Antioxidants in fruit tea infusions. J Food Biochem 35:195–212. https://doi.org/10.1111/j.1745-4514.2010.00375.x
Zhang KX, Tan JB, Xie CL, Zheng RB, Huang XD, Zhang MM, Zhao M (2020) Antioxidant effects and cytoprotective potentials of herbal tea against H2O2-induced oxidative damage by activating heme oxygenase1 pathway. Biomed Res Int 2020:1–10. https://doi.org/10.1155/2020/7187946
Agagunduz D (2020) Determination of the total antioxidant and oxidant status of some galactagogue and herbal teas. Food Sci Human Wellness 9:377–382. https://doi.org/10.1016/j.fshw.2020.06.002
Sanchez M, González-Burgos E, Divakar PK, Gómez-Serranillos MP (2020) DNA-based authentication and metabolomics analysis of medicinal plants samples by DNA barcoding and ultra-high-performance liquid chromatography/triple quadrupole mass spectrometry (UHPLC-MS). Plants 9:1601. https://doi.org/10.3390/plants9111601
Mannani N, Tabarani A, Abdennebi EH, Zinedine A (2020) Assessment of aflatoxin levels in herbal green tea available on the Moroccan market. Food Control 108:106882. https://doi.org/10.1016/j.foodcont.2019.106882
Kilic S, Soylak M (2020) Determination of trace element contaminants in herbal teas using ICP-MS by different sample preparation method. J Food Sci Technol 57:927–933. https://doi.org/10.1007/s13197-019-04125-6
Karak T, Abollino O, Bhattacharyya P, Das KK, Paul RK (2011) Fractionation and speciation of arsenic in three tea gardens soil profiles and distribution of As in different parts of tea plant (Camellia sinensis L.). Chemosphere 85:948–960. https://doi.org/10.1016/j.chemosphere.2011.06.061
Chadha J, Moudgil G, Harjai K (2024) Synergism between α-terpineol and terpinen-4-ol potentiates antivirulence response against Pseudomonas aeruginosa. Indian J Microbiol. https://doi.org/10.1007/s12088-024-01189-7
AYUSH. 2020. AYUSH reiterates immunity boosting measures for self-care during COVID 19 crises. https://pib.gov.in/PressReleaseIframePage.aspx?PRID=1613129, as on 15th Feb. 2023.
Acknowledgements
Authors are thankful to each organization for cooperation with us in collecting and extracting the data.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
DDJ: Conceptualization, Writing—Original draft preparation, RS: Methodology, Data curation, Reviewing and Editing, LD: Visualization, Investigation, KK: Reference Compilation, BGS: Editing and Visualization, VSR: Reviewing and Editing. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Joshi, D.D., Deb, L., Kaul, K. et al. Relevance of Indian Traditional Herbal Brews for Gut Microbiota Balance. Indian J Microbiol (2024). https://doi.org/10.1007/s12088-024-01251-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12088-024-01251-4