Abstract
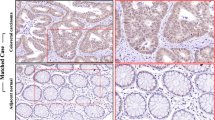
Colorectal cancer (CRC) ranks as the third most prevalent cancer type globally. Nevertheless, the fundamental mechanisms driving CRC progression remain ambiguous, and the prognosis for the majority of patients diagnosed at an advanced stage is dismal. YWHA/14-3-3 proteins serve as central nodes in several signaling pathways and are closely related to tumorigenesis and progression. However, their exact roles in CRC are still poorly elucidated. In this study, we revealed that YWHAG was the most significantly upregulated member of the YWHA/14-3-3 family in CRC tissues and was associated with a poor prognosis. Subsequent phenotypic experiments showed that YWHAG promoted the proliferation, migration, and invasion of CRC cells. Mechanistically, RNA-seq data showed that multiple signaling pathways, including Wnt and epithelial-mesenchymal transition, were potentially regulated by YWHAG. CTTN was identified as a YWHAG-associated protein, and mediated its tumor-promoting functions by activating the Wnt/β-catenin signaling in CRC cells. In summary, our data indicate that YWHAG facilitates the proliferation, migration, and invasion of CRC cells by modulating the CTTN-Wnt/β-catenin signaling pathway, which offers a novel perspective for the treatment of CRC.






Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics 2020. CA: Cancer J Clin. 2020;70(1):30.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2021;71(3):209–49.
Mackintosh C. Dynamic interactions between 14-3-3 proteins and phosphoproteins regulate diverse cellular processes. Biochem J. 2004;381(Pt 2):329–42.
Morrison DK. The 14-3-3 proteins: integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol. 2009;19(1):16–23.
Pawson T, Nash P. Protein-protein interactions define specificity in signal transduction. Genes Dev. 2000;14(9):1027–47.
Muslin AJ, Tanner JW, Allen PM, Shaw AS. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell. 1996;84(6):889–97.
Yaffe MB, Rittinger K, Volinia S, Caron PR, Aitken A, Leffers H, et al. The structural basis for 14-3-3: phosphopeptide binding specificity. Cell. 1997;91(7):961–71.
Aitken A, Baxter H, Dubois T, Clokie S, Mackie S, Mitchell K, et al. Specificity of 14-3-3 isoform dimer interactions and phosphorylation. Biochem Soc Trans. 2002;30(4):351–60.
Fan X, Cui L, Zeng Y, Song W, Gaur U, Yang M. 14-3-3 proteins are on the crossroads of cancer, aging, and age-related neurodegenerative disease. Int J Mol Sci. 2019;20(14):3518.
Fan T, Li R, Todd NW, Qiu Q, Fang H-B, Wang H, et al. Up-regulation of 14-3-3zeta in lung cancer and its implication as prognostic and therapeutic target. Cancer Res. 2007;67(16):7901–6.
Maxwell SA, Cherry EM, Bayless KJ. Akt, 14-3-3ζ, and vimentin mediate a drug-resistant invasive phenotype in diffuse large B-cell lymphoma. Leuk Lymphoma. 2011;52(5):849–64.
Maxwell SA, Li Z, Jaya D, Ballard S, Ferrell J, Fu H. 14-3-3zeta mediates resistance of diffuse large B cell lymphoma to an anthracycline-based chemotherapeutic regimen. J Biol Chem. 2009;284(33):22379–89.
Teo Z, Sng MK, Chan JSK, Lim MMK, Li Y, Li L, et al. Elevation of adenylate energy charge by angiopoietin-like 4 enhances epithelial-mesenchymal transition by inducing 14-3-3γ expression. Oncogene. 2017;36(46):6408–19.
Lee JXT, Tan WR, Low ZS, Lee JQ, Chua D, Yeo WDC, et al. YWHAG deficiency disrupts the EMT-associated network to induce oxidative cell death and prevent metastasis. Adv Sci (Weinh). 2023;10(31): e2301714.
Zhang J, Cui K, Huang L, Yang F, Sun S, Bian Z, et al. SLCO4A1–AS1 promotes colorectal tumourigenesis by regulating Cdk2/c-Myc signalling. J Biomed Sci. 2022;29(1):4.
Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014;7(344):re8.
Moon SJ, Choi HJ, Kye YH, Jeong GY, Kim HY, Myung JK, et al. CTTN overexpression confers cancer stem cell-like properties and trastuzumab resistance via DKK-1/WNT signaling in HER2 positive breast cancer. Cancers (Basel). 2023;15(4):1168.
Sekiba K, Otsuka M, Funato K, Miyakawa Y, Tanaka E, Seimiya T, et al. HBx-induced degradation of Smc5/6 complex impairs homologous recombination-mediated repair of damaged DNA. J Hepatol. 2022;76(1):53–62.
Radhakrishnan VM, Martinez JD. 14-3-3gamma induces oncogenic transformation by stimulating MAP kinase and PI3K signaling. PLoS ONE. 2010;5(7):e11433.
Ko B-S, Lai IR, Chang T-C, Liu T-A, Chen S-C, Wang J, et al. Involvement of 14-3-3γ overexpression in extrahepatic metastasis of hepatocellular carcinoma. Hum Pathol. 2011;42(1):129–35.
Lee Y-S, Lee JK, Bae Y, Lee B-S, Kim E, Cho C-H, et al. Suppression of 14-3-3γ-mediated surface expression of ANO1 inhibits cancer progression of glioblastoma cells. Sci Rep. 2016;6:26413.
Xu J, Wang J, He Z, Chen P, Jiang X, Chen Y, et al. LncRNA CERS6-AS1 promotes proliferation and metastasis through the upregulation of YWHAG and activation of ERK signaling in pancreatic cancer. Cell Death Dis. 2021;12(7):648.
Wang P, Deng Y, Fu X. MiR-509-5p suppresses the proliferation, migration, and invasion of non-small cell lung cancer by targeting YWHAG. Biochem Biophys Res Commun. 2017;482(4):935–41.
Garan LAW, Xiao Y, Lin WC. 14-3-3tau drives estrogen receptor loss via ERalpha36 induction and GATA3 inhibition in breast cancer. Proc Natl Acad Sci USA. 2022;119(43):e2209211119.
Liou JY, Ghelani D, Yeh S, Wu KK. Nonsteroidal anti-inflammatory drugs induce colorectal cancer cell apoptosis by suppressing 14-3-3epsilon. Cancer Res. 2007;67(7):3185–91.
Ding J, Zhu YT, Yang L, Tang J, Wang YY, Chen Y, et al. 14-3-3zeta is involved in the anticancer effect of metformin in colorectal carcinoma. Carcinogenesis. 2018;39(3):493–502.
Hiraoka E, Mimae T, Ito M, Kadoya T, Miyata Y, Ito A, et al. Breast cancer cell motility is promoted by 14-3-3gamma. Breast Cancer. 2019;26(5):581–93.
Ren XL, Qiao YD, Li JY, Li XM, Zhang D, Zhang XJ, et al. Cortactin recruits FMNL2 to promote actin polymerization and endosome motility in invadopodia formation. Cancer Lett. 2018;419:245–56.
Li W, Lei T, Song X, Deng C, Lu J, Zhang W, et al. CBLC inhibits the proliferation and metastasis of breast cancer cells via ubiquitination and degradation of CTTN. J Recept Signal Transduct Res. 2022;42(6):588–98.
Jing X, Wu H, Ji X, Wu H, Shi M, Zhao R. Cortactin promotes cell migration and invasion through upregulation of the dedicator of cytokinesis 1 expression in human colorectal cancer. Oncol Rep. 2016;36(4):1946–52.
Wu H, Cheng X, Ji X, He Y, Jing X, Wu H, et al. Cortactin contributes to the tumorigenicity of colorectal cancer by promoting cell proliferation. Oncol Rep. 2016;36(6):3497–503.
Wei C-Y, Zhu M-X, Yang Y-W, Zhang P-F, Yang X, Peng R, et al. Downregulation of RNF128 activates Wnt/β-catenin signaling to induce cellular EMT and stemness via CD44 and CTTN ubiquitination in melanoma. J Hematol Oncol. 2019;12(1):21.
Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7(1):3.
Tang Q, Chen J, Di Z, Yuan W, Zhou Z, Liu Z, et al. TM4SF1 promotes EMT and cancer stemness via the Wnt/β-catenin/SOX2 pathway in colorectal cancer. J Exp Clin Cancer Res. 2020;39(1):232.
Funding
This study was partially supported by grants from the National Natural Science Foundation of China (82173063), Wuxi Taihu Lake Talent Plan for Leading Talents in Medical and Health Profession, and Wuxi Medical Key Discipline (ZDXK2021002).
Author information
Authors and Affiliations
Contributions
ZHH conceived and designed the project. YBW, YLC, YC, HC, and ZAL performed all experiments. KSC, YBW, YLC, and MNW performed the statistical analyses. YYF and BJF were responsible for clinical sample collection. ZHH, KSC, and YBW prepared, wrote, and/or revised the paper. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
The present study was approved by the Clinical Research Ethics Committees of the participating institutions.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Cao, Y., Chen, Y. et al. YWHAG promotes colorectal cancer progression by regulating the CTTN-Wnt/β-catenin signaling axis. Med Oncol 41, 100 (2024). https://doi.org/10.1007/s12032-024-02349-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-024-02349-x