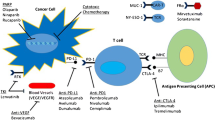
Abstract
Gynecological malignancies are most leading causes of death among women worldwide. The high prevalence of gynecologic malignancies remains significant, necessitating to turn the novel treatment approach like immunotherapy, wherein cancer cells are killed by the invasion of immune system. In recent year, immunotherapy has mostly an advanced treatment approach to repressing the tumor cells survival, proliferation, and invasion via the activation of immune systems. Moreover, various types of immune cells including T-cells, B-cells, and dendritic cells are associated with the immunotherapeutic strategy in cancer treatment. Although the significant role of T-cells against cancer is well established, while B-cells and dendritic cells also play an important role against different gynecological cancer by regulating the immune system. This review focuses on that arena and highlight the role of immune cells in the treatment of gynaecological cancer. Various immune cell-based anticancer therapies such as T-cell therapies, Adoptive Cellular transfer, B-cell therapies as well as approaches to Dendritic Cell therapies have been discussed in detail. Furthermore, the clinical settings and future avenues regarding immunotherapy on gynecological cancer have also been reviewed and illuminated in the recent study.




Similar content being viewed by others
Data availability
Not applicable.
References
Decker WK, da Silva RF, Sanabria MH, Angelo LS, Guimarães F, Burt BM, et al. Cancer immunotherapy: historical perspective of a clinical revolution and emerging preclinical animal models. Front Immunol. 2017;8:829.
Oiseth SJ, Aziz MS. Cancer immunotherapy: a brief review of the history, possibilities, and challenges ahead. J Cancer Metastasis Treat. 2017;3:250–61.
Halliday GM, Patel A, Hunt MJ, Tefany FJ, Barnetson RS. Spontaneous regression of human melanoma/nonmelanoma skin cancer: association with infiltrating CD4+ T cells. World J Surg. 1995;19:352–8.
Rosenberg SA. Raising the bar: the curative potential of human cancer immunotherapy. Sci Transl Med. 2012;4:127ps8.
Nikanjam M, Mullen J, Yacoub C, Daniels GA. Combination high-dose interleukin-2 and nivolumab for programmed cell death-1 refractory metastatic melanoma: a case series. J Med Case Rep. 2022;16(1):337.
Lynam S, Lugade AA, Odunsi K. Immunotherapy for gynecologic cancer: current applications and future directions. Clin Obstet Gynecol. 2020;63(1):48–63.
Quezada SA, Peggs KS, Simpson TR, Allison JP. Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication. Immunol Rev. 2011;241(1):104–18.
Jiang G, Wu Q, Li B. Evaluation of immunotherapy efficacy in gynecologic cancer. Front Immunol. 2023;14:1061761.
Nishio H, Iwata T, Aoki D. Current status of cancer immunotherapy for gynecologic malignancies. Jpn J Clin Oncol. 2021;51(2):167–72.
Lorusso D, Ceni V, Daniele G, Pietragalla A, Salutari V, Muratore M, et al. Immunotherapy in gynecological cancers. Explor Target Antitumor Ther. 2021;2(1):48–64.
Ventriglia J, Paciolla I, Pisano C, Cecere SC, Di Napoli M, Tambaro R, et al. Immunotherapy in ovarian, endometrial and cervical cancer: State of the art and future perspectives. Cancer Treat Rev. 2017;59:109–16.
Ascierto ML, Idowu MO, Zhao Y, Khalak H, Payne KK, Wang XY, Dumur CI, Bedognetti D, Tomei S, Ascierto PA, Shanker A, Bear HD, Wang E, Marincola FM, De Maria A, Manjili MH. Molecular signatures mostly associated with NK cells arepredictive of relapse free survival in breast cancer patients. J Transl Med. 2013;11(1):145.
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells withinhuman colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–4.
Ginsburgs VH, Goodill SW. A dance/movement therapy clinical model for women with gynecologic cancer undergoing high dose rate brachytherapy. Am J Dance Ther. 2009;31(2):136–58.
Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32(19–20):1267–84.
Parrott DV, de Sousa MA, East J. Thymus-dependent areas in the lymphoid organs of neonatally thymectomized mice. J Exp Med. 1966;123:191–204.
LeBien TW, Tedder TFB. lymphocytes: how they develop and function. Blood. 2008;112:1570–80.
Chen C, Liu X, Chang CY, Wang HY, Wang RF. The interplay between T cells and cancer: the basis of immunotherapy. Genes. 2023;14:1008.
Lanza R, Russell DW, Nagy A. Engineering universal cells that evade immune detection. Nat Rev Immunol. 2019;19(12):723–33.
Speiser DE, Ho PC, Verdeil G. Regulatory circuits of T cell function in cancer. Nat Rev Immunol. 2016;16(10):599–611.
Donadon M, Hudspeth K, Cimino M, Di Tommaso L, Preti M, Tentorio P, et al. Increased infiltration of natural killer and T cells in colorectal liver metastases improves patient overall survival. J Gastrointest Surg. 2017;21(8):1226–36.
Kumar BV, Connors TJ, Farber DL. Human T cell development, localization, and function throughout life. Immunity. 2018;48:202–13.
van den Broek T, Borghans JAM, van Wijk F. The full spectrum of human naive T cells. Nat Rev Immunol. 2018;18(6):363–73.
Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–32.
Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat Rev Immunol. 2004;4:123–32.
Wilson IA, Garcia KC. T-cell receptor structure and TCR complexes. Curr Opin Struct Biol. 1997;7:839–48.
Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–80.
Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–68.
Legut M, Dolton G, Mian AA, Ottmann OG, Sewell AK. CRISPR-mediated TCR replacement generates superior anticancer transgenic T cells. Blood. 2018;131(3):311–22.
Yuen GJ, Demissie E, Pillai S. B lymphocytes and cancer: a love–hate relationship. Trends in cancer. 2016;2(12):747–57.
Chen Z, Zhu Y, Du R, Pang N, Zhang F, Dong D, et al. Role of regulatory B cells in the progression of cervical cancer. Mediat Inflamm. 2019;2019:1–8.
Zegallai HM, Abu-El-Rub E, Mejia EM, Sparagna GC, Cole LK, Marshall AJ, et al. Tafazzin deficiency attenuates anti-cluster of differentiation 40 and interleukin-4 activation of mouse B lymphocytes. Cell Tissue Res. 2022. https://doi.org/10.1007/s00441-022-03692-z.
Tan R, Nie M, Long W. The role of B cells in cancer development. Front Oncol. 2022;12: 958756.
Sarvaria A, Madrigal JA, Saudemont A. B cell regulation in cancer and anti-tumor immunity. Cell Mol Immunol. 2017;14(8):662–74.
Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH, et al. CD20+ tumor-infiltrating lymphocytes have an atypical CD27− memory phenotype and together with CD8+ T-cells promote favorable prognosis in ovarian cancer. Clin Cancer Res. 2012;18(12):3281–92.
Katsnelson A. Kicking off adaptive immunity: the discovery of dendritic cells. J Exp Med. 2006;203(7):1622.
Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20:7–24.
Wylie B, Macri C, Mintern JD, Waithman J. Dendritic cells and cancer: from biology to therapeutic intervention. Cancers (Basel). 2019;11(4):521.
Freudenthal PS, Steinman RM. The distinct surface of human blood dendritic cells, as observed after an improved isolation method. Proc Natl Acad Sci. 1990;87(19):7698–702.
Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014;15(7):e257–67.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252.
Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–82.
Gou Q, Dong C, Xu H, Khan B, Jin J, Liu Q, et al. PD-L1 degradation pathway and immunotherapy for cancer. Cell Death Dis. 2020;11(11):955.
Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7–1 and B7–2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21(3):401–13.
Shiravand Y, Khodadadi F, Kashani SMA, Hosseini-Fard SR, Hosseini S, Sadeghirad H, et al. Immune checkpoint inhibitors in cancer therapy. Curr Oncol. 2022;29(5):3044–60.
Bellmunt J, De Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–26.
Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA. 2003;100(8):4712–7.
Boyiadzis MM, Kirkwood JM, Marshall JL, Pritchard CC, Azad NS, Gulley JL. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. J Immunother Cancer. 2018;6(1):35.
Santin AD, Deng W, Frumovitz M, Buza N, Bellone S, Huh W, et al. Phase II evaluation of nivolumab in the treatment of persistent or recurrent cervical cancer (NCT02257528/NRG-GY002). Gynecol Oncol. 2020;157(1):161–6.
Makker V, Taylor MH, Aghajanian C, Oaknin A, Mier J, Cohn AL, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38(26):2981.
Guo Z, Cheng D, Xia Z, Luan M, Wu L, Wang G, et al. Combined TIM-3 blockade and CD137 activation affords the long-term protection in a murine model of ovarian cancer. J Transl Med. 2013;11(1):1–11.
Wu B, Zhong C, Lang Q, Liang Z, Zhang Y, Zhao X, et al. Poliovirus receptor (PVR)-like protein cosignaling network: new opportunities for cancer immunotherapy. J Exp Clin Cancer Res. 2021;40(1):1–16.
Smith M, Lara OD, O’Cearbhaill R, Knisely A, McEachron J, Gabor L, et al. Inflammatory markers in gynecologic oncology patients hospitalized with COVID-19 infection. Gynecol Oncol. 2020;159(3):618–22.
Li J, Cao C, Xiang Y, Hong Z, He D, Zhong H, et al. TLT2 suppresses Th1 response by promoting IL-6 production in monocyte through JAK/STAT3 signal pathway in tuberculosis. Front Immunol. 2020;11:2031.
Lee JB, Ha S-J, Kim HR. Clinical insights into novel immune checkpoint inhibitors. Front Pharmacol. 2021. https://doi.org/10.3389/fphar.2021.681320.
Anderson K, Eskander RN. Immune checkpoint inhibition in the treatment of gynecologic cancer. Curr Obstet Gynecol Rep. 2018;7(1):6–19.
Barani M, Bilal M, Sabir F, Rahdar A, Kyzas GZ. Nanotechnology in ovarian cancer: diagnosis and treatment. Life Sci. 2021;266: 118914.
Bojadzic D, Chen J, Alcazar O, Buchwald P. Design, synthesis, and evaluation of novel immunomodulatory small molecules targeting the CD40–CD154 costimulatory protein-protein interaction. Molecules. 2018;23(5):1153.
Heong V, Ngoi N, Tan DSP. Update on immune checkpoint inhibitors in gynecological cancers. J Gynecol Oncol. 2017. https://doi.org/10.3802/jgo.2017.28.e20.
Solinas C, Migliori E, De Silva P, Willard-Gallo K. LAG3: the biological processes that motivate targeting this immune checkpoint molecule in human cancer. Cancers. 2019;11(8):1213.
Goode EL, Block MS, Kalli KR, Vierkant RA, Chen W, Fogarty ZC, et al. Dose-response association of CD8+ tumor-infiltrating lymphocytes and survival time in high-grade serous ovarian cancer. JAMA Oncol. 2017;3(12): e173290.
Son J, George GC, Nardo M, Krause KJ, Jazaeri AA, Biter AB, et al. Adoptive cell therapy in gynaecologic cancers: a systematic review and meta-analysis. Gynecol Oncol. 2022;165(3):664–70.
Wu JWY, Dand S, Doig L, Papenfuss AT, Scott CL, Ho G, et al. T-Cell receptor therapy in the treatment of ovarian cancer: a mini review. Front Immunol. 2021;12: 672502.
Zhu Y, Zhou J, Zhu L, Hu W, Liu B, Xie L. Adoptive tumor infiltrating lymphocytes cell therapy for cervical cancer. Hum Vaccin Immunother. 2022;18:5.
Zhao L, Cao YJ. Engineered T cell therapy for cancer in the clinic. Front Immunol. 2019;10:2250.
Morotti M, Albukhari A, Alsaadi A, Artibani M, Brenton JD, Curbishley SM, et al. Promises and challenges of adoptive T-cell therapies for solid tumours. Br J Cancer. 2021;124(11):1759–76.
Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. N Engl J Med. 1988;319(25):1676–80.
Kazemi MH, Sadri M, Najafi A, Rahimi A, Baghernejadan Z, Khorramdelazad H, et al. Tumor-infiltrating lymphocytes for treatment of solid tumors: it takes two to tango? Front Immunol. 2022;13:1018962.
Wallen H, Thompson JA, Reilly JZ, Rodmyre RM, Cao J, Yee C. Fludarabine modulates immune response and extends in vivo survival of adoptively transferred CD8 T cells in patients with metastatic melanoma. PLoS ONE. 2009;4(3): e4749.
Shafer P, Kelly LM, Hoyos V. Cancer therapy with TCR-engineered T cells: current strategies, challenges, and prospects. Front Immunol. 2022;13: 835762.
Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233.
Howlader N, Noone A, Krapcho M, Miller D, Brest A, Yu M, et al. SEER Cancer Statistics Review, 1975–2016, National Cancer Institute. Bethesda, MD National Cancer Institute Bethesda, MD; 2019.
Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(9):1274–84.
Barber A, Zhang T, Sentman CL. Immunotherapy with chimeric NKG2D receptors leads to long-term tumor-free survival and development of host antitumor immunity in murine ovarian cancer. J Immunol. 2008;180(1):72–8.
Liu H, Wang S, Xin J, Wang J, Yao C, Zhang Z. Role of NKG2D and its ligands in cancer immunotherapy. Am J Cancer Res. 2019;9(10):2064–78.
Serritella AV, Saenz-Lopez P, Dhar P, Liu S, Wu J. The clinical impact of soluble natural killer cell group 2-member D (NKG2D) receptor ligands on tumor tumorigenicity and anti-tumor immunity. J Clin Oncol. 2023;41(16):e14557.
Uppendahl LD, Dahl CM, Miller JS, Felices M, Geller MA. Natural killer cell-based immunotherapy in gynecologic malignancy: a review. Front Immunol. 2018;8:1825.
Wright SE, Rewers-Felkins KA, Quinlin IS, Phillips CA, Townsend M, Philip R, et al. Cytotoxic T-lymphocyte immunotherapy for ovarian cancer: a pilot study. J Immunother (Hagerstown). 2012;35(2):196.
Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci USA. 2010;107(17):7875–80.
Qin R, Ren W, Ya G, Wang B, He J, Ren S, et al. Role of chemokines in the crosstalk between tumor and tumor-associated macrophages. Clin Exp Med. 2022. https://doi.org/10.1007/s10238-022-00888-z.
De Jong R, Leffers N, Boezen H, ten Hoor KA, van der Zee AG, Hollema H, et al. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114(1):105–10.
Ott PA, Bang YJ, Berton-Rigaud D, Elez E, Pishvaian MJ, Rugo HS, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positiveendometrial cancer: results from the KEYNOTE-028 study. J ClinOncol. 2017;35(22):2535–41.
Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res. 2019;25(13):3753–8.
Bonneville R, Krook MA, Chen HZ, Smith A, Samorodnitsky E, Wing MR, et al. Detection of microsatellite instability biomarkers via next-generation sequencing. Methods Mol Biol. 2020;2055:119–32.
Li K, Luo H, Huang L, Luo H, Zhu X. Microsatellite instability: a review of what the oncologist should know. Cancer Cell Int. 2020;20:16.
Lower SS, McGurk MP, Clark AG, Barbash DA. Satellite DNA evolution: old ideas, new approaches. Curr Opin Genet Dev. 2018;49:70–8.
Makker V, Rasco D, Vogelzang NJ, Brose MS, Cohn AL, Mier J, et al. Lenvatinib plus pembrolizumabin patients with advanced endometrial cancer: an interim analysis of amulticentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20(5):711–8.
Santin A, Hermonat P, Ravaggi A, Bellone S, Cowan C, Coke C, et al. Development and therapeutic effect of adoptively transferred T cells primed by tumor lysate-pulsed autologous dendritic cells in a patient with metastatic endometrial cancer. Gynecol Obstet Invest. 2000;49(3):194–203.
Oaknin A, Gilbert L, Tinker AV, Brown J, Mathews C, Press J, et al. Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: interim results from GARNET-a phase I, single-arm study. J Immunother Cancer. 2022;10(1):e003777.
Oaknin A, Duska L, Sullivan R, Pothuri B. Preliminary safety, efficacy, and pharmacokinetic/pharmacodynamic characterization from GARNET, a phase I/II clinical trial of the anti–PD-1 monoclonal antibody, TSR-042, in patients with recurrent or advanced MSI-h and MSS endometrial cancer. Gynecol Oncol. 2019;154:17.
Doran SL, Stevanović S, Adhikary S, Gartner JJ, Jia L, Kwong MLM, et al. T-cell receptor gene therapy for human papillomavirus-associated epithelial cancers: a first-in-human, phase I/II study. J Clin Oncol. 2019;37(30):2759–68.
Ge Y, Zhang Y, Zhao KN. Emerging therapeutic strategies of different immunotherapy approaches combined with PD-1/PD-L1 blockade in cervical cancer. Drug Des Devel Ther. 2022;16:3055–70.
Mazzotti L, Gaimari A, Bravaccini S, Maltoni R, Cerchione C, Juan M. T-cell receptor repertoire sequencing and its applications: focus on infectious diseases and cancer. Int J Mol Sci. 2022;23(15):8590.
Bryson P, Jia Q, Chen G, Li S, Fang J, Zhao L, et al. 1227p-HPV16 E6-SpecificTCR-T armored with checkpoint blockade in the treatment of cervical cancer. J Immunother Cancer. 2019;30: v502.
Litwin TR, Irvin SR, Chornock RL, Sahasrabuddhe VV, Stanley M, Wentzensen N. Infiltrating T-cell markers in cervical carcinogenesis: a systematic review and meta-analysis. Br J Cancer. 2021;124(4):831–41.
Yu L, Lanqing G, Huang Z, Xin X, Minglin L, Fa-hui L, et al. T cell immunotherapy for cervical cancer: challenges and opportunities. Front Immunol. 2023;14:1105265.
He Y, Li X, Yin C, Wu YM. Killing cervical cancer cells by specific ChimeriAntigen receptor-modified T cells. J Reprod Immunol. 2020;139: 103115.
Zhang Y, Li X, Zhang J, Mao L. Novel cellular immunotherapy UsingNKG2D CAR-T for the treatment of cervical cancer. Bio Med Pharmacother. 2020;131: 110562.
Xu Y, Jiang J, Wang Y, Wang W, Li H, Lai W, et al. Engineered T cell therapy for gynecologic malignancies: challenges and opportunities. Front Immunol. 2021;12: 725330.
Oaknin A, Tinker AV, Gilbert L, Samouëlian V, Mathews C, Brown J, et al. Clinical activity and safety of the anti-PD-1 monoclonal antibody dostarlimab for patients with recurrent or advanced dMMR endometrial cancer. Future Oncol. 2021;17:3781–5.
Broderick JM. FDA Grants LN-145 Breakthrough Designation for Cervical Cancer. 2019. https://www.onclive.com/view/fda-grants-ln145-breakthrough-designation-for-cervical-cancerAccessed 20 Dec 2020.
Kerkar SP, Wang Z, Lasota J, Park T, Patel K, Groh E, et al. MAGE-A is more highly expressed than NY-ESO-1 in a systematic immunohistochemical analysis of 3668 cases. J Immunother. 2016;39:181–7.
Rodriguez-Garcia A, Sharma P, Poussin M, Boesteanu AC, Minutolo NG, Gitto SB, et al. CAR T cells targeting MISIIR for the treatment of ovarian cancer and other gynecologic malignancies. Mol Ther. 2020;28:548–60.
Tanyi JL, Bobisse S, Ophir E, Tuyaerts S, Roberti A, Genolet R, et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci Transl Med. 2018;10(436):eaao5931.
Chiang CL, Kandalaft LE, Tanyi J, Hagemann AR, Motz GT, Svoronos N, et al. A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: from bench to bedside. Clin Cancer Res. 2013;19:4801.
Fader AN, Diaz LA, Armstrong DK, Tanner EJ, Uram JN, Eyring A, et al. Preliminary results of a phase II study: PD-1 blockade in mismatch repair–deficient, recurrent or persistent endometrial cancer. Gynaecol Oncol. 2016;141:206–7.
Yang C, Lee H, Jove V, Deng J, Zhang W, Liu X, et al. Prognostic significance of B-cells and pSTAT3 in patients with ovarian cancer. PLoS ONE. 2013;8(1): e54029.
Wouters MC, Nelson BH. Prognostic significance of tumor-infiltrating B-cells and plasma cells in human cancer. Clin Cancer Res. 2018;24(24):6125–35.
Lundgren S, Berntsson J, Nodin B, Micke P, Jirström K. Prognostic impact of tumour-associated B-cells and plasma cells in epithelial ovarian cancer. J Ovarian Res. 2016;9(1):1–9.
Gupta P, Chen C, Chaluvally-Raghavan P, Pradeep S. B-cells as an immune-regulatory signature in ovarian cancer. Cancers. 2019;11(7):894.
Biagi E, Rousseau R, Yvon E, Schwartz M, Dotti G, Foster A. Responses to human CD40 ligand/human interleukin-2 autologous cell vaccine in patients with B cell chronic lymphocytic leukemia. Clin Cancer Res. 2005;11(19):6916–23.
Kugler A, Seseke F, Thelen P, Kallerhoff M, Müller GA, Stuhler G, et al. Autologous and allogenic hybrid cell vaccine in patients with metastatic renal cell carcinoma. Br J Urol. 1998;82(4):487–93.
Trefzer U, Weingart G, Chen Y, Herberth G, Adrian K, Winter H, et al. Hybrid cell vaccination for cancer immune therapy: first clinical trial with metastatic melanoma. Int J Cancer. 2000;85(5):618–26.
Mandal G, Biswas S, Anadon CM, Yu X, Gatenbee CD, Prabhakaran S, et al. IgA-dominated humoral immune responses govern patients’ outcome in endometrial cancer. Cancer Res. 2021. https://doi.org/10.1158/0008-5472.CAN-21-2376.
Dzopalic T, Rajkovic I, Dragicevic A, Colic M. The response of human dendritic cells to co-ligation of pattern-recognition receptors. Immunol Res. 2012;52:20–33.
Murthy V, Moiyadi A, Sawant R, Sarin R. Clinical considerations in developing dendritic cell vaccine-based immunotherapy protocols in cancer. Curr Mol Med. 2009;9:725–31.
Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, Brugger W. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood. 2000;96:3102–8.
Bhargava A, Srivastava RK, Mishra DK, Tiwari RR, Sharma RS, Mishra PK. Dendritic cell engineering for selective targeting of female reproductive tract cancers. Indian J Med Res. 2018;148(Suppl):S50–63.
Chen B, Liu L, Xu H, Yang Y, Zhang L, Zhang F. Effectiveness of immune therapy combined with chemotherapy on the immune function and recurrence rate of cervical cancer. Exp Ther Med. 2015;9:1063–7.
Coosemans A, Vanderstraeten A, Tuyaerts S, Verschuere T, Moerman P, Berneman Z, et al. Immunological response after WT1 mRNA-loaded dendritic cell immunotherapy in ovarian carcinoma and carcinosarcoma. Anticancer Res. 2013;33:3855–9.
Gray H, Benigno B, Berek J, Chang J, Mason J, Mileshkin L, et al. Progression-free and overall survival in ovarian cancer patients treated with CVac, a mucin 1 dendritic cell therapy in a randomized phase 2 trial. J Immunother Cancer. 2016;4(1):34.
Jiang L, Liu G, Ni W, Zhang N, Jie J, Xie F, et al. The combination of MBP and BCG-induced dendritic cell maturation through TLR2/TLR4 promotes Th1 activation in vitro and vivo. Mediat Inflamm. 2017;2017:1–14.
Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604.
Goyvaerts C, Breckpot K. The journey of in vivo virus engineered dendritic cells from bench to bedside: a bumpy road. Front Immunol. 2018. https://doi.org/10.3389/fimmu.2018.02052.
Moyer TJ, Zmolek AC, Irvine DJ. Beyond antigens and adjuvants: formulating future vaccines. J Clin Investig. 2016;126(3):799–808.
Idoyaga J, Lubkin A, Fiorese C, Lahoud MH, Caminschi I, Huang Y, et al. Comparable T helper 1 (Th1) and CD8 T-cell immunity by targeting HIV gag p24 to CD8 dendritic cells within antibodies to Langerin, DEC205, and Clec9A. Proc Natl Acad Sci. 2011;108(6):2384–9.
Birkholz K, Schwenkert M, Kellner C, Gross S, Fey G, Schuler-Thurner B, et al. Targeting of DEC-205 on human dendritic cells results in efficient MHC class II–restricted antigen presentation. Blood J Am Soc Hematol. 2010;116(13):2277–85.
Mastelic-Gavillet B, Balint K, Boudousquie C, Gannon PO, Kandalaft LE. Personalized dendritic cell vaccines—recent breakthroughs and encouraging clinical results. Front Immunol. 2019;10:766.
Roddie C, O’Reilly M, Dias Alves Pinto J, Vispute K, Lowdell M. Manufacturing chimeric antigen receptor T cells: issues and challenges. Cytotherapy. 2019;21:327–40.
Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. ‘Off- the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19:185–99.
Ren J, Liu X, Fang C, Jiang S, June CH, Zhao Y. Multiplex genome editing togenerate universal CAR T cells resistant to PD1 inhibition. Clin Cancer Res. 2017;23:2255–66.
Lund FE, Randall TD. Effector and regulatory B-cells: modulators of CD4+ T-cell immunity. Nat Rev Immunol. 2010;10(4):236–47.
Irvine DJ, Maus MV, Mooney DJ, Wong WW. The future of engineered immune cell therapies. Science. 2022;378(6622):853–8.
Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non–small cell lung cancer. Clin Cancer Res. 2008;14(16):5220–7.
Nedergaard BS, Ladekarl M, Nyengaard JR, Nielsen K. A comparative study of the cellular immune response in patients with stage IB cervical squamous cell carcinoma. Low numbers of several immune cell subtypes are strongly associated with relapse of disease within 5 years. Gynecol Oncol. 2008;108(1):106–11.
Acknowledgements
The authors are highly grateful to the NSHM Knowledge Campus, Kolkata, Maulana Abul Kalam Azad University of Technology, Jadavpur University and Chittaranjan National Cancer Institute, Kolkata for their continuous support.
Author information
Authors and Affiliations
Contributions
SD, SG, TC, NA, RP, and SM participated in data collection and manuscript writing. SD and VN drafted and SR revised the final manuscript. All authors have fully read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing financial and non-financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dasgupta, S., Gayen, S., Chakraborty, T. et al. Potential role of immune cell therapy in gynecological cancer and future promises: a comprehensive review. Med Oncol 41, 98 (2024). https://doi.org/10.1007/s12032-024-02337-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-024-02337-1