Abstract
Purpose
Intracardiac transcatheter interventions allow for reducing trauma and hospitalization stays as compared to standard surgery. In the treatment of mitral regurgitation, the most widely adopted transcatheter approach consists in deploying a clip on the mitral valve leaflets by means of a catheter that is run through veins from a peripheral access to the left atrium. However, precise manipulation of the catheter from outside the body while copying with the path constraints imposed by the vessels remains challenging.
Methods
We proposed a path tracking control framework that provides adequate motion commands to the robotic steerable catheter for autonomous navigation through vascular lumens. The proposed work implements a catheter kinematic model featuring nonholonomic constraints. Relying on the real-time measurements from an electromagnetic sensor and a fiber Bragg grating sensor, a two-level feedback controller was designed to control the catheter.
Results
The proposed method was tested in a patient-specific vessel phantom. A median position error between the center line of the vessel and the catheter tip trajectory was found to be below 2 mm, with a maximum error below 3 mm. Statistical testing confirmed that the performance of the proposed method exhibited no significant difference in both free space and the contact region.
Conclusion
The preliminary in vitro studies presented in this paper showed promising accuracy in navigating the catheter within the vessel. The proposed approach enables autonomous control of a steerable catheter for transcatheter cardiology interventions without the request of calibrating the intuitive parameters or acquiring a training dataset.
Similar content being viewed by others
Introduction
Mitral regurgitation (MR) is a type of heart valve disease in which the valve between the left atrium (LA) and the left ventricle (LV) does not completely close, allowing blood to leak backward. MR increases the pressure in the pulmonary venous channel and the left atrial chamber, weakening the heart walls, causing shortness of breath, fatigue, and in chronic cases, heart failure. According to the report on the population-based studies in USA, 1.7% of adult population and 9.3% of adults over the age of 75 suffer from MR [1]. Moreover, the annual mortality rate is about 34%. Open-chest surgery can provide immediate relief unlike medication, but 50 % of the MR patients are not recommended open-heart surgery due to their age and possibilities of postoperative complications [2].
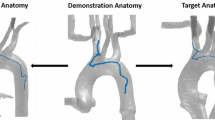
Transcatheter mitral valve repair approaches are gaining popularity due to reduced invasiveness and shorter recovery time. Moreover, transcatheter approaches offer an alternative treatment for patients who cannot undergo open-chest surgery. With systems such as the MitraClip\(^{TM}\) (MC) device (Abbott Laboratories, IL), mitral valve competence can be restored by enforcing leaflet coaptation through the implantation of a clip. The cardiologist inserts a steerable catheter, commonly called sheath catheter, from the femoral vein, passing a sequence of narrow and rugged vessels to reach the right atrium (RA). From there, the LA is accessed through a puncture in the atrial septum (Fig. 1). After successfully accessing the LA, the operator inserts the delivery catheter through the sheath catheter to the mitral valve and implants the clip [3]. However, to visualize the catheter during the procedure, both patients and operators would be exposed to damaging radiation [4]. In addition, given the poor image quality and the lack of depth, a significant risk of embolization or perforation exists [5].
To address these challenges in maneuvering the MC steerable sheath catheter, we proposed a control framework for a customized-built actuator to autonomously advance it in a continuous fashion along a pre-planned path. By providing the appropriate steering commands based on the catheter kinematic model, the tip of the catheter tracks the center line of the vessel to avoid intense contact between the acute catheter tip and the fragile vessel wall. To ensure precise steering, a feedback controller is implemented to control the tension on the tendon and reject the error of the steering angle in the joint space. Experiments were carried out in a vessel phantom to evaluate the performance of the system.
Related work
Robotic technology has emerged as an important tool for catheters deployment. In 2011, CorPath 200 (Corindus Inc., USA) was introduced as the first robot-assisted system to control coronary guidewires and stents for coronary angioplasty procedure [6]. Subsequently, the Sensei Robotic Navigation System (Hansen Medical Inc., USA) was developed and evaluated for catheter ablation of atrial fibrillation [7] and ventricular arrhythmias [8]. The most recent Sensei X Robotic System (Hansen Medical Inc., USA) expands its application on collecting electrophysiological data inside the heart chambers [9]. Furthermore, researchers have developed catheter prototypes with haptic interfaces to enhance the safety of robot-assisted percutaneous coronary interventions (PCI) [10,11,12]. Despite successful clinical studies, robot-assisted PCI is only limited to a few clinics with skilled surgeons because of the steep learning curve and expensive hardware [13].
The adoption of task autonomy, where the surgeon supervises the procedure while the robot performs the task autonomously, could address this challenge [14]. Fagogenis et al. contributed to the field by autonomously navigating a concentric tube robot inside the beating heart [15]. Sganga proposed an autonomous navigation method for flexible robots in the lungs to diagnose cancer [16]. Yang et al. introduced an autonomous control algorithm for magnetic microrobot navigation in a large workspace [17]. Although robotic catheters are considered ideal for medical applications due to their particular structure, their compliance can pose difficulties in tracking a constrained path inside the body.
Open-loop control relies on model inversion to determine the appropriate actuation values for achieving the desired robot state. In the context of controlling robotic catheters, some researchers have proposed approaches based on specific kinematic models. [18,19,20]. Greigarn et al. introduce the pseudo-rigid-body (PRB) model on a robotic catheter by approximating the catheter as rigid links connected by flexible joints [21]. Bailly et al. proposed a differential model-based control scheme for a continuum robot [22]. Although these methods could control catheters in free space, it is still difficult to reach precise motion in realistic scenarios where catheters move in a constrained environment (i.e. the vessels) where contact with the vessel wall is inevitable with open-loop approaches [23].
The data-driven approaches were investigated to overcome the challenges posed by the high complexity of continuum robot kinematics, Michael et al. proposed a model-less control method for controlling a tendon-driven continuum manipulator [24]. In addition, Di et al. introduced a deep learning-based compliant motion controller for a robotic catheter [25]. However, the low compatibility rate of change in the environment and disturbances, as well as the complexity of learning approaches, may limit their application in medical scenarios where surgical instruments are single use and patients have different anatomies [26].
Schematic of the catheter actuation system: A top view of the steerable catheter shows a 310-mm-long catheter body and a 47-mm-long steerable segment with a maximum steering angle of \(60^{\circ }\); B cross-section view of the steerable catheter indicates the outer diameter of the catheter and the position of the steering wires; C the robotic system has three actuated DOFs in total, including the rotational motion, the translational motion, and the steering motion
The development of pose-tracking techniques and shape sensors have demonstrated great potential to close the control loop of robotic catheters and compensate for łinaccuracies of the model. In particular, electromagnetic (EM) tracking techniques have been widely applied to track the robotic catheter within the human body [27, 28]. Loschak et al. developed a robotic ultrasound imaging catheter to control the position and orientation based on EM [29]. Omisore et al. proposed a robotic catheter with adaptive compensation of backlash with an EM sensor fixed at the tip of the catheter [30]. In addition, relying on the shape reconstruction from fiber Bragg grating (FBG) sensor, Sefati et al. designed an optimization-based control algorithm to position the continuum manipulators interacting with unknown obstacles [31].
Method
Steerable catheter kinematics
The tendon-driven steerable catheter is composed by a 310-mm-long catheter body and a 47-mm-long steerable segment at the distal tip side space(Fig. 2A). The steerable segment is able to generate a \(60^{\circ }\) steering angle from the straight position. Two antagonistic steering wires travel along the length of the catheter body up to the tip of the 25-Fr steerable catheter to actuate the steerable segment (Fig. 2B). Expect for the tendon-driven steering motion, based on our previous work [32], a catheter driver system with the sleeve-based grippers and the spur gear is used to generate decoupled 2 degrees of freedom (DOFs) (i.e., translation and rotation) (Fig. 2C).
The “bicycle” model is often used in autonomous driving by characterizing the system with two main wheels, capturing both lateral and longitudinal motion. Regarding the catheter, assuming that the translational motion and the rotational motion propagate ideally from the base through the catheter body to the tip, the distal steerable segment of the catheter can be modeled as a kinematic nonholonomic system, an extension of the “bicycle" model [33], including all the three actuated DOFs. As shown in Fig. 3, the front wheel (A) of the bicycle is attached to the tip of the catheter, and the rear wheel (B) is located on the proximal end of the steerable segment. Generally speaking, advancing the catheter can be approximated as “cycling” forward at a speed, \(\textbf{u}\), and bending the catheter with an angle, \(\theta \), is like steering the front wheel of the bicycle. The combination of these two motions will generate a circular trajectory with a constant curvature radius, r, and a rotation center at point C. Furthermore, by rotating the catheter about its longitudinal axis with a rotation angle, \(\varphi \), one can control the angle of the planar trajectory.
Non-holonomic motion of the catheter modeled with a so-called bicycle model showing the steerable segment of the catheter showing the front and rear “wheels” at frame A and frame B of a bicycle model, including three DOFs: rotation angle \({\varvec{{\varphi }}}\), insertion speed \(\textbf{u}\), and in-plane steering angle \(\theta \)
Control strategy
This work combines two control strategies: 1) path tracking control, a high-level controller, is used to correct the motion of the steerable catheter when it deviates from the path; 2) feedback control, a low-level controller, is used to drive the actuation system to reach a desired steering angle (Fig. 4).
High-level (Path tracking) controller
The path tracking controller is a nonlinear feedback controller which reduces the tracking error between the measured tip position \(\textbf{p}_{\textrm{m}} \) and the closest point \(\textbf{p}_k\) on the desired path \(\textbf{p}_i [ x_i,y_i,\gamma _i] \). The control law consists of two parts, which account for the orientation error, \(e_{\theta } \left( t \right) \), and the distance offset error, \(e_{\delta } \left( t \right) \), as shown in Eqs. (1), (2), and (3). These two terms are the steering control elements based on the “Stanly method” [34]. Within the control loop, \(e_{\theta } \left( t \right) \) is intuitively aligning the orientation of the tip, \(\gamma _m \left( t \right) \), to match the orientation of the desired path, \(\gamma _k\left( t \right) \), and the second term adjusts the steering angle in (nonlinear) proportion to the distance error \(e_{d}\left( t \right) \). In other words, it controls the steering angle, \(\theta _{d} (t)\), such that the intended trajectory intersects the path at point \(\textbf{p}_d\) at the next time step \(t+1\) (Fig. 5). Additionally, the contribution of \(e_{\theta } \left( t \right) \) and \(e_{\delta } \left( t \right) \) can be adjusted by tuning the orientation gain factor, \(k_{\theta }\), for adapting to different paths. The steering gain factor, \(k_{d}\), determines the rate of convergence toward the path.
Control framework: The high-level controller accepts the preoperative desired path \(\textbf{p}_i\) as input and finds the closest point \(\textbf{p}_k\) with respect to the measured tip pose \( \textbf{p}_m [ x(t)_m,y(t)_m,\gamma (t)_m ]\) from the pose sensor. Then it computes the distance error \(e_d(t)\) and the orientation error \(e_\theta (t)\) between \(\textbf{p}_k\) and \(\textbf{p}_m\). Combining the insertion velocity \(\textbf{u}(t)\) in the nonlinear part and the orientation error \(e_\theta (t)\) with the orientation gain \(k_\theta \), the path tracking controller outputs the desired angle \(\theta _d(t)\) to the steering controller. On the low-level side, the PID feedback controller obtains the measured angle \(\theta _m(t)\) from the curvature sensor and outputs the tension on the tendon \(\textrm{t} _c(t)\) to the plant
Path tracking strategy: based on the current position \(\textbf{p}_m \) and the desired path \(\textbf{p}_i [x_i,y_i,\gamma _i] \), the steering angle \(\theta \) can be computed by considering the distance error \(e_{d}\) and the orientation error \(e_{\theta }\) with respect to the closed point \(\textbf{p}_k\) on the desired path. At the next time step, the catheter will intersect with the path on point \(\textbf{p}_d\) with a steady speed \(\textbf{u}\)
According to the bicycle model [35], the time derivative of the distance error, \(\dot{e}_{d}\left( t \right) \), can be written as follows:
and hence, for a small distance error \( e_{d }\),
Thus, the distance error converges exponentially to zero.
Low-level (feedback) controller
The desired steering angle is then sent to the low-level controller, which is a PID feedback controller in the actuation space (Eq. (6)).
where \(t_C(t)\) is the tension applied on the tendon, e(t) is the error between the desired steering angle, \(\theta _d(t)\), and the measured steering angle, \(\theta _m(t)\), and \(K_p, K_i, K_d\) are the proportional, integral and derivative gains, respectively.
Experimental setup and protocols
Experimental setup
The experiment was performed by actuating and controlling a tendon-driven steerable catheter using a sleeve-based robotic catheter driver. Moreover, a 6-DOFs EM sensor (NDI, Canada) was attached to the tip of the steerable catheter as the pose sensor, and an FBG stylet (FBGS, Technologies GmbH. Germany) with 1 cm spacing was placed in the channel of the catheter as the curvature sensor (Fig. 6). All the data communication was conducted and synchronized on the ROS (robot operating system).
Experimental setup: A tendon-driven steerable catheter, equipped with an EM sensor and a FBG style of 1 cm spacing. The catheter drive advances the steerable catheter into the vessel phantom, which is placed on top of the EM generator. In addition, eight calibration pillars are used to find the transformation matrix \(\textbf{T}\), between the EM reference frame \(\{\textbf{EM}\}\), and the phantom reference frame \(\{\textbf{PH}\}\). A camera is mounted above to record the trajectory as the ground truth
The EM generator was placed below the catheter to generate the magnetic field for tracking the EM sensor, which acquires the pose at 40 Hz with a standard deviation of 1.4 mm, A calibration procedure was implemented to compute the registration matrix between the vessel phantom and the EM coordinate. The position of eight calibration pillars was measured, which were pre-set on the phantom with a 6-DOFs EM probe (NDI, Canada). Each position was acquired ten times to reduce the acquisition error and reject the noise from the EM sensor. Then the transformation matrix, \(\textbf{T}\), between the pillar’s positions in phantom space \(\{\textbf{PH}\}\) and the pillar position in the EM space \(\{\textbf{EM}\}\) was calculated based on the singular value decomposition approach [36].
The wavelengths from the FBG interrogator Were obtained (FBGS, Technologies GmbH. Germany) and converted to a point cloud depicting the shape of the catheter [37]. To measure the steering angle, two points were selected at the distal end of the FBG stylet to represent the orientation of the tip and two points behind to represent the orientation of the base. Those last two points were selected by calibrating the steering angle with a protractor.
As the ground truth to evaluate the accuracy of the proposed approach, a camera (Prosilica GT, Allied Vision Technologies GmbH. Germany) with a resolution of 31 megapixels and frame rates of 53 frames per second was mounted on a shelf above the phantom to record the trajectory of the catheter (Fig. 6). One piece of green tape was attached to the tip of the catheter as the tracking markers. Before each test, a checkboard with \(25\times 25\) mm squares was put below the camera for calibration. The camera data was processed by converting the unit from pixel to millimeter based on the calibration data.
The vessel phantom had the laser cutting boundary for the shape of the vessel and two acrylic planes for constraining motion in a plane (Fig. 6). The 3D anatomical model of the vessel was manually segmented and reconstructed using 3D slicer (Harvard University, National Institutes of Health), based on the CT scan images that we obtained from the hospital (Ospedale San Raffaele, Milan, Italy). Then, the 3D anatomy was projected in the coronal plane in Matlab (Mathworks, Massachusetts, USA), similar to the fluoroscopy image in the clinical procedure. The center line was extracted from the boundary of the vessel as the desired path (Fig. 7A). To reduce the friction resulting from the radial compression of the wooden boundary against the catheter body, a scaling factor of 1.5 to the vessel phantom was applied. The experiment was carried out in the first 60 mm section of the vessel phantom, which is the projection of the femoral vein and the iliac vein and the most tortuous part.
Experimental protocols
To evaluate the accuracy of the control framework at each measured point j, we calculated the tracking error, \(e_j\), which was the minimum Euclidean distance between the measured tip position of the catheter \(\textbf{p}_{j} \left( x_{j},y_{j} \right) \) from the camera data and the pre-defined desired path \(\textbf{p}_{i} \left( x_{i},y_{i} \right) \), which contains I points (Eq. 7).
However, the distribution of errors along the entire path is not normal, because the level of challenge in navigating the catheter under various environmental constraints is different due to the insertion speed \(\textbf{u}\), and the path geometry. To comprehensively evaluate tracking accuracy throughout the entire path, all the measurement was divided into ten equal sections, which contain n measured points. The associated median value (MED) in each section was calculated, which allowed us to intuitively compare the performance of the controller under different settings. Matlab (Mathworks, USA) was also used to calculate the maximum value (Max), and interquartile range (IQR). We examined the data set distribution with the Shapiro-Wilk normality test and applied corresponding descriptive statistics to compare different groups. In total, eight tests were carried out, and the velocity dependency was analyzed by setting the insertion speed \(\textbf{u}\) at 1 mm/s, 2 mm/s, and 3 mm/s.
The proposed autonomous control framework was compared with a hybrid control system, which used a joystick controller (PS4, SONY Inc.) as the control element. In this system, the experiment participant was asked to maintain the tip of the catheter at the center of the vessel by controlling the steering DOF of the catheter with the joystick, while the catheter driver autonomously progressed the catheter at a constant insertion speed of 1 mm/s. After three tests, the most confident trajectory was selected as the hybrid control group to compare with the proposed autonomous control framework.
The controller was validated in both a free space and a constrained environment. In the first half of the path, there is no contact between the catheter and the boundary of the phantom (Fig. 7B). Then the catheter body collided with the upper boundary but navigation continues (Fig. 7C). The MED, Max, IQR was computed to evaluate the tracking accuracy in the region with and without environmental constraints.
Experimental results and discussion
Results
With the proposed controller, the tip of the robotic catheter successfully follows the center line of the vessel phantom. Figure 8A–H presents the trajectories recorded by the camera corresponding with different insertion speed and control gains, in which the real trajectories of the catheter tip in blue follow the centerline in red, and away from the black vessel border. The results of the Shapiro–Wilk normality test on the tracking error (p-value \(< 0.05\)) show that the error distributes abnormally on the path. The Kruskal–Wallis test shows that the insertion speed is significantly affecting the tracking performance (p-value \(< 0.05\)). The tracking results in the box plots indicate that the increase in the insertion speed will raise the tracking error because the robot doesn’t have enough time to respond and converge to the desired path. When the catheter is inserted at 1 mm/s, the controller has the most accurate performance with a MED along the entire path of 1.43 mm, and a Max of 2.19 mm (Fig. 9A). Compared with the hybrid control system, which has a MED value of 2.65 mm and a Max of 5.51 mm, the result in group H with the optimal control gain has a median value below 2 mm and a maximum error below 3 mm (Fig. 9B). The Mann–Whitney U test indicates a highly significant difference between the performances of those two methods (p-value \(< 0.05\)).
The contact between the catheter body and the vessel wall was identified at approximately \(50\%\) of the trajectory where a salient drop was observed. To show the precision of the controller in both free space and the contact region, the MED, Max, and IQR in those regions were calculated and compared (Table 1). No significant difference was identified with the Mann–Whitney U test, indicating that the controller performed consistently in both free space and contact regions (p-value \(> 0.05\)), across different insertion speeds regarding groups H, G, and C.
Discussion
We proposed a control framework to allow a safer and more autonomous insertion by avoiding contact between the catheter tip and the vessel wall. Our method achieves a convincing result and the insertion velocity has been considered in the control loop. In cardiovascular applications, the required precision that clinicians indicate as being acceptable is typically in the order of 1–3 mm [38, 39]. Our results from the in vitro studies showed that fidelity of the tracking could satisfy this requirement. Note that the test path does not cover the entire vessel which is 330 mm, because of the limited length of the catheter. A shortcoming of this work is the lack of adaptive methodology for regulating the insertion speed and the control gain factors. The insertion speed affects the performance of the controller and the final procedure time. The control gain factors are sensitive to the radius of the path and the contact region. Given these same wrenches, a Bayesian optimization approach may be used to choose those parameters for the patient-specific path.
In the future, the proposed controller can be extended in these two directions. 1) Path tracking with 3-D path: combing the rotation of the working plane could allow the catheter to achieve any points in the cartesian space. Furthermore, the working plane should be properly chosen to compensate for the gravity effect. 2) Adaptive control: The insertion velocity and the control gain factors could be regulated automatically based on the Bayesian optimization method to self-adapt different paths, which can reduce tracking errors and minimize the procedure time.
Conclusion
In this work, we proved the feasibility of controlling a robotic catheter to track a given path with the proposed control framework. A tendon-driven steerable catheter was actuated and tested in vitro. The results suggest the potential for increasing the level of autonomy in robotic catheters to revolutionize transcatheter cardiology interventions. Compared with other model-based or model-less feedback control methods, our method achieves satisfying accuracy without the request of calibrating the intuitive parameters nor acquiring a training dataset. Moreover, nonholonomic motion planning and control have been extensively explored in robotics literature, allowing us to leverage a vast array of existing research in applying our model.
References
Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M (2006) Burden of valvular heart diseases: a population-based study. The Lancet 368(9540):1005–1011
Mirabel M, Iung B, Baron G, Messika-Zeitoun D, Détaint D, Vanoverschelde J-L, Butchart EG, Ravaud P, Vahanian A (2007) What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 28(11):1358–1365
Isaac YW, Matthew BB, Rebecca TH (2018) The mitraclip procedure–a comprehensive review for the cardiac anesthesiologist. J Cardiothorac Vasc Anesth 32(6):2746–2759
Simon HS, Patric B, Jürg G, Michael G, Christian F, Christian F, Volkmar F, Roberto C (2014) Safety and feasibility of novel technology fusing echocardiography and fluoroscopy images during mitraclip interventions. EuroIntervention 9(10):1210–1216
Chhatriwalla AK, Vemulapalli S, Holmes DRJ, Dai D, Li Z, Ailawadi G, Glower D, Kar S, Mack MJ, Rymer J, Kosinski AS, Sorajja P (2019) Institutional experience with transcatheter mitral valve repair and clinical outcomes: insights from the TVT registry. JACC Cardiovasc Interv 12(14):1342–1352
Granada JF, Delgado JA, Uribe MP, Fernandez A, Blanco G, Leon MB, Weisz G (2011) First-in-human evaluation of a novel robotic-assisted coronary angioplasty system. JACC Cardiovasc Interv 4(4):460–465
Rillig A, Schmidt B, Di Biase L, Lin T, Scholz L, Heeger CH, Metzner A, Steven D, Wohlmuth P, Willems S, Trivedi C, Galllinghouse JG, Natale A, Ouyang F, Kuck K-H, Tilz RR (2017) Manual versus robotic catheter ablation for the treatment of atrial fibrillation: the man and machine trial. JACC: Clin Electrophys 3(8):875–883
Valderrábano M, Dave AS, Báez-Escudero JL, Rami T (2011) Robotic catheter ablation of left ventricular tachycardia: initial experience. Heart Rhythm 8(12):1837–1846
Bassil G, Markowitz SM, Liu CF, Thomas G, Ip JE, Lerman BB, Cheung JW (2020) Robotics for catheter ablation of cardiac arrhythmias: current technologies and practical approaches. J Cardiovasc Electrophysiol 31(3):739–752
Shi P, Guo S, Zhang L, Jin X, Hirata H, Tamiya T, Kawanishi M (2021) Design and evaluation of a haptic robot-assisted catheter operating system with collision protection function. IEEE Sens J 21(18):20807–20816
Woo J, Song H-S, Cha H-J, Yi B-J (2019) Advantage of steerable catheter and haptic feedback for a 5-dof vascular intervention robot system. Appl Sci 9(20):4305
Zhang L, Guo S, Yu H, Song Y, Tamiya T, Hirata H, Ishihara H (2018) Design and performance evaluation of collision protection-based safety operation for a haptic robot-assisted catheter operating system. Biomed Microdevice 20(2):1–14
Dupont PE, Nelson BJ, Goldfarb M, Hannaford B, Menciassi A, O’Malley MK, Simaan N, Valdastri P, Yang G-Z (2021) A decade retrospective of medical robotics research from 2010 to 2020. Sci Robot 6(60):8017
Yang G-Z, Cambias J, Cleary K, Daimler E, Drake J, Dupont PE, Hata N, Kazanzides P, Martel S, Patel RV, Santos VJ, Taylor RH (2017) Medical robotics—Regulatory, ethical, and legal considerations for increasing levels of autonomy. American Association for the Advancement of Science
Fagogenis G, Mencattelli M, Machaidze Z, Rosa B, Price K, Wu F, Weixler V, Saeed M, Mayer JE, Dupont PE (2019) Autonomous robotic intracardiac catheter navigation using haptic vision. Sci Robot 4(29):1977
Sganga J, Eng D, Graetzel C, Camarillo DB (2019) Autonomous driving in the lung using deep learning for localization. arXiv preprint arXiv:1907.08136
Yang Z, Yang L, Zhang L (2021) Autonomous navigation of magnetic microrobots in a large workspace using mobile-coil system. IEEE/ASME Trans Mechatron 26(6):3163–3174
Ganji Y, Janabi-Sharifi F (2009) Catheter kinematics for intracardiac navigation. IEEE Trans Biomed Eng 56(3):621–632
Greigarn T, Poirot NL, Xu X, Çavuşoğlu MC (2018) Jacobian-based task-space motion planning for MRI-actuated continuum robots. IEEE Robot Automat Lett 4(1):145–152
Rucker DC, Webster RJ (2011) Computing jacobians and compliance matrices for externally loaded continuum robots. In: 2011 IEEE international conference on robotics and automation, pp. 945–950. IEEE
Greigarn T, Jackson R, Liu T, Çavuşoğlu MC (2017) Experimental validation of the pseudo-rigid-body model of the mri-actuated catheter. In: 2017 IEEE International conference on robotics and automation (ICRA). IEEE, pp. 3600–3605
Bailly Y, Amirat Y, Fried G (2011) Modeling and control of a continuum style microrobot for endovascular surgery. IEEE Trans Rob 27(5):1024–1030
Coevoet E, Escande A, Duriez C (2017) Optimization-based inverse model of soft robots with contact handling. IEEE Robot Autom Lett 2(3):1413–1419
Yip MC, Camarillo DB (2014) Model-less feedback control of continuum manipulators in constrained environments. IEEE Trans Rob 30(4):880–889
Wu D, Ha XT, Zhang Y, Ourak M, Borghesan G, Niu K, Trauzettel F, Dankelman J, Menciassi A, Vander Poorten E (2022) Deep-learning-based compliant motion control of a pneumatically-driven robotic catheter. IEEE Robot Autom Lett 7(4):8853–8860
Chikhaoui MT, Burgner-Kahrs J (2018) Control of continuum robots for medical applications: State of the art. In: ACTUATOR 2018; 16th International conference on new actuators. VDE, pp. 1–11
Shi C, Luo X, Qi P, Li T, Song S, Najdovski Z, Fukuda T, Ren H (2016) Shape sensing techniques for continuum robots in minimally invasive surgery: a survey. IEEE Trans Biomed Eng 64(8):1665–1678
Dore A, Smoljkic G, Vander Poorten E, Sette M, Vander Sloten J, Yang G-Z (2012) Catheter navigation based on probabilistic fusion of electromagnetic tracking and physically-based simulation. In: 2012 IEEE/RSJ International conference on intelligent robots and systems. IEEE, pp. 3806–3811
Loschak PM, Brattain LJ, Howe RD (2016) Algorithms for automatically pointing ultrasound imaging catheters. IEEE Trans Rob 33(1):81–91
Omisore OM, Han SP, Ren LX, Wang GS, Ou FL, Li H, Wang L (2018) Towards characterization and adaptive compensation of backlash in a novel robotic catheter system for cardiovascular interventions. IEEE Trans Biomed Circuits Syst 12(4):824–838
Sefati S, Murphy RJ, Alambeigi F, Pozin M, Iordachita I, Taylor RH, Armand M (2018) Fbg-based control of a continuum manipulator interacting with obstacles. In: 2018 IEEE/RSJ International conference on intelligent robots and systems (IROS). IEEE, pp. 6477–6483
Al-Ahmad O, Ourak M, Smits J, Jeanquart S, Deserranno N, Bernhard F, Kassahun Y, Yu B, Vander Poorten E (2018) Development of an innovative sleeve-based robotic catheter driver. In: Joint workshop on new technologies for computer/robot assisted surgery, Date: 2018/09/10-2018/09/11, Location: London
Webster RJ III, Kim JS, Cowan NJ, Chirikjian GS, Okamura AM (2006) Nonholonomic modeling of needle steering. Int J Robot Res 25(5–6):509–525
Thrun S, Montemerlo M, Dahlkamp H, Stavens D, Aron A, Diebel J, Fong P, Gale J, Halpenny M, Hoffmann G, Lau K, Oakley C, Palatucci M, Pratt V, Stang P (2006) Stanley: The robot that won the darpa grand challenge. J Field Robot 23(9):661–692
Fallahi B, Khadem M, Rossa C, Sloboda R, Usmani N, Tavakoli M (2015) Extended bicycle model for needle steering in soft tissue. In: 2015 IEEE/RSJ International conference on intelligent robots and systems (IROS). IEEE, pp. 4375–4380
Boles M, Fu J, Iovene E, Francesco C, Ferrigno G, De Momi E (2022) Augmented reality and robotic navigation system for spinal surgery. In: Proceeding of the 11th joint workshop on new technologies for computer/robot assisted surgery, pp. 96–97
Al-Ahmad O, Ourak M, Van Roosbroeck J, Vlekken J, Vander Poorten E (2020) Improved fbg-based shape sensing methods for vascular catheterization treatment. IEEE Robot Automat Lett 5(3):4687–4694
Nijland H, Gerbers J, Bulstra S, Overbosch J, Stevens M, Jutte P (2017) Evaluation of accuracy and precision of ct-guidance in radiofrequency ablation for osteoid osteoma in 86 patients. PLoS ONE 12(4):0169171
Bourier F, Reents T, Ammar-Busch S, Buiatti A, Grebmer C, Telishevska M, Brkic A, Semmler V, Lennerz C, Kaess B, Kottmaier M, Kolb C, Deisenhofer I, Hessling G (2015) Sensor-based electromagnetic navigation (mediguide®): how accurate is it? a phantom model study. J Cardiovascul Electrophys 26(10):1140–1145
Acknowledgements
The authors would like to thank Madhan Chirumamilla from FBGS Technologies GmbH. for providing the sensors and the RAS group at KU Leuven for their suggestions on the experimental setup.
Funding
Open access funding provided by Politecnico di Milano within the CRUI-CARE Agreement. This work was supported by the European Union’s Horizon 2020 research and innovation program, under the project ARTERY, Grant No. 101017140.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, X., Sridhar, A., Ha, X.T. et al. Path tracking control of a steerable catheter in transcatheter cardiology interventions. Int J CARS 19, 757–766 (2024). https://doi.org/10.1007/s11548-024-03069-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-024-03069-3