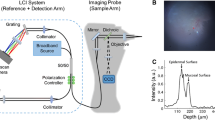
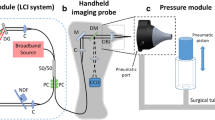
Abstract
In middle ear ailments, the thickness of tympanic membrane (TM) could change due to biofilm depositions. It is useful to measure the vibrations of the TM as well as its thickness and curvature. In the presence of unavoidable human motion, this becomes challenging and requires the use of expensive devices. We demonstrate a low-cost method for measuring these in real-time using a custom-built line-field spectral-domain optical coherence tomography device. Linearity of the amplitude response of the TM as well as its frequency response over the 1–2.5 kHz region is demonstrated. While an in-vivo sensitivity of 2 nm is achieved for the TM, a sensitivity of 200 pm is demonstrated on a membrane phantom. Our device enables a depth range of 6.2 mm using a line-field B-scan that covers a lateral extent of 3 mm with a lateral and depth resolution of 18 µm each.







Similar content being viewed by others
References
Liu Y-F, Liu Q, Li Y-Q, Huang P, Yao J-Y, Hu N and Fu S-Y 2020 Spider-inspired ultrasensitive flexible vibration sensor for multifunctional sensing. ACS Appl. Mater. Interfaces. 12: 30871–30881
Liu Y, Norton J J S, Qazi R, Zou Z, Ammann K R and Liu H et al 2016 Epidermal mechano-acoustic sensing electronics for cardiovascular diagnostics and human-machine interfaces. Sci. Adv.. 2: e1601185
Hsieh H-H, Hsu F-C and Chen Y-F 2018 Energetically autonomous, wearable, and multifunctional sensor. ACS Sensors. 3: 113–120
Rosowski J J, Nakajima H H and Merchant S N 2008 Clinical utility of laser-doppler vibrometer measurements in live normal and pathologic human ears. Ear Hear. 29: 3–19
Kirsten L, Schindler M, Morgenstern J, Erkkila M T, Golde J and Walther J et al 2018 Endoscopic optical coherence tomography with wide field-of-view for the morphological and functional assessment of the human tympanic membrane. J. Biomed. Opt. 24: 1–11
Park K, Cho N M, Jeon M, Lee S H, Jang J H and Boppart S A et al 2018 Optical assessment of the in vivo tympanic membrane status using a handheld optical coherence tomography-based otoscope. Acta Otolaryngol. 138: 367–374
Hamra M, Shinnawi S, Vaizer M C and Yelin D 2020 Rapid imaging of tympanic membrane vibrations in humans. Biomed. Opt. Express 11: 6470–6479
Cheng J T, Hamade M, Merchant S N, Rosowski J J, Harrington E and Furlong C 2013 Wave motion on the surface of the human tympanic membrane: Holographic measurement and modeling analysis. J. Acoust. Soc. Am. 133: 918
Tang H, Razavi P, Pooladvand K, Psota P, Maftoon N and Rosowski J J et al 2019 High-speed holographic shape and full-field displacement measurements of the tympanic membrane in normal and experimentally simulated pathological ears. Appl. Sci. 9: 14
Won J, Hong W, Khampang P, Spillman D R, Marshall S and Yan K et al 2021 Longitudinal optical coherence tomography to visualize the in vivo response of middle ear biofilms to antibiotic therapy. Sci Rep. 11: 5176
Tan H E I, Maria P L S, Wijesinghe P, Kennedy B F and Allardyce B J et al 2018 Optical coherence tomography of the tympanic membrane and middle ear: a review. Otolaryngol. Head Neck Surg. 159: 424–438
Lee J, Wijesinghe R E, Jeon D, Kim P and Choung Y-H et al 2018 Clinical utility of intraoperative tympanomastoidectomy assessment using a surgical microscope integrated with an optical coherence tomography. Sci. Rep. 8: 17432
Huang D, Swanson E A, Lin C P, Schuman J S, Stinson W J and Chang W et al 1991 Optical coherence tomography. Science. 254: 1178–1181
De Boer J F, Leitgeb R and Wojtkowski M 2017 Twenty-five years of optical coherence tomography: the paradigm shift in sensitivity and speed provided by Fourier domain OCT. Biomed. Opt. Express. 8: 3248–3280
Subhash H M, Choudhury N, Chen F, Wang R K, Jacques S L and Nuttall A L 2013 Depth-resolved dual-beamlet vibrometry based on Fourier domain low coherence interferometry. J. Biomed. Opt. 18: 36003
Burkhardt A, Kirsten L, Bornitz M, Zahnert T and Koch E 2014 Investigation of the human tympanic membrane oscillation ex vivo by Doppler optical coherence tomography. J. Biophotonics. 7: 434–441
MacDougall D, Farrell J, Brown J, Bance M and Adamson R 2016 Long-range, wide-field sweptsource optical coherence tomography with GPU accelerated digital lock-in Doppler vibrography for real-time, in vivo middle ear diagnostics. Biomed. Opt. Express. 7: 4621–4635
Kim W, Kim S, Huang S, Oghalai J S and Applegate B E 2019 Picometer scale vibrometry in the human middle ear using a surgical microscope based optical coherence tomography and vibrometry system. Biomed. Opt. Express. 10: 4395–4410
Guo C, Yang X, Wu J-P, Guo X, He Y and Shen Z et al. 2019 Image-guided vibrometry system integrated with spectral- and time-domain optical coherence tomography. Appl. Opt. 58: 1606–1613
Lui C G, Kim W, Dewey J B, Macias-Escriva F D, Ratnayake K and Oghalai J S et al. 2021 In vivo functional imaging of the human middle ear with a hand-held optical coherence tomography device. Biomed. Opt. Express. 12: 5196–5213
Song G, Chu K K, Kim S, Crose M, Cox B and Jelly E T et al. 2019 First clinical application of low-cost OCT. Transl. Vis. Sci. Technol. 8: 61
Won J, Monroy G L, Dsouza R I, Spillman D R, McJunkin J and Porter R G et al. 2021 Handheld briefcase optical coherence tomography with real-time machine learning classifier for middle ear infections. Biosensors. 11: 143
Yaqoob Z, Choi W, Oh S, Lue N, Park Y and Fang-Yen C et al. 2009 Improved phase sensitivity in spectral domain phase microscopy using line-field illumination and self phase-referencing. Opt. Express. 17: 10681–10687
Zhao Z, Zhang Z, Lawman S, Yin Z, Hu Y and Xu J et al. 2021 Characterization of electrical–thermal–mechanical deformation of bonding wires under silicone gel using LF-OCT. IEEE Trans. Power Electron. 9384327: 11045–11054
Nandakumar H, Mallick S P and Srivastava S 2020 Sensing high frequency sub-nanometer vibrations using optical coherence tomography with real-time profilometry of multiple inner layers. Opt. Lasers Eng. 127: 105992
Nandakumar H, Parameshwaran S, Gamini R and Srivastava S 2019 Artifact-free robust single-shot background subtraction for optical coherence tomography. OSA Continuum. 2: 1556–1564
George D M M, Nandakumar H and Srivastava S 2021 Enhancement of dynamic range and complete elimination of self-interference artifacts in a spectral domain OCT: potential for high performance at much lowered-cost. Results Opt. 5: 100144
Dsouza R, Won J, Monroy G L, Spillman D R and Boppart S A 2018 Economical and compact briefcase spectral-domain optical coherence tomography system for primary care and point-of-care applications. J. Biomed Opt. 23: 096003
George D M M, “J0vibro.” Jan. 13, 2022. Accessed: Jan. 13, 2022. https://github.com/dennymmg/J0vibro
Acknowledgements
The authors thank the founder chancellor of their university, Bhagawan Sri Sathya Sai Baba, for the constant source of inspiration and all the research facilities provided.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest to disclose.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
George, D.M.M., Nandakumar, H., Koushik, V. et al. In-vivo sensing of the vibrations and thickness of the human tympanum with real-time profilometry using low-cost line-field spectral domain optical coherence tomography. Sādhanā 49, 122 (2024). https://doi.org/10.1007/s12046-024-02473-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12046-024-02473-4