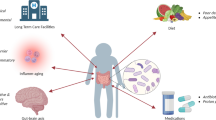
Abstract
Globally, colorectal cancer (CRC) is a leading cause of cancer-related mortality. Dietary habits, inflammation, hereditary characteristics, and gut microbiota are some of its causes. The gut microbiota, a diverse population of bacteria living in the digestive system, has an impact on a variety of parameters, including inflammation, DNA damage, and immune response. The gut microbiome has a significant role in colon cancer susceptibility. Many studies have highlighted dysbiosis, an imbalance in the gut microbiota’s makeup, as a major factor in colon cancer susceptibility. Dysbiosis has the potential to produce toxic metabolites and pro-inflammatory substances, which can hasten the growth of tumours. The ability of the gut microbiota to affect the host’s immune system can also influence whether cancer develops or not. By better comprehending these complex interactions between colon cancer predisposition and gut flora, new preventive and therapeutic techniques might be developed. Targeting the gut microbiome with dietary modifications, probiotics, or faecal microbiota transplantation may offer cutting-edge approaches to reducing the risk of colon cancer and improving patient outcomes. The complex connection between the makeup of the gut microbiota and the emergence of colorectal cancer is explored in this narrative review.




Similar content being viewed by others
References
Bürtin F, Mullins CS, Linnebacher M (2020) Mouse models of colorectal cancer: past, present and future perspectives. World J Gastroenterol 26:1394–1426. https://doi.org/10.3748/wjg.v26.i13.1394
Kersten K, de Visser KE, van Miltenburg MH, Jonkers J (2017) Genetically engineered mouse models in oncology research and cancer medicine. EMBO Mol Med 9:137–53. https://doi.org/10.15252/emmm.201606857
Heijstek MW, Kranenburg O, Borel Rinkes IHM (2005) Mouse models of colorectal cancer and liver metastases. Dig Surg 22:16–25. https://doi.org/10.1159/000085342
Taketo MM, Edelmann W (2009) Mouse models of colon cancer. Gastroenterology 136:780–798. https://doi.org/10.1053/j.gastro.2008.12.049
O’Rourke KP, Loizou E, Livshits G et al (2017) Transplantation of engineered organoids enables rapid generation of metastatic mouse models of colorectal cancer. Nat Biotechnol 35:577–582. https://doi.org/10.1038/nbt.3837
McIntyre RE, Buczacki SJA, Arends MJ, Adams DJ (2015) Mouse models of colorectal cancer as preclinical models. BioEssays 37:909–920. https://doi.org/10.1002/bies.201500032
Nguyen TLA, Vieira-Silva S, Liston A, Raes J (2015) How informative is the mouse for human gut microbiota research? Dis Model Mech 8:1–16. https://doi.org/10.1242/dmm.017400
Oliveira RC, Abrantes AM, Tralhão JG, Botelho MF (2020) The role of mouse models in colorectal cancer research-The need and the importance of the orthotopic models. Animal Model Exp Med 3:1–8. https://doi.org/10.1002/ame2.12102
de la Cueva A, Ramírez de Molina A, Alvarez-Ayerza N et al (2013) Combined 5-FU and ChoKα inhibitors as a new alternative therapy of colorectal cancer: evidence in human tumor-derived cell lines and mouse xenografts. PLoS ONE 8:e64961. https://doi.org/10.1371/journal.pone.0064961
Abuqayyas L, Balthasar JP (2012) Application of PBPK modeling to predict monoclonal antibody disposition in plasma and tissues in mouse models of human colorectal cancer. J Pharmacokinet Pharmacodyn 39:683–710. https://doi.org/10.1007/s10928-012-9279-8
Bradshaw-Pierce EL, Pitts TM, Kulikowski G et al (2013) Utilization of quantitative in vivo pharmacology approaches to assess combination effects of everolimus and irinotecan in mouse xenograft models of colorectal cancer. PLoS ONE 8:e58089. https://doi.org/10.1371/journal.pone.0058089
Lim C, Broqueres-You D, Brouland JP et al (2013) Hepatic ischemia-reperfusion increases circulating bone marrow-derived progenitor cells and tumor growth in a mouse model of colorectal liver metastases. J Surg Res 184:888–897. https://doi.org/10.1016/j.jss.2013.04.069
Shen F, Li JL, Cai WS et al (2013) Interleukin-12 prevents colorectal cancer liver metastases in mice. Onco Targets Ther 6:523–526. https://doi.org/10.2147/OTT.S44161
Sebolt-Leopold J (2018) Development of preclinical models to understand and treat colorectal cancer. Clin Colon Rectal Surg 31:199–204. https://doi.org/10.1055/s-0037-1602240
Mullins CS, Micheel B, Matschos S et al (2019) Integrated biobanking and tumor model establishment of human colorectal carcinoma provides excellent tools for preclinical research. Cancers 11:1520. https://doi.org/10.3390/cancers11101520
Young M, Ordonez L, Clarke AR (2013) What are the best routes to effectively model human colorectal cancer? Mol Oncol 7:178–189. https://doi.org/10.1016/j.molonc.2013.02.006
Francia G, Cruz-Munoz W, Man S, Xu P, Kerbel RS (2011) Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat Rev Cancer 11:135–141. https://doi.org/10.1038/nrc3001
Priolli DG, Abrantes AM, Neves S, Batista JN, Cardinalli IA, Botelho MF (2012) A novel model of distal colon cancer in athymic mice. Acta Cir Bras 27:355–360. https://doi.org/10.1590/s0102-86502012000600001
Huang X, Zou Y, Lian L et al (2013) Changes of T cells and cytokines TGF-β1 and IL-10 in mice during liver metastasis of colon carcinoma: implications for liver anti-tumor immunity. J Gastrointest Surg 17:1283–1291. https://doi.org/10.1007/s11605-013-2194-5
Luca AC, Mersch S, Deenen R et al (2013) Impact of the 3D microenvironment on phenotype, gene expression, and EGFR inhibition of colorectal cancer cell lines. PLoS ONE 8:e59689. https://doi.org/10.1371/journal.pone.0059689
Kruse J, von Bernstorff W, Evert K et al (2013) Macrophages promote tumour growth and liver metastasis in an orthotopic syngeneic mouse model of colon cancer. Int J Colorectal Dis 28:1337–1349. https://doi.org/10.1007/s00384-013-1703-z
Mittal VK, Bhullar JS, Jayant K (2015) Animal models of human colorectal cancer: current status, uses and limitations. World J Gastroenterol 21:11854–11861. https://doi.org/10.3748/wjg.v21.i41.11854
Katsiampoura A, Raghav K, Jiang ZQ et al (2017) Modeling of patient-derived xenografts in colorectal cancer. Mol Cancer Ther 16:1435–1442. https://doi.org/10.1158/1535-7163.MCT-16-0721
Burgenske DM, Monsma DJ, Dylewski D et al (2014) Establishment of genetically diverse patient-derived xenografts of colorectal cancer. Am J Cancer Res 4:824–37
Caetano-Oliveira R, Gomes MA, Abrantes AM et al (2018) Revisiting colorectal cancer animal model-an improved metastatic model for distal rectosigmoid colon carcinoma. Pathophysiology 25:89–99. https://doi.org/10.1016/j.pathophys.2018.02.002
Bhome R, Goh RW, Bullock MD et al (2017) Exosomal microRNAs derived from colorectal cancer-associated fibroblasts: role in driving cancer progression. Aging 9:2666–94. https://doi.org/10.18632/aging.101355
Wang J, Chen C, Wang S et al (2015) Bufalin inhibits HCT116 colon cancer cells and its orthotopic xenograft tumor in mice model through genes related to apoptotic and PTEN/AKT pathways. Gastroenterol Res Pract 2015:457193. https://doi.org/10.1155/2015/457193
Buc E, Dubois D, Sauvanet P et al (2013) High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS One 8:e56964. https://doi.org/10.1371/journal.pone.0056964
Collins D, Hogan AM, Winter DC (2011) Microbial and viral pathogens in colorectal cancer. Lancet Oncol 12:504–512. https://doi.org/10.1016/S1470-2045(10)70186-8
Chen YS, Li J, Menon R et al (2021) Dietary spinach reshapes the gut microbiome in an Apc-mutant genetic background: mechanistic insights from integrated multi-omics. Gut Microbes 13:1972756. https://doi.org/10.1080/19490976.2021.1972756
Wilkinson JE, Franzosa EA, Everett C et al (2021) A framework for microbiome science in public health. Nat Med 27:766–774. https://doi.org/10.1038/s41591-021-01258-0
Dai Z, Coker OO, Nakatsu G et al (2018) Multi-cohort analysis of colorectal cancer metagenome identified altered bacteria across populations and universal bacterial markers. Microbiome. https://doi.org/10.1186/s40168-018-0451-2
He Y, Wu W, Zheng HM et al (2018) Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat Med 24:1532–1535. https://doi.org/10.1038/s41591-018-0164-x
Peters BA, Dominianni C, Shapiro JA et al (2016) The gut microbiota in conventional and serrated precursors of colorectal cancer. Microbiome. https://doi.org/10.1186/s40168-016-0218-6
Flemer B, Lynch DB, Brown JMR et al (2017) Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut 66:633–643. https://doi.org/10.1136/gutjnl-2015-309595
Feng Q, Liang S, Jia H et al (2015) Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat Commun 6:6528. https://doi.org/10.1038/ncomms7528
Abu-Ghazaleh N, Chua WJ, Gopalan V (2021) Intestinal microbiota and its association with colon cancer and red/processed meat consumption: microbiota, meat and colon cancers. J Gastroenterol Hepatol 36:75–88. https://doi.org/10.1111/jgh.15042
Kostic AD, Chun E, Robertson L et al (2013) Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14:207–215. https://doi.org/10.1016/j.chom.2013.07.007
Yachida S, Mizutani S, Shiroma H et al (2019) Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat Med 25:968–976. https://doi.org/10.1038/s41591-019-0458-7
Kostic AD, Gevers D, Pedamallu CS et al (2012) Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res 22:292–298. https://doi.org/10.1101/gr.126573.111
Yang Y, Weng W, Peng J et al (2017) Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear Factor−κB, and up-regulating expression of MicroRNA-21. Gastroenterology 152:851-866.e24. https://doi.org/10.1053/j.gastro.2016.11.018
Wu S, Rhee KJ, Albesiano E et al (2009) A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med 15:1016–1022. https://doi.org/10.1038/nm.2015
Wu S, Lim KC, Huang J, Saidi RF, Sears CL (1998) Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc Natl Acad Sci USA 95:14979–14984. https://doi.org/10.1073/pnas.95.25.14979
Kim JM, Jung HY, Lee JY, Youn J, Lee CH, Kim KH (2005) Mitogen-activated protein kinase and activator protein-1 dependent signals are essential for Bacteroides fragilis enterotoxin-induced enteritis. Eur J Immunol 35:2648–2657. https://doi.org/10.1002/eji.200526321
Arthur JC, Perez-Chanona E, Mühlbauer M et al (2012) Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338:120–123. https://doi.org/10.1126/science.1224820
Cuevas-Ramos G, Petit CR, Marcq I, Boury M, Oswald E, Nougayrède JP (2010) Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc Natl Acad Sci USA 107:11537–11542. https://doi.org/10.1073/pnas.1001261107
Smith JL, Bayles DO (2006) The contribution of cytolethal distending toxin to bacterial pathogenesis. Crit Rev Microbiol 32:227–248. https://doi.org/10.1080/10408410601023557
Lara-Tejero M, Galán JE (2002) Cytolethal distending toxin: limited damage as a strategy to modulate cellular functions. Trends Microbiol 10:147–152. https://doi.org/10.1016/s0966-842x(02)02316-8
Doye A, Mettouchi A, Bossis G et al (2002) CNF1 exploits the ubiquitin-proteasome machinery to restrict Rho GTPase activation for bacterial host cell invasion. Cell 111:553–564. https://doi.org/10.1016/s0092-8674(02)01132-7
Falzano L, Quaranta MG, Travaglione S et al (2003) Cytotoxic necrotizing factor 1 enhances reactive oxygen species-dependent transcription and secretion of proinflammatory cytokines in human uroepithelial cells. Infect Immun 71:4178–4181. https://doi.org/10.1128/IAI.71.7.4178-4181.2003
Boyer L, Travaglione S, Falzano L et al (2004) Rac GTPase instructs nuclear factor-κB activation by conveying the SCF complex and IkBα to the ruffling membranes. Mol Biol Cell 15:1124–1133. https://doi.org/10.1091/mbc.e03-05-0301
Giamboi-Miraglia A, Travaglione S, Filippini P, Fabbri A, Fiorentini C, Falzano L (2007) A multinucleating Escherichia coli cytotoxin perturbs cell cycle in cultured epithelial cells. Toxicol In Vitro 21:235–239. https://doi.org/10.1016/j.tiv.2006.08.013
Fiorentini C, Matarrese P, Straface E et al (1998) Rho-dependent cell spreading activated by E. coli cytotoxic necrotizing factor 1 hinders apoptosis in epithelial cells. Cell Death Differ 5:921–9. https://doi.org/10.1038/sj.cdd.4400422
McCOY WC, Mason JM 3rd (1951) Enterococcal endocarditis associated with carcinoma of the sigmoid; report of a case. J Med Assoc State Ala 21:162–166
Klein RS, Catalano MT, Edberg SC, Casey JI, Steigbigel NH (1979) Streptococcus bovis septicemia and carcinoma of the colon. Ann Intern Med 91:560–562. https://doi.org/10.7326/0003-4819-91-4-560
Hoppes WL, Lerner PI (1974) Nonenterococcal group-D streptococcal endocarditis caused by Streptococcus bovis. Ann Intern Med 81:588–593. https://doi.org/10.7326/0003-4819-81-5-588
Deng Q, Wang C, Yu K et al (2020) Streptococcus bovis contributes to the development of colorectal cancer via recruiting CD11b+TLR-4+ cells. Med Sci Monit 26:e921886. https://doi.org/10.12659/msm.921886
Abdulamir AS, Hafidh RR, Mahdi LK, Al-jeboori T, Abubaker F (2009) Investigation into the controversial association of Streptococcus gallolyticus with colorectal cancer and adenoma. BMC Cancer 9:403. https://doi.org/10.1186/1471-2407-9-403
Abdulamir AS, Hafidh RR, Bakar FA (2010) Molecular detection, quantification, and isolation of Streptococcus gallolyticus bacteria colonizing colorectal tumors: inflammation-driven potential of carcinogenesis via IL-1, COX-2, and IL-8. Mol Cancer 9:249. https://doi.org/10.1186/1476-4598-9-249
Martins M, Porrini C, du Merle L et al (2016) The Pil3 pilus of Streptococcus gallolyticus binds to intestinal mucins and to fibrinogen. Gut Microbes 7:526–532. https://doi.org/10.1080/19490976.2016.1239677
Bezawada N, Song M, Wu K et al (2014) Urinary PGE-M levels are associated with risk of colorectal adenomas and chemopreventive response to anti-inflammatory drugs. Cancer Prev Res 7:758–765. https://doi.org/10.1158/1940-6207.CAPR-14-0120
Butt J, Varga MG, Blot WJ et al (2019) Serologic response to Helicobacter pylori proteins associated with risk of colorectal cancer among diverse populations in the United States. Gastroenterology 156:175-186.e2. https://doi.org/10.1053/j.gastro.2018.09.054
McClain M, Beckett A, Cover T (2017) Helicobacter pylori vacuolating toxin and gastric cancer. Toxins 9:316. https://doi.org/10.3390/toxins9100316
Kountouras J, Zavos C, Chatzopoulos D, Katsinelos P (2008) New aspects of Helicobacter pylori infection involvement in gastric oncogenesis. J Surg Res 146:149–158. https://doi.org/10.1016/j.jss.2007.06.011
Thorburn CM, Friedman GD, Dickinson CJ, Vogelman JH, Orentreich N, Parsonnet J (1998) Gastrin and colorectal cancer: a prospective study. Gastroenterology 115:275–280. https://doi.org/10.1016/s0016-5085(98)70193-3
Strofilas A, Lagoudianakis EE, Seretis C et al (2012) Association of helicobacter pylori infection and colon cancer. J Clin Med Res 4:172–176. https://doi.org/10.4021/jocmr880w
Davenport JR, Cai Q, Ness RM et al (2016) Evaluation of pro-inflammatory markers plasma C-reactive protein and urinary prostaglandin-E2 metabolite in colorectal adenoma risk. Mol Carcinog 55:1251–1261. https://doi.org/10.1002/mc.22367
Cai Q, Gao YT, Chow WH et al (2006) Prospective study of urinary prostaglandin E2 metabolite and colorectal cancer risk. J Clin Oncol 24:5010–5016. https://doi.org/10.1200/JCO.2006.06.4931
Wang X, Yang Y, Moore DR, Nimmo SL, Lightfoot SA, Huycke MM (2012) 4-hydroxy-2-nonenal mediates genotoxicity and bystander effects caused by Enterococcus faecalis-infected macrophages. Gastroenterology 142:543–551. https://doi.org/10.1053/j.gastro.2011.11.020
Huycke MM, Abrams V, Moore DR (2002) Enterococcus faecalis produces extracellular superoxide and hydrogen peroxide that damages colonic epithelial cell DNA. Carcinogenesis 23:529–536. https://doi.org/10.1093/carcin/23.3.529
Wang X, Huycke MM (2007) Extracellular superoxide production by Enterococcus faecalis promotes chromosomal instability in mammalian cells. Gastroenterology 132:551–561. https://doi.org/10.1053/j.gastro.2006.11.040
Wang X, Allen TD, May RJ, Lightfoot S, Houchen CW, Huycke MM (2008) Enterococcus faecalis induces aneuploidy and tetraploidy in colonic epithelial cells through a bystander effect. Cancer Res 68:9909–9917. https://doi.org/10.1158/0008-5472.CAN-08-1551
Yang Y, Wang X, Moore DR, Lightfoot SA, Huycke MM (2012) TNF-α mediates macrophage-induced bystander effects through Netrin-1. Cancer Res 72:5219–5229. https://doi.org/10.1158/0008-5472.CAN-12-1463
Boonanantanasarn K, Gill AL, Yap Y, Jayaprakash V, Sullivan MA, Gill SR (2012) Enterococcus faecalis enhances cell proliferation through hydrogen peroxide-mediated epidermal growth factor receptor activation. Infect Immun 80:3545–3558. https://doi.org/10.1128/iai.00479-12
van Dalen PJ, van Steenbergen TJ, Cowan MM, Busscher HJ, de Graaff J (1993) Description of two morphotypes of Peptostreptococcus micros. Int J Syst Bacteriol 43:787–793. https://doi.org/10.1099/00207713-43-4-787
Xu J, Yang M, Wang D et al (2020) Alteration of the abundance of Parvimonas micra in the gut along the adenoma-carcinoma sequence. Oncol Lett 20:106. https://doi.org/10.3892/ol.2020.11967
Loftus M, Hassouneh SAD, Yooseph S (2021) Bacterial community structure alterations within the colorectal cancer gut microbiome. BMC Microbiol 21:98. https://doi.org/10.1186/s12866-021-02153-x
Zhao L, Zhang X, Zhou Y, Fu K, Lau HCH, Chun TWY et al (2022) Parvimonas micra promotes colorectal tumorigenesis and is associated with prognosis of colorectal cancer patients. Oncogene 41:4200–4210. https://doi.org/10.1038/s41388-022-02395-7
Lo CH, Wu DC, Jao SW et al (2022) Enrichment of Prevotella intermedia in human colorectal cancer and its additive effects with Fusobacterium nucleatum on the malignant transformation of colorectal adenomas. J Biomed Sci 29:88. https://doi.org/10.1186/s12929-022-00869-0
Fenner L, Roux V, Ananian P, Raoult D (2007) Alistipes finegoldii in blood cultures from colon cancer patients. Emerg Infect Dis 13:1260–1262. https://doi.org/10.3201/eid1308.060662
Lin Y, Lau HC-H, Liu Y et al (2022) Altered mycobiota signatures and enriched pathogenic Aspergillus rambellii are associated with colorectal cancer based on multicohort fecal metagenomic analyses. Gastroenterology 163:908–921. https://doi.org/10.1053/j.gastro.2022.06.038
Moyes DL, Naglik JR (2012) The mycobiome: influencing IBD severity. Cell Host Microbe 11:551–552. https://doi.org/10.1016/j.chom.2012.05.009
Qin X, Gu Y, Liu T et al (1875) (2021) Gut mycobiome: a promising target for colorectal cancer. Biochim Biophys Acta Rev Cancer 1:188489. https://doi.org/10.1016/j.bbcan.2020.188489
Marongiu L, Allgayer H (2022) Viruses in colorectal cancer. Mol Oncol 16:1423–1450. https://doi.org/10.1002/1878-0261.13100\
HPV and cancer. National Cancer Institute (2019). https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-and-cancer. Accessed 3 February 2024
Boguszakova L, Hirsch I, Brichacek B et al (1988) Absence of cytomegalovirus, Epstein-Barr virus, and papillomavirus DNA from adenoma and adenocarcinoma of the colon. Acta Virol 32:303–308
Koulos J, Symmans F, Chumas J et al (1991) Human papillomavirus detection in adenocarcinoma of the anus. Mod Pathol 4:58–61
Shah KV, Daniel RW, Simons JW et al (1992) Investigation of colon cancers for human papillomavirus genomic sequences by polymerase chain reaction. J Surg Oncol 51:5–7. https://doi.org/10.1002/jso.2930510104
Ramprasad C, Major VJ, Zhang Y et al (2019) 293 receptive anal sex in women and risk of colorectal cancer (2009–2014): a retrospective analysis of NHANES: 293. Am J Gastroenterol 114:S170. https://doi.org/10.14309/01.ajg.0000590704.93495.66
Burnett-Hartman AN, Newcomb PA, Potter JD (2008) Infectious agents and colorectal cancer: a review of Helicobacter pylori, Streptococcus bovis, JC virus, and human papillomavirus. Cancer Epidemiol Biomarkers Prev 17:2970–2979. https://doi.org/10.1158/1055-9965.EPI-08-05711
Chuang L-C, Chen H-C, You S-L et al (2010) Association between human papillomavirus and adenocarcinoma of rectum and recto-sigmoid junction: a cohort study of 10,612 women in Taiwan. Cancer Causes Control 21:2123–2128. https://doi.org/10.1007/s10552-010-9631-5
Chaturvedi AK, Engels EA, Gilbert ES et al (2007) Second cancers among 104,760 survivors of cervical cancer: evaluation of long-term risk. J Natl Cancer Inst 99:1634–1643. https://doi.org/10.1093/jnci/djm201
Chan DSM, Lau R, Aune D et al (2011) Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS ONE 6:e20456. https://doi.org/10.1371/journal.pone.0020456
Song M, Garrett WS, Chan AT (2015) Nutrients, foods, and colorectal cancer prevention. Gastroenterology 148:1244–60.e16. https://doi.org/10.1053/j.gastro.2014.12.035
Sonnenburg JL, Bäckhed F (2016) Diet–microbiota interactions as moderators of human metabolism. Nature 535:56–64. https://doi.org/10.1038/nature18846
Singh N, Gurav A, Sivaprakasam S et al (2014) Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40:128–139. https://doi.org/10.1016/j.immuni.2013.12.007
Bultman SJ (2017) Interplay between diet, gut microbiota, epigenetic events, and colorectal cancer. Mol Nutr Food Res 61:1500902. https://doi.org/10.1002/mnfr.201500902
Zackular JP, Baxter NT, Iverson KD et al (2013) The gut microbiome modulates colon tumorigenesis. MBio 4:e00692-e713. https://doi.org/10.1128/mBio.00692-13
Saus E, Iraola-Guzmán S, Willis JR, Brunet-Vega A, Gabaldón T (2019) Microbiome and colorectal cancer: roles in carcinogenesis and clinical potential. Mol Aspects Med 69:93–106. https://doi.org/10.1016/j.mam.2019.05.001
Montalban-Arques A, Scharl M (2019) Intestinal microbiota and colorectal carcinoma: implications for pathogenesis, diagnosis, and therapy. EBioMedicine 48:648–655. https://doi.org/10.1016/j.ebiom.2019.09.050
Zou S, Fang L, Lee MH (2018) Dysbiosis of gut microbiota in promoting the development of colorectal cancer. Gastroenterol Rep 6:1–12. https://doi.org/10.1093/gastro/gox031
Wong SH, Yu J (2019) Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol 16:690–704. https://doi.org/10.1038/s41575-019-0209-8
Ternes D, Karta J, Tsenkova M, Wilmes P, Haan S, Letellier E (2020) Microbiome in colorectal cancer: how to get from meta-omics to mechanism? Trends Microbiol 28:401–423. https://doi.org/10.1016/j.tim.2020.01.001
Singh S, Sharma P, Sarma DK et al (2023) Implication of obesity and gut microbiome dysbiosis in the etiology of colorectal cancer. Cancers 15:1913. https://doi.org/10.3390/cancers15061913
Chattopadhyay I, Dhar R, Pethusamy K et al (2021) Exploring the role of gut microbiome in colon cancer. Appl Biochem Biotechnol 193:1780–1799. https://doi.org/10.1007/s12010-021-03498-9
de Souza JB, Brelaz-de-Castro MCA, Cavalcanti IMF (2022) Strategies for the treatment of colorectal cancer caused by gut microbiota. Life Sci 290:120202. https://doi.org/10.1016/j.lfs.2021.120202
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kamath, H.S., Shukla, R., Shah, U. et al. Role of Gut Microbiota in Predisposition to Colon Cancer: A Narrative Review. Indian J Microbiol (2024). https://doi.org/10.1007/s12088-024-01242-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12088-024-01242-5