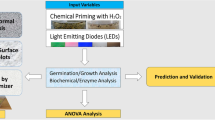
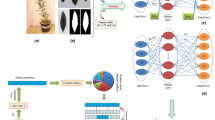
Abstract
The influence of hydropriming and Light Emitting Diodes (LED) on germination and growth indices, followed by optimizing and validation via artificial intelligence-based models was carried out in this research. White LEDs (W-LEDs) were more effective by yielding the most effective growth indices, such as mean germination time (MGT) (1.11 day), coefficient of variation of germination time (CVt) (20.72%), mean germination rate (MR) (0.81 day−1), uncertainty (U) (0.40 bit), and synchronization (Z values) (0.79); the optimum MGT (1.09 day), CVt (15.97%), MR (0.77 day−1), U (0.32 bit), and Z (0.55) values were found after 2 h of hydropriming, which was responsible for all efficient growth indicators. W-LEDs with 1 h hydropriming proved to be the ideal LED and hydropriming combination. Results on growth indices for in vitro seedlings were completely different from those on germination indices, and the most desirable germination indices were linked to red LEDs (R-LEDs). Whereas 4 h hydropriming was most effective for the post-germination process. Pareto charts, normal plots, contour plots, and surface plots were created to optimize the input variables. Finally, the data were predicted using Arificial Neural Network (ANN) inspired multilayer perceptron (MLP) and machine learning-based random forest (RF) algorithms. For both models, plant height was correlated with maximum R2 values. Whereas, all output variables had relatively low mean absolute error (MAE), mean square error (MSE), and mean absolute percentage error (MAPE) scores, indicating that both models performed well. The results of this investigation disclosed a link between certain LEDs and hydropriming treatment for in vitro germination indices and plant growth.
Graphical Abstract
Graphical presentation of actual and predicted values for germination indices in chickpea

Similar content being viewed by others
Introduction
Successful seed germination is the first step towards any plant's efficacious growth in either a field or in a controlled environment. The entire germination process is a consequence of the manipulation of both internal (genetic) and external (environmental) factors. This process is rather complicated, and various biological and environmental elements play a role in its regulation (Aasim et al. 2023a). These molecular mechanisms, in turn, control the germination process due to these biotic and abiotic stimuli. Temperature, moisture content, gases, light, and other abiotic elements are some key factors that control seed germination (Kumar et al. 2015). Light is one of the factors that most plants use to regulate seed germination. Most cultivated plants can germinate either in the presence or absence of light and seeds can be categorized according to their sensitivity to light during the germination test. The three components of light sensitivity are light intensity, wavelength, and irradiation time (Lone et al. 2014). Plants use five different classes of pigments called phytochromes to detect light, and each phytochrome recognizes a different ratio and wavelength of light (Takaki 2001) to control germination, growth and flowering (Quail 2002). Lately, Light Emitting Diodes (LEDs) have gained popularity as a substitute source of light for indoor agriculture and horticulture in climate-controlled environments like greenhouses (Morrow 2008) or for in vitro studies due to its significant benefits for boosting plant regeneration (Karataş et al. 2016; Aasim et al. 2022a). However, the way plants react to LEDs differs depending on the type of LEDs used and how they are mixed (Karataş et al. 2016).
Priming is the process of exposing seeds to an agent for a specific period before planting, which can range from a few minutes to a few days. For improving seed germination and plant establishment under unfavourable conditions, hydropriming is used in conjunction with other priming strategies such as osmo-priming, halo-priming, thermo-priming, or hormonal-priming (Tzortzakis 2009). The most popular priming method among these is hydropriming, which is employed for many economic crops under field conditions (Asl and Taheri 2012). Additionally, some investigations demonstrated the utilization of hydropriming methods for better in vitro seed germination (Tzortzakis 2009; Arun et al. 2013; Čanak et al. 2014) and vigor (Phat et al. 2017). The benefit of using hydropriming in vitro is that the entire germination process can be monitored and visualized easily.
Studies on germination frequently focus on how seeds behave during the germination process and their capacity to germinate in addition to quantitative analyses (Ranal and Santana 2006; Aasim et al. 2023a). A few examples of quantitative parameters in the germination process include time, rate, homogeneity, and synchronization. Physiologists, seed technologists, and ecologists must meticulously understand the impact on these germination indices in order to better comprehend the germination process for improved future field plans from germination through harvesting. The use of biological/physiological processes or mathematical expressions is essential to fathom the germination process from a scientific perspective (Aasim et al. 2023a). There exist many documented mathematical formulas to understand the germination process and measurement from different perspectives. Due to recent improvements in the diversity of expressions for the measurement of germination, it is now more challenging because the same parameter has multiple names or acronyms (Ranal et al. 2009; Aasim et al. 2023a).
Machine learning (ML) is the sub-field of artificial intelligence (AI) and is being widely used in plant breeding and other plant sciences. Researchers can examine and interpret enormous volumes of data produced from diverse sources by integrating AI technologies. Accurate trait prediction has been demonstrated to be possible using decision tree-based machine learning (ML) algorithms such as Random Forest (RF) or artificial neural network (ANN)-based multi layer perceptron (MLP) models. Plant biotechnology has evolved to a new level because of the rapid development of artificial intelligence technology and other cutting-edge technologies. In recent years, a substantial use of ML-based modeling is witnessed in plant biotechnology. Newly, the use of AI models for industrial hemp’s in vitro germination and growth indices of industrial hemp (Hesami et al. 2021; Pepe et al. 2021a, b) and chickpea (Aasim et al. 2023a) has been reported for prediction, validation, and optimization. The use of AI for LEDs is very limited in plant tissue culture (Aasim et al. 2022a), and to date no report has been registered for investigating the impact on germination. In general, understanding the impacts of stress and making decisions based on scientific evidence may be challenging. The issue can be resolved by utilizing various models and algorithms to provide predictions that are significantly more precise and accurate.
Moreover, the two stages of seed germination and seedling establishment are crucial for a successful plant growth cycle. There is virtually little usage of mathematical formulas to comprehend the germination process under in vitro conditions (Aasim et al. 2023a). Plant tissue culture has several advantages, including making it easier and more efficient to measure how efficiently light, temperature, and other stresses impact germination and plant development (Phat et al. 2017). The purpose of this study was to improve the in vitro germination indices and seedling growth of desi chickpea seeds when exposed to LEDs and hydropriming under controlled settings, and then to optimize the input variables using various statistical approaches and AI models.
Material and Methods
Seed Sterilization and Hydropriming
The seeds of desi chickpea were procured from the Department of Field Crops, Faculty of Agriculture, Dicle University, Diyarbakir. Damaged seeds were removed, and manually chosen uniform seeds were subjected to commercial bleach (NaOCl—5% w/v) for 15 min before being stirred in sterilized distilled water for five minutes. The entire procedure was performed three times. After that, seeds were hydroprimed for 1, 2, and 4 h in sterile water before being cultured on MS medium without any plant growth variations. Additionally, unprimed seeds were grown as a control group. Both primed and unprimed (control) seeds were placed under different LEDs like white (W-LEDs), red (R-LEDs) and blue (B-LEDs) to investigate the impact of both input variables.
In Vitro Culture Conditions and Medium Preparation
Surface sterilized seeds of desi chickpea were placed on Murashige and Skoog (MS) containing culture medium (Murashige and Skoog 1962) for tabulating the in vitro germination and gowth parameters. The MS medium was prepared as a standard medium augmented with MS (0.44%), sucrose (3.0%), and solidifying agent as agar (0.65%). The MS medium was prepared without the application of any plant growth regulators. The agar was added after adjusting the pH approximately to 5.8 by using 1N HCl (hydrochloric acid) or 1N NaOH (sodium hydroxide) solution. The medium was autoclaved for 20 min (121 °C; 1.5 kg cm−2 pressure) followed by pouring the medium into Magentas GA7 vessels. The vessels containing seeds were kept under 16/8 h light/dark photoperiod using W-LEDs, R-LEDs and B-LEDs with temperature of 24 ± 1 °C.
Germination Indices
The seeds were checked 12 and 24 h after the first culture of the inoculated seeds on MS medium for germination. The seeds were then monitored after every 24 h for the following 4 days without interruption Radicle emergence (2–3 mm) was used as the baseline for germination, and the data were collected every day for up to 4 days. Six different germination indices (G: germination percentage, MGT: mean germination time, CVt: coefficient of variation of the germination time, MR: mean germination rate, \(U\): uncertainty, and \(Z\): synchronization index) were computed to understand the germination process. The data analysis was tallied using the previously discussed formulae and methodology (Ranal et al. 2009).
Mean Germination Time (MGT or \(\overline{t }\))
It is the most popular germination metric, also referred as MET (mean emergence time), MLIT (mean length of incubation time), or Mdays (mean days) for germination. Thus, the weighted mean of germination (MGT), which is utilized as the reciprocal of germination rate in various plants, is determined by using germination rate and time-spread (Bewley et al. 2012). It is helpful to estimate the normal amount of time required for a seed lot to germinate at its best (Ranal et al. 2009).
Germination Percentage (G)
The germination percentage (G), often known as the germination rate, is a measurement used by scientists to determine how swiftly seeds germinate. A prediction of the viability of a population of seeds is offered as an alternative (Ranal et al. 2009).
The Coefficient of Variation of the Germination Time (CVt)
It gauges how uniformly or widely germination occurs in proportion to the mean germination time (Ranal et al. 2009).
CVt in percentage is written as:
Mean Germination Rate (MR)
It is the reciprocal of the mean germination time, and its value can be expressed as 0 < MR ≤ 1 day−1. The value close to 1 reflects more seed vigor, germination speed or rate (Ranal and Santana 2006).
Uncertainty (U)
It is employed to determine the degree of uncertainty related to the distribution of the relative frequency of germination. Uncertainty (U) values are expressed as \(0\le U\le {\text{log}}_{2}n\) (bit) where low U values reflects more synchronized germination (Ranal and Santana 2006). It indicates the degree of uncertainty about germination frequency, and low U values indicate more concentrated germination time. Germination of even a single seed may change the U value. Low values of U indicate more synchronized germination (Ranal et al. 2009).
Synchronization Index (Z)
It was originally used to gauge how much a population's flowering cycles overlapped with one another. It indicates the degree of germination overlapping when when germination of two seeds occurs simultaneously (Ranal and Santana 2006). The \(Z\) give the value range of \(0\le Z\le 1\)(a unit less number) and a higher germination synchronization index is represented by the greatest Z value of 1 or near to 1.
Description about all abbreviations that are used in the formulas (Eqs. 1–7) are given below.
\({C}_{{n}_{i}, 2}\) combination of seeds germinated in the ith time, fi = relative frequency of germination, \({s}_{t}\) standard deviation of the germination time, \(\overline{t }\) mean germination time, ith day and ni is the number of seeds germinated on the ith day, N total number of seeds that were planted in a petri dish, ni number of seeds that germinated on the ith day or the daily germination percentage at time (ti) from sowing, ti time from the start of the experiment to the ith day, k last day of observation.
In Vitro Seedling Growth Indices
After three weeks of in vitro culture, various plant growth metrics, including shoot or root length, shoot:root length ratio, and shoot and root fresh weight or dry weight, were recorded. To calculate the specified growth parameters, ten plants per replication were chosen randomly. A measuring scale was used to measure the length of the shoots and roots, followed by weighing on sensitive electric weighing balance. Shoot and root samples were wrapped in an aluminum folio and oven-dried at 65 °C for 72 h to determine the dry weight,
Statistical Analysis
The experiment was designed as factorial randomized complete design. Hydropriming and LEDs were used as input parameters. The data regarding germination indices and in vitro seedling growth were analyzed with Minitab 20.0 statistical program. Similarly, factorial regression analysis, Pareto chart, and normal plots were also constructed by using the same statistical program. On the other hand, contour plots and surface plots were created with the aid of Design Expert (DX 17) statisitcal program.
Machine Learning Analysis
Eight different germination indices were used as output parameters and two input parameters (LEDs light intensity and hydropriming time) were computed for decision tree-based machine learning algorithms and artificial neural network based multilayer perceptron model. The popularity of decision tree-based algorithms can be attributed to their remarkable accuracy, speed, stability, and ease of use. They are significantly more effective than linear models at mapping intricate non-linear relationships. Random Forest (Aggarwal 2018) is the widely used advanced decision tree models in the realm of data science. To train several trees at once, RF uses bagging, also known as bootstrap aggregation, and most of the trained trees determine the outcome. Equation 1 presents the fundamental idea of the RF model (Pavlov 2019).
y value of the data point, n number of samples.
Multilayer perceptron is a particular kind of feedforward neural network that consists of many layers of processing nodes coupled in a feedforward fashion. It has one or more input, output, and hidden levels that are all perfectly integrated. To update the error-related weights and biases, backpropagation is utilized to train the data up until Eq. 4 is reduced (Katırcı et al. 2021).
y observed value of data point k, k number of samples.
Seed germination, an intricate biological process, is influenced by numerous intrinsic and extrinsic factors that demand optimization for improved outcomes. Adding to the challenge, these variables often interact, leading to unpredictable responses. Machine learning, a branch of Artificial Intelligence, provides an innovative approach to recognize patterns in diverse datasets and anticipate optimal combinations for desired results. This data-driven modeling excels in deciphering non-normal, non-linear, and non-deterministic data, avoiding irrelevant spectral bands and multicollinearity. A custom code was written in Python programming language (Van Rossum and Drake 2009) to implement machine learning models with the aid of sklearn library (Pedregosa et al. 2011). The link (https://github.com/seyidali/PGR-Chickpea) provides access to Python code, the data file, and some outputs. Moreover, leave-one-out cross-validation (LOO-CV) technique was used to evaluate the performance of the models (Webb et al. 2011). Leave-one-out cross-validation is a special form of cross-validation in which the dataset includes precisely as many instances as there are folds. The learning method is applied to each instance individually, with the selected instance acting as the single-item test set and the remaining instances acting as the training set. LOO-CV is more suitable when the sample size is small since more training samples are used in each iteration, allowing the model to gain a deeper understanding of the data. To find the optimal hyperparameters and build the best model, a grid search approach was used.
Four different performance indicators were used to evaluate the effectiveness of each model. The degree to which the model and the dependent variables are correlated can be determined by computing the coefficient of determination (R2) presented in Eq. 10. Its values range from \(0\le {R}^{2}\le 1\), with levels closer to 1 indicating higher model performance. The mean square error (MSE) shows how well a regression line corresponds to the generated data points (Eq. 11), and values range from \(0\le {\text{MSE}}\le \infty\). The mean absolute error (MAE) gauges how far an observed value deviates from its expected value on average (Eq. 12). Using mean absolute percent error (MAPE), the accuracy is displaced as a proportion of the error (Eq. 13) and presented as \({\text{MAPE}}\ge 0\). Values of 50% or greater are indicative of inaccurate predictions (Lewis 1982).
where \({Y}_{i}\) measured value, \({\widehat{Y}}_{i}\) predicted value, \(\overline{Y}\) measured value’s mean, n count of samples.
In machine learning data scaling is typically used during the data preparation stage. It enables the inputs to be transformed into dimensionless and/or comparable distributions, which ultimately helps to improve the data quality and algorithm performance. In this work, all input variables were standardized to have values centered around the zero mean with a unit standard deviation (Eq. 14).
\({X}^{\prime}\) standardized value, \({X}_{i}\) actual data, \(\mu\) mean of the feature values, \(\sigma\) standard deviation of the featured values.
Results
In Vitro Germination Indices
Sterilized seeds hydroprimed with sterilized distilled water were cultured on MS medium followed by placing magentas under different LEDs for germination. The emergence of 2–3 mm radicle was considered as germinated seeds. Data regarding germination percentage, mean germination time, percentage coefficient of variation of the germination time, mean germination rate, uncertainty, and synchronization index of germination under in vitro conditions was tabulated upon recording 100% radicle emergence. In contrast, 100% seedling emergence was recorded within 4 days for shoot emergence, which took somewhat longer. The data regarding growth indices were tabulated after three weeks of total culture.
Impact of LEDs on Germination Indices
Germination in response to different LEDs recorded as B-LEDs (88.89%) > W-LEDs (81.94%) > R-LEDs (76.60%) (Data not presented in figures) at the end of day 1 and 100% germination was documented at the end of day 2. The MGT response to various LEDs ranged from 1.11 to 1.26 days with a falling order of B-LEDs > R-LEDs > W-LEDs. In comparison to other LEDs, the results clearly demonstrate the benefits of W-LEDs and show them to be more suitable for quick germination. Like MGT, CVt values showed a pattern of B-LEDs (30.69%) > R-LEDs (29.60%) > W-LEDs (20.72%). However, both W-LEDs and R-LEDs generated statistically similar CVt% values. Results on MR values under LEDs revealed the association with GMT and CVt% values, and it was discovered that they were inversely proportional to those values. The recorded MR values for the various LEDs were 0.91 (W-LEDs) > 0.86 (R-LEDs) > 0.81 (B-LEDs). In response to LEDs, the various patterns of U and Z values were recorded. The order of the U values for LEDs was B-LEDs (0.70), R-LEDs (0.59), and W-LEDs (0.40). While Z values were listed as W-LEDs (0.79), R-LEDs (0.68), and B-LEDs (0.61), respectively. Based on germination indices, the overall conclusion shows that W-LEDs are superior to other illumination systems with low MGT, CVt (%), and U values (Fig. 1).
Impact of LEDs on Germination Indices
Seed germination approached 100% within 2 days in response to different hydropriming treatments. The Germination (%) on day 1 was totally different and hydropriming for 1 h (87.03%) and 2 h (90.74%) was proved to be more efficient for enhancing seed germination compared to control. Hydropriming for 4 h was unfavorable and exhibited a significantly lower value of only 68.25% and less than the controlled environment (79.63%). On the other hand, hydropriming exerted a significant effect on MGT values and 4 h hydropriming was least effective with highest MGT value of 1.31 day (Fig. 1) compared to other treatments. The MGT values in order were recorded as 2 h (1.09 day) > 1 h (1.13 day) > 0 h (1.20 day) > 4 h (1.31 day). A similar pattern was also recorded for CVt (%). The CVt (%) values were recorded as 2 h (15.97%) > 1 h (20.27%) > 0 h (35.82%) > 4 h (36.96). However, CVt (%) values in response to hydropriming for 1 h and 2 h were statistically similar and less than control. Hydropriming for 4 h was statistically similar with control. MR values in response to hydropriming were recorded from highest to lowest in case of 2 h (0.93 day) > 1 h (0.90 day) > 0 h (0.83 day) > 4 h (0.77 day). The U values of 1 h and 2 h hydropriming were statistically same but 2 h hydropriming had less U value (0.32) compared to 1 h (0.40). The U values of 4 h and 0 h was almost double to 1 h and 2 h which was recorded as 0.81 (4 h) and 0.71 (0 h) (Fig. 1). The Z values were contrary to the U values and were recorded in order of 2 h (0.83) > 1 h (0.77) > 0 h (0.62) > 4 h (0.55). The low MGT, CVt (%) and U values along with high MR and Z values reveals the supremacy of 2 h treatment over other treatments.
Impact of LEDs × Hydropriming on Germination Indices
Individual response of LEDs or hydropriming exerted statistically significant effect on germination percentage, but interaction of LEDs × hydropriming had no effect after 1 day. Germination was maximum under blue LEDs with all hydropriming treatments and control compared with other LEDs. The combination of B-LEDs × 1 h generated 100% germination (Fig. 2). Response of R-LEDs was lowest with all hydropriming treatments. The germinability (%) of desi chickpea seeds were recorded insignificant due to 100% germination in response to LEDs, hydropriming or interaction of LEDs × Hydropriming after 2 days. The MGT and MR values of LEDs × hydropriming were in line with individual factors and ranged 1.0–1.40 day and 0.74–1.00 day respectively. The best combination (1.0 day MGT) was recorded as W-LEDs × 1 h hydropriming followed by 1.06 day (MGT) for W-LEDs × 2 h hydropriming and R-LEDs × 2 h hydropriming. On the contrary, provision of B-LEDs with all hydropriming treatments was less effective than all other treatments. As MGT value in response to 1 h hydropriming under B-LEDs was 1 day, the CVt% value was recorded 0 which reflects the minimum variation coefficient in germination time. The next best combination (0.95) expressing CVt (%) was associated with W-LEDs × 2 h hydropriming and R-LEDs × 2 h hydropriming. Contrarily, the highest CVt% values were linked with 4 h hydropriming and control under all LEDs. Similar observations were also documented for U values. Interaction of B-LEDs × 1 h hydropriming reveals the U value of 0 when seeds were hydro primed for 1 h and cultured under B-LEDs. In general, 4 h hydropriming under all LEDs lights had a high U value along with 0 h. Whereas, 1 h and 2 h hydropriming in combination with LEDs generated low U values (Fig. 2). The results for Z values were contrary to U values and high Z values were documented for 1 h and 2 h hydropriming in combination with all LEDs. The highest Z value (1.0) was documented for W-LEDs × 1 h hydropriming followed by 0.89 for W-LEDs × 2 h hydropriming and R-LEDs × 2 h hydropriming.
In Vitro Plant Growth Indices
LEDs lights used in this study exerted statistically significant effect on shoot length (p ≤ 0.01), root length (p ≤ 0.01), root numbers (p ≤ 0.01), fresh shoots wt (p ≤ 0.01) and shoot:root (p ≤ 0.05), which were in general highest under W-LEDs and lowest under B-LEDs. Contrarily, root fresh wt, shoot dry wt and root dry wt were recorded statistically insignificant when exposed to different LEDs (Fig. 3). It was notable that all growth parameters showed the same pattern of maximum to minimum in order of W-LEDs > R-LEDs > B-LEDs. The results recorded for different parameters were ranged 11.07–16.57 cm (shoot length), 11.67–14.97 cm (root length), 18.90–23.38 (root number), 0.448–0.514 g (fresh shoot weight), 0.297–0.338 g (root fresh wt), 0.040–0.042 g (dry shoot wt) and 0.027–0.029 g (root dry wt) (Fig. 3).
Hydropriming application at different time periods affected the shoot length (p ≤ 0.01) and root dry weight (p ≤ 0.05). Shoot length in response to hydropriming ranged 13.78–14.88 cm. Hydropriming for 4 h proved to be beneficial for enhancing shoot length (14.88 cm) compared to other hydropriming treatments and control (Fig. 4). Hydropriming in comparison to control resulted in relatively low for root dry weight and ranged 0.026–0.032 g. All other parameters taken were unaffected by hydropriming application compared to seeds where priming was not performed. Hydropriming has no effects on shoots dry wt and ranged 0.040–0.042 g. Shoots fresh wt (0.486–0.503 g) and root length (12.45–13.16 cm) remained unaffected by hydropriming timings compared to control. Root length (12.45–1316), root fresh wt (0.293–0.348 g) and root numbers (18.33–21.85) were statistically insignificant and unaffected by hydropriming. In general, all parameters increased with an increase in hydropriming timing and were recorded comparatively higher than control treatment.
Interaction of LEDs × hydropriming significantly controlled the shoot length (p ≤ 0.05), root length (p ≤ 0.05), root numbers (p ≤ 0.01), root fresh wt (p ≤ 0.05), and shoot dry wt (p ≤ 0.05). Contrarily, shoot fresh wt and root dry wt were unaffected by the combination of LEDs × hydropriming. Shoot length in response to LEDs × hydropriming revealed the importance of longer exposure of seeds to water and use of W-LEDs with longer shoots of 18.02 cm (Fig. 5). In general, hydropriming of seeds for 4 h generated longer shoots under all LEDs. Comparatively longer shoots were observed under W-LEDs with both primed and non-primed seeds. B-LEDs with all hydropriming combinations inhibited the shoot length. Combination of LEDs × hydropriming exerted variable effects on root length of in vitro germinated seedlings. Root length under all LEDs was relatively more from non-primed seeds as compared to hydroprimed seeds. Root length was maximum under W-LEDs and minimum under B-LEDs with all primed or non-primed seeds (Fig. 5). These results suggested that LEDs lights control the shoot and root length of primed and non-primed seeds but may vary with hydropriming exposure time (Fig. 5).
LEDs × hydropriming combination significantly affected the fresh shoot wt that was ranged 0.367–0.534 g (Fig. 5). Hydropriming for 2 h under R-LEDs or W-LEDs and hydropriming for 4 h under B-LEDs resulted in low fresh shoot wt. Contrarily, 4 h hydropriming under R-LEDs and W-LEDs yielded more fresh shoot wt. Results further revealed that fresh root wt remained unaffected statistically and ranged 0.238–0.369 g. In general, hydropriming exerted negative effects on root fresh wt under all LEDs. Results on dry wt of shoots and roots were totally opposite to their respective fresh wt. Dry wt of shoots was statistically similar and recorded as 0.036–0.047 g. In general, dry shoot wt decreased when hydro primed seeds were cultured under B-LEDs and R-LEDs and increased under W-LEDs. Contrarily, hydropriming of seeds resulted in decreased dry wt of roots cultured under all lighting systems. It was also noted that 1 h and 2 h hydropriming yielded low dry root wt compared to 4 h hydropriming under all LEDs (Fig. 5).
Root numbers in response to LEDs × hydropriming ranged 14.89–27.72 with no clear relationship between LEDs and hydropriming timing was observed. Hydropriming for 1 h under W-LEDs generated 27.72 roots followed by 2 h hydropriming under same LEDs. Whereas lowest root numbers (14.89) were achieved when seeds were primed for 2 h and cultured under R-LEDs (Fig. 5). In general, root numbers were lowest with all hydropriming under R-LEDs and maximum under W-LEDs. The results on shoot:root ratio was statistically significant and ranged 0.796–1.291 (Fig. 5). The highest shoot:root ratio under specific LEDs was associated with specific hydropriming time. The highest shoot:root ratio under W-LEDs, B-LEDs and R-LEDs was recorded for 2 h, control and 1 h respectively.
Factorial Regression Analysis
Results of factorial regression analysis revealed the non-significant impact of LEDs and hydropriming on all germination indices. Whereas variable impact was recorded for growth indices (Table S1). Results illustrated the significant impact of LEDs on shoot length (p 0.000), root length (p 0.047), and fresh shoot wt (p 0.022). Hydropriming regulated the fresh root wt (p 0.032) and dry root wt (p 0.025). Whereas, statistically signficant impact of LEDs x Hydropriming was attributed only to fresh shoot wt. Root numbers and shoot:root remained unaffected by all input parameters. The results on R-sq scores (actual and predicted) is presented in Table S2 for all growth indices parameters. Maximum R2-act. (85.53%) and R2-pred. (70.08%) were attributed to shoot length followed by fresh shoot wt (R2-act. = 54.53%; R2-pred. = 24.25%), and dry root wt (R2-act. = 52.76%; R2-pred. = 14.85%). Whereas other output parameters exhibited relatively low R2-act. below 50.0% (Table S2). The regression equation of all output parameters is alo presented in Table S2.
Pareto Chart and Normal Plot Analysis
The results of Pareto chart is presented in Fig. 6A–H. The central point line was automated at 2.306, and significant input parameter is expressed as bold. The placement of input parameters from top to bottom of all parameters were recorded as A > B > AB for shoot length (Fig. 6A), A > AB > B for root length (Fig. 6B) and fresh shoot wt (Fig. 6C), B > A > AB for fresh root wt (Fig. 6D) and dry root wt (Fig. 6F), and AB > B > A for dry shoot wt (Fig. 6E). Whereas, non-significant impact for both root numbers (Fig. 6G) and shoot:root (Fig. 6H) exhibited the order of A > B > AB. The data presented in Pareto chart were also examined with normal plots (Fig. 7A–H), with distribution of input parameters (A, B, AB) on the lef tor right side of the central line. For shoot length, root length, and fresh shoot wt, the impact of LEDs was statistically significant, and normal plot placed it on the left side of the central line. Increase in light intensity (Lux) exerted negative impact on shoot length, root length, and fresh shoot wt. Similarly, all other significant input parameters (B and AB) were also distributed on the left side of the central line. Increase in hydropriming duration exerted negative impact on fresh root wt and dry root wt. Whereas interaction of both LEDs × hydropriming affected the dry shoot wt. It was also interesting to note that non-significant impact caused by input factors were also on the left side of the line. The results revealed that the increase of input factor generally led to the negative impact on their respective output parameter.
Contour Plots and Surface Plot Analysis
Contour plots and surface plots analysis illustrated the impact of two input factors on output parameters. Results on shoot length revealed the range of 9.71–18.02 cm, and contour plots illustrated the maximum of 17.5 cm shoot length from 165 lx LEDs and approximately 4.0 h hydropriming (Fig. 8a). The results were confirmed by surface plots and expressed in orange line on the bottom surface (Fig. 9a). The range of root length was recorded as 10.4–16.23 cm. controur plots optimized slightly more than 15.0 cm long roots from the combination of 165–210 lx LEDs with hydropriming of 3.15–4.0 h (Fig. 8b), confirmed by surface plots (Fig. 9b). The maximum fresh shoot wt. of slightly more than 0.54 g was optimized from LEDs range of 165–750 lx and 3.6–4.0 h hydropriming (Fig. 8c), comfirmed by constructing surface plots (Fig. 9c). Fresh root wat range was observed as 0.238–0.361 g with maximum achieveable value of 0.34 g. Results revealed two different combinations of 165–400 lx × 0–0.7 h hydropriming and 1825–2025 lx × 0–0.3 h hydropriming (Fig. 8d). The results can be confirmed by constructing surface plots (Fig. 9d). Dry shoot wt revealed the probabality of 0.044 g or above wt with maximum of approximately 0.456 g from the combination of 1580–2030 lx and 0–0.67 h hydropriming (Fig. 8e). The investigation of surfae plots confirmed the results (Fig. 9e). Dry root wt exhibited the optimum conditions of 435–1950 lx LEDs with hydropriming of 0–0.32 h with maximum dry root wt of 0.32–0.33 g (Fig. 8f), confirmed with constructing surface plots (Fig. 9f). Results on root numbers also exhibited the combination of 165–335 lx light and 0–0.30 h hydropriming. Results also revealed the approximately maximum of 24.32 roots from 167 lx light and 0.58 h hydropriming (Fig. 8g). It was confirmed by constructing surface plots (Fig. 9g). Maximum shoot:root of 1.20 can be obtained from 165–240 lx light and 3.6–4.0 h hydropriming (Fig. 8h), consequently confirmed by constructing surface plots (Fig. 9h).
Machine Learning Analysis
Analysis of growth indices with the help of MLP and RF models illustrated the maximum R2 scores of shoot length (R2 = 0.831) from MLP model closely followed by RF model (R2 = 0.789). Considering all growth indices, the pattern based on R2 scores exhibited as shoot length (R2 = 0.831) > root length (R2 = 0.0.550) > root numbers (R2 = 0.426) > fresh shoot wt (R2 = 0.0.403) > fresh root wt (R2 = 0.201) > dry root wt (R2 = 0.228) > shoot:root (R2 = 0.090) > dry shoot wt (R2 = 0.069). The analysis based on model revealed the maximum R2 scores from shoot length, fresh shoot wt, root numbers, and dry shoot wt were attributed to MLP model. Contrarily, the highest R2 scores from root length, fresh root wt, and dry root wt were computed for RF model. Whereas, both models calculated similar R2 scores for shoot:root. The results were further confirmed by computing MSE (MLP: 1e-05–6.479; RF: 1e-05–9.458) and MAE (MLP: 0.002–11.707; RF: 0.002–2.470) metrics (Table 1). Overall, relatively low scores were registered from both models for all growth indices. The validity of the results was also confirmed by using MAPE metric which revealed the range of 5.764–10.806% for MLP model and 6.719–12.072% for RF model (Table 1). The overall analysis of both models showed the better performance of MLP model compared to RF model.
Discussion
Germination Indices
LEDs, a low-cost and effective lighting system, offer an alternative to traditional lighting systems. LEDs provide specific light illumination, controlling in vitro germination, seedling establishment, and plant growth (Olvera-González et al. 2013). However, factors like type, photoperiod, and intensity can alter the germination and growth pattern of each plant under specific environmental conditions (Tang et al. 2010). The condition for the establishment of plants or crops for their life cycle is uniform germination. The germination process is regulated by a variety of internal and external elements, and any deviation or delayed germination may result in variable plant growth and, eventually, reduced crop output. Therefore, scientists use different techniques to obtain uniform and rapid germination by exposing or treating seeds to different stimuli. In the present study, seeds were exposed to two different stimuli of LEDs and hydropriming and their impact on germination and growth indices were examined by employing different mathematical expressions.
Germination is a complex process influenced by various factors, including light (Hasan et al. 2017). The first and most noticeable effect of light is on plant germination, which is followed by the corresponding plant growth. However, it varies with genotype and LEDs type or illumination. Exposing seeds to LEDs may affect a plant's enzymatic activity, metabolic processes, and growth and productivity. Estimating the mean germination percentage in response to any external stimuli or subjecting the seed to any stressor is the most popular technique used to assess the seed vigor and viability. Results show that 100% germination is observed in response to different LEDs. The studies on LEDs exhibits the variable response on germination and growth indices (Lim et al. 2020; Sorgato et al. 2020). The results revealed that the type of LEDs does not affect germination percentage, but it can alter the germination process. The variable MGT, MR, CVt %, U, and Z values of desi chickpea seeds exposed to different LEDs were confirmed.
Mean germination time (MGT), another crucial mathematical expression for germination, is the weighted average of germination dependent on germination rate in response to time (Bewley et al. 2012). MGT has been utilized successfully for various kinds of plants and is a reciprocal of germination rate (Demir et al. 2008; Khajeh-Hosseini et al. 2009). High MGT reflects the slow emergence with probability of low emergence (Mavi et al. 2010). Regardless of high G (%) levels, seed exposure to LEDs had an impact on MGT, which highlights the importance of the precise light wavelength that LEDs give. It has been demonstrated that light wavelength has a substantial impact on the MGT of pitaya seeds (Lone et al. 2014), and demonstrated the beneficial association between light color and MGT. However, their study also emphasized the significance of genotype for MGT values. The coefficient of variation of the germination time (CVt) can be used to determine whether seeds associated with MGT germinate uniformly or unevenly (Cruz et al. 2001; Dorneles et al. 2005). A low CVt value indicates more evenly distributed seed germination at that time, and vice versa. In response to LEDs, CVt of desi chickpea displayed the same pattern as MGT. Plants frequently have varying germination times, which can be controlled by factors including light intensity, irradiation, and photoperiod. The presence of phytochrome in seeds in varying amounts and its contribution to seed germination under various lighting conditions could be the reason behind it (Marcos Filho 2015).
The mean germination rate (MR), which is the inverse of the mean germination time, often expresses the vigor and germination speed of a particular seed lot. The range of MR is 0 to 1, with values close to 1 suggesting more vigorous seeds that germinate more quickly over a shorter period of time, and vice versa (Ranal and Santana 2006). The MR values were completely at odds with the MGT and CVt values, highlighting the importance of LEDs and the fact that W-LEDs were more efficient at producing high MR values. Uncertainty (U) values ranged from 0 ≤ U ≤ \({\text{log}}_{2} \left( n \right)\), and a value of “0” or very near to “0” indicates more synchronized germination and vice versa (Bewley et al. 2012). A single seed in a specific seed batch is extremely important and may cause doubt uncertainty (U). Seed germination means asynchronization and it can be quantified as uncertainty associated to the distribution of the relative frequency of germination. This asynchronization of germination shows germination's inherent uncertainty, and quantification of this uncertainty can be related to relative germination frequency. The synchronization index (Z), on the other hand, measures how closely two seeds germinate at the same time and is expressed as 0 ≤ Z ≤ 1 (Bewley et al. 2012). The Z values differ from the U values in that a Z value of 1 or close to 1 indicates better synchronized germination, whereas a Z value of “0” indicates no germination and values close to “0” indicate asynchronous germination of a specific seed lot. Both U and Z values reflect the uncertainty and synchronization of the whole germination process (Aasim et al. 2023a). Results indicate the superiority of W-LEDs over other LEDs due to low U and high Z values. The variable impact of different LEDs lighting system on germination of desi chickpea seeds is possibly due to the ABA and GA3 signalling and metabolism in response to the activation of photoreceptor phytochrome B (PHYB) under different LEDs. The activation of phytochrome B in turn regulates the whole germination process (Lim et al. 2020) by removing the repressive histone arginine methylations.
Hydropriming significantly affected all germination indices of desi chickpea. Results revealed 100% germination for all hydropriming exposure time after 2 days (Nimac et al. 2018). However, variable germination (%) was recorded in response to hydropriming time after day 1. Results revealed that 4 h hydropriming was highly detrimental for G (%) and MGT (Selvarani and Umarani 2011; Jagosz 2015). Decrease MGT values in response to priming techniques have been documented for Quercus castaneifolia (Hadinezhad et al. 2013). Results also revealed that hydropriming for 1 h and 2 h was beneficial than 4 h hydropriming treatment (Selvarani and Umarani 2011; Jagosz 2015) and control. The results suggest that 2 h hydropriming is more suitable with positive impact on germination indices.
There is no report to date available which reflects the effect of LEDs × hydropriming on germination indices in desi chickpea. The combination of LEDs × hydropriming regulates the germination indices of desi chickpea and results clearly indicated the role of LEDs light on germination percentage and B-LEDs in combination with 1 h hydropriming yielded relatively high germination percentage (100%). However, after day 2, all combinations of LEDs × hydropriming yielded 100% germination and these results suggest the role of LEDs at initial stage of germination. Desi chickpea seeds responded to MGT and MR values in a manner that was consistent with how LEDs and hydropriming affected those values individually. The results on MR revealed that combination of two factors are highly significant (Homrani-Bakali 2015).
Results on MGT clearly showed that the pairing of W-LEDs with 1 h and 2 h hydropriming and R-LEDs with 2 h hydropriming was most effective. The effects of many parameters, including seed size, vigor, aging, and the application of external factors, such as light exposure, temperature, or priming techniques, on MGT of diverse plants have also been highlighted in earlier studies (Demir et al. 2008; Selvarani and Umarani 2011; Jagosz 2015). The impact of MGT was also reflected on CVt % with minimum variation for both B-LEDs and R-LEDs with 2 h hydropriming. The impact of hydropriming on CVt % values was substantiated by the fact that 4 h hydropriming was least effective with higher CVt % values (Shahverdi et al. 2017). In contrast, sea fennel seeds have not been shown to be affected by hydropriming or priming with NaCl in terms of CVt % (Nimac et al. 2018). The U and Z indices of desi chickpea seeds were controlled by the combination of LEDs and hydropriming. Combining B-LEDs with 1-h hydropriming produced the lowest U value, and this was because two distinct elements were combined to reduce uncertainty. Instead, Z values were completely the opposite of U values, and all LEDs combined with 1 h and 2 h hydropriming produced significant Z values. Priming strategies have been shown to have negligible influence on Z value (Nimac et al. 2018) or to provide the highest Z value in the control group when compared to other priming techniques (Homrani-Bakali 2015).
Growth Indices
Studies on LEDs used either single or a combinations of different LEDs to regulate the vegetative, physiological and biochemical characteristics of plants (Cho et al. 2019; Xu et al. 2020). However, other factors like plant type, species or other culture conditions may also alter plant growth. The results achieved in this study discerned the significance of specific light illumination on individual growth parameter of desi chickpea grown under different LEDs. The results also revealed that W-LEDs was more effective than other LEDs light and yielded more values possibly due to wide light spectrum offered by W-LEDs. Contrary to earlier research, which showed that certain LED lights were connected with certain development characteristics (Lim et al. 2020). Zantedeschia albomaculata grown under R-LEDs or B-LEDs, yielded comparatively more shoots, fresh weight, and dry weight (Chang et al. 2003). Additionally, the largest dry weight of potato plantlets grown under B-LEDs or B:R-LEDs in comparison to FL was recorded (Sivakumar et al. 2006), or the highest fresh weight of Phalaenopsis ‘Vivien’ was recorded under R-LEDs (Ouzounis et al. 2014). According to these findings, R-LEDs control in vitro plant development more effectively than conventional FL or W-LEDs, whether used alone or in combination with B-LEDs. On the contrary, positive impact of FL or W-LEDs on plant height, roots numbers and length, fresh and dry weight, and dry matter contents of V. Planifolia (Bello-Bello et al. 2016), or variable impact on plant growth indices of Cunninghamia lanceolata (Lamb.) Hook (Xu et al. 2020) have also been documented. The results of the following studies clearly revealed that plant growth and development of plants may change with specific light illumination and plant type.
Hydropriming of seeds generally resulted in elevated plant growth compared to control. However, other factors (internal and external) in the experiment also regulate the germination and plant growth. Results achieved in response to different hydropriming clearly exhibit the need of specific hydropriming time for individual growth parameters. Shoot length of desi chickpea was maximum when seeds were hydro primed for 4 h (Ghasemi-Golezani et al. 2013; Sepehri and Rouhi 2017). Contrarily, similar or no difference of hydropriming and control treatment on plant height of Abies hickelli (Zulueta-Rodríguez et al. 2015). The small amount of dry weight calculated from hydro primed seeds demonstrated that hydropriming had no impact on the accumulation of dry matter, contrary to the of hydro primed lentil seeds with relatively more dry wt of seedlings (Ghassemi-Golezani et al. 2008). Results on root numbers remained unaffected in response to different hydropriming time. Previously, twofold more roots than control under hydroprimed seeds for 3 days under in vitro conditions has been documented (Arun et al. 2013). The positive impact of hydropriming on germination and plant growth is supposed to be due to the activation of pre-germinative metabolic events that help and enable the plant embryo to grow quickly with relatively more germination speed and seedlings vigor (Afzal et al. 2012).
Plots and Charts Analysis
Pareto and normal plots provide a graphical means to investigate the significance level and the influence of input components on output parameters. The approach relies on the distribution of input variables on the left and right sides of a standard line based on statistically significant or non-significant. Variables positioned far from the line show the greater impact of input variables on the corresponding output parameters. The location of the input variables near the line indicates the low impact. Normal plots illustrate the data statistically significant (right, square, red) or non-significant (left, round, blue). Direct proportionate impact of each input variable on its corresponding output variable is shown by the input variables placed on the right side and vice versa inverse proportionate on the left side (Katirci 2015; Aasim et al. 2023b). Results of Pareto chart and normal plots exhibited the similar pattern and distributed the input parameters on the basis of significant input factor and significance level (Aasim et al. 2023b). Considering the Pareto chart, both LEDs and hydropriming duration regulated the in vitro growth indices of chickpea. LEDs regulated the length and fresh wt of shoot. Whereas hydropriming controlled the root growth. The combination of LEDs × hydropriming regulated the dry wt of root. Results further revealed that root numbers and shoot:root were not affected by any input factor. The Pareto chart reflected the significance level of input factors efficiently, the normal plots distributed the input parameters data based on negative/positive impact or direct/inverse proportional impact on their respective output parameter. Results exhibited the negative or inverse proportional impact of all significant input parameters on output parameters. Regardless of its importance, the usage of Pareto charts and normal plots in plant sciences is limited. However, successful use of both charts have been employed for optimizing IAA generation utilizing Streptomyces fradiae (Myo et al. 2019), biosynthesis of gold nanoparticles (Keijok et al. 2019), and in vitro regeneration of sorghum (Aasim et al. 2023b).
Prediction of output parameters by considering two input factors can be attained by placing one factor on x-axis and other on y-axis. In this way, input parameters can be derived by setting the desired output target. Contour plots distribute the data into sub-data sets, and it enables the researcher to find the best optimum conditions for setting target of output parameters. The results can be further verified by using surface plots, which distribute the data in 3-D form. Both contour plots and surface plots are powerful statistical tools used for the prediction analysis and to optimize the responses between two input variables (Aasim et al. 2023b), expressed with different colors (Kasman et al. 2019). The use of both contour and surface plots have been documented for phytoremediation (Jaskulak et al. 2020; Kasman et al. 2019; Mohamad Thani et al. 2020; Aasim et al. 2023c), and in vitro regeneration of sorghum (Aasim et al. 2023b).
Machine Learning Analysis
Understanding the effects of various stresses on growth metrics and germination indices is prerequisite to understanding the relationship between the input and output variables. This might be done with the assistance of statistical or computer-aided algorithms. Recently, AI/ML-based models have been used to validate, predict, and optimize data, and the performance of these models has been evaluated using a variety of performance indicators. For certain crops, ML or ANN models have also been used to predict germination or growth indices in response to different stresses or growth promoting treatments (Škrubej et al. 2015). In these investigations, deep learning models were typically used for seedling establishment (Samiei et al. 2020) and germination detection (Genze et al. 2020). Studies conducted on plant biotechnology demonstrated the precision and dependability of various ML in a variety of fields, including in vitro germination to regeneration (Hesami et al. 2020; Hesami and Jones 2021; Pepe et al. 2021a, b; Türkoğlu et al. 2023; Şimşek et al. 2024). Whereas classification based ML/ANN models were employed to validate the in vitro germination and seedling growth parameter of industrial hemp (Aasim et al. 2022b). In this present work, the MLP and RF models were employed to forecast the in vitro growth parameters using four different performance criteria in response to LEDs and hydropriming. The four performance measures that were used confirmed the results and correctly predicted the results.
Results of MLP and RF models predicted the growth indices of chickpea sufficiently with maximum prediction for shoot length. These results are contrary to the recently reported findings in chickpea (Aasim et al. 2023a), where relatively low R2 scores for shoot length and vice versa high R2 scores for remaining growth indices in response to NaCl induced salinity. The performance of both models was almost like each other, relatively high R2 scores were associated with MLP model. The significance of results was also analyzed by using three other performance metrics along with R2. The low MSE and MAE scores confirmed the performance of R2. Application of MAPE is another strong metric to evaluate the performance of the model. The MAPE scores below 50% are acceptable for the model with values of 0 or near to zero reflect the better performance of the model. Therefore, opting for two more performance metrics offers better model prediction compared to using single performance metric. Application of AI/ML models for the prediction, validation and optimization of in vitro growth indices have been documented for different crops like chickpea (Aasim et al. 2023a), and industrial hemp (Aasim et al. 2022b; Hesami et al. 2021; Pepe et al. 2021a, b). These results revealed the excellent performance of both MLP and RF models. Previous studies on MLP and RF models exhibited the variable response, based mainly on the design of the experiment (input and output variables), and used models (Hesami et al. 2019; Salehi et al. 2021).
Conclusion
This research provides a thorough comprehension of how LEDs and hydropriming interact to influence chickpea germination and growth. White LEDs (W-LEDs) were more effective, promoting rapid germination with high indices such as MGT, CVt, and Z values. R-LEDs, on the other hand, were more effective in promoting seedling development. The hydropriming treatment duration proved to be a crucial factor, with the 2 h treatment period exhibiting superior results characterized by lower MGT, CVt, and U values, accompanied by the highest Z values. Whereas hydropriming for 4 h enhanced the in vitro plant growth parameters. The combination of W-LEDs with hydropriming durations of 1 h and 2 h proved highly effective, showcasing a synergistic impact on the optimization of germination and growth. From an anticipatory standpoint, the research not only found relationships between LED type and hydropriming times, but it also made use of sophisticated analytical instruments like Pareto charts, normal plots, and optimization techniques like surface and contour plots. These research techniques aided in the improvement of results and laid the groundwork for more advanced techniques in the fields of plant sciences and agronomy. An important development in outcome prediction is the use of machine learning (ML) and artificial intelligence (AI) models. The study shows that hydropriming and LEDs may be effectively linked by using four performance criteria. The results point to the prospect of using optimization methods in addition to predictive modeling for chickpea cultivation. The study enlightens the complex relationships that exist between hydropriming conditions and light sources, exhibiting a range of responses in growth indices. This thorough comprehension opens the door for further research into the underlying processes driving these reactions. In this study, two models based on different principles were employed for ML modeling. It is possible and recommended to integrate more advanced AI techniques like deep learning or hybrid models for more efficient and robust prediction, followed by enhancing the optimization and validation. It is also recommended to employ these high-tech technologies under field or greenhouse conditions. The study’s overall findings demonstrate the use of intelligent modeling approaches and technology for plant science research.
References
Aasim M, Ali SA, Bekiş P, Nadeem MA (2022a) Light-emitting diodes induced in vitro regeneration of Alternanthera reineckii mini and validation via machine learning algorithms. Vitr Cell Dev Biol 58:1–10
Aasim M, Katırcı R, Akgur O et al (2022b) Machine learning (ML) algorithms and artificial neural network for optimizing in vitro germination and growth indices of industrial hemp (Cannabis sativa L.). Ind Crops Prod 181:114801
Aasim M, Akin F, Ali SA et al (2023a) Artificial neural network modeling for deciphering the in vitro induced salt stress tolerance in chickpea (Cicer arietinum L.). Physiol Mol Biol Plants. https://doi.org/10.1007/s12298-023-01282-z
Aasim M, Ali SA, Altaf MT et al (2023b) Artificial neural network and decision tree facilitated prediction and validation of cytokinin-auxin induced in vitro organogenesis of sorghum (Sorghum bicolor L.). Plant Cell Tissue Organ Cult 153:1–14
Aasim M, Ali SA, Aydin S et al (2023c) Artificial intelligence–based approaches to evaluate and optimize phytoremediation potential of in vitro regenerated aquatic macrophyte Ceratophyllum demersum L. Environ Sci Pollut Res 30:1–12
Afzal I, Hussain B, Basra SMA, Rehman H (2012) Priming with moringa leaf extract reduces imbibitional chilling injury in spring maize. Seed Sci Technol 40:271–276
Aggarwal CC (2018) Neural networks and deep learning, vol 10(978). Springer, New York, p 3
Arun K, Uma S, Saraswathi S et al (2013) Effects of whole seed priming on the in vitro germination of hybrid banana embryos (Musa spp.). Seed Sci Technol 41:439–451
Asl MBA, Taheri G (2012) Survey the effect of seed priming on germination and physiological indices of cotton khordad cultivar. Ann Biol Res 3:1003–1009
Bello-Bello JJ, Martínez-Estrada E, Caamal-Velázquez JH, Morales-Ramos V (2016) Effect of LED light quality on in vitro shoot proliferation and growth of vanilla (Vanilla planifolia Andrews). Afr J Biotechnol 15:272–277
Bewley JD, Bradford K, Hilhorst H (2012) Seeds: physiology of development, germination and dormancy. Springer, New York
Čanak P, Jocković M, Ćirić M et al (2014) Effect of seed priming with various concentrations of KNO3 on sunflower seed germination parameters in in vitro drought conditions. Res Crop 15:154–158
Chang HS, Charkabarty D, Hahn EJ, Paek KY (2003) Micropropagation of calla lily (Zantedeschia albomaculata) via in vitro shoot tip proliferation. Vitr Cell Dev Biol 39:129–134
Cho KH, Laux VY, Wallace-Springer N et al (2019) Effects of light quality on vegetative cutting and in vitro propagation of coleus (Plectranthus scutellarioides). HortScience 54:926–935
Cruz ED, Martins FDEO, Carvalho JEUDE (2001) Biometria de frutos e sementes e germinação de jatobá-curuba (Hymenaea intermedia Ducke, Leguminosae-Caesalpinioideae). Braz J Bot 24:161–165
Demir I, Ermis S, Mavi K, Matthews S (2008) Mean germination time of pepper seed lots (Capsicum annuum L.) predicts size and uniformity of seedlings in germination tests and transplant modules. Seed Sci Technol 36:21–30
Dorneles MC, Ranal MA, Santana DG (2005) Germinação de diásporos recém-colhidos de Myracrodruon urundeuva Allemão (Anacardiaceae) ocorrente no cerrado do Brasil Central. Braz J Bot 28:399–408
Genze N, Bharti R, Grieb M et al (2020) Accurate machine learning-based germination detection, prediction and quality assessment of three grain crops. Plant Methods 16:1–11
Ghasemi-Golezani K, Japparpour-Bonyadi Z, Shafagh-Kolvanagh J, Nikpour-Rashidabad N (2013) Effects of water stress and hydro-priming duration on field performance of lentil. Int J Farming Allied Sci 2:922–925
Ghassemi-Golezani K, Aliloo AA, Valizadeh M, Moghaddam M (2008) Effects of hydro and osmo-priming on seed germination and field emergence of lentil (Lens culinaris Medik.). Not Bot Horti Agrobot Cluj-Napoca 36:29–33
Hadinezhad P, Payamenur V, Mohamadi J, Ghaderifar F (2013) The effect of priming on seed germination and seedling growth in Quercus castaneifolia. Seed Sci Technol 41:121–124
Hasan MM, Bashir T, Ghosh R et al (2017) An overview of LEDs’ effects on the production of bioactive compounds and crop quality. Molecules 22:1420
Hesami M, Jones AMP (2021) Modeling and optimizing callus growth and development in Cannabis sativa using random forest and support vector machine in combination with a genetic algorithm. Appl Microbiol Biotechnol 105:5201–5212
Hesami M, Naderi R, Tohidfar M (2019) Modeling and optimizing In vitro sterilization of chrysanthemum via multilayer perceptron-non-dominated sorting genetic algorithm-II (MLP-NSGAII). Front Plant Sci 10:1–13. https://doi.org/10.3389/fpls.2019.00282
Hesami M, Naderi R, Tohidfar M (2020) Introducing a hybrid artificial intelligence method for high-throughput modeling and optimizing plant tissue culture processes: the establishment of a new embryogenesis medium for chrysanthemum, as a case study. Appl Microbiol Biotechnol 104:10249–10263
Homrani-Bakali A (2015) Effect of various pre-treatments and alternating temperature on seed germination of Artemisia herba-alba Asso. J Plant Stud 4:12
Jagosz B (2015) Improving onion seed germination using priming treatments. Infrastrukt i Ekol Teren Wiej IV/4: 1437–1447.
Jaskulak M, Grobelak A, Vandenbulcke F (2020) Modeling and optimizing the removal of cadmium by Sinapis alba L. from contaminated soil via response surface methodology and artificial neural networks during assisted phytoremediation with sewage sludge. Int J Phytoremediation 22:1321–1330
Karataş M, Aasim M, Dazkirli M (2016) Influence of light-emitting diodes and benzylaminopurin on adventitious shoot regeneration of water hyssop (Bacopa monnieri (L.) Pennell) in vitro. Arch Biol Sci. https://doi.org/10.2298/ABS150803039K
Kasman M, Riyanti A, Salmariza S, Aslamia RTSS (2019) Response surface methodology approach for analysis of phytoremediation process of Pb (II) from aqueous solution using Echinodorus palaefolius. IOP Conf Ser 546:22009
Katirci R (2015) Statistical approach to optimizing A Zn–Ni bath containing ed and tea. Surf Rev Lett 22:1550015
Katırcı R, Yılmaz EK, Kaynar O, Zontul M (2021) Automated evaluation of Cr-III coated parts using Mask RCNN and ML methods. Surf Coat Technol 422:127571. https://doi.org/10.1016/j.surfcoat.2021.127571
Keijok WJ, Pereira RHA, Alvarez LAC et al (2019) Controlled biosynthesis of gold nanoparticles with Coffea arabica using factorial design. Sci Rep 9:1–10
Khajeh-Hosseini M, Lomholt A, Matthews S (2009) Mean germination time in the laboratory estimates the relative vigour and field performance of commercial seed lots of maize (Zea mays L.). Seed Sci Technol 37:446–456
Kumar S, Patra AK, Datta SC et al (2015) Phytotoxicity of nanoparticles to seed germination of plants. Int J Adv Res 3:854–865
Lewis CD (1982) Industrial and business forecasting methods: a practical guide to exponential smoothing and curve fitting. Butterworth-Heinemann, London
Lim CH, Guan TS, Chan Hong E et al (2020) Effect of different LED lights spectrum on the ‘in vitro’ germination of gac seed ‘(Momordica cochinchinensis)’. Aust J Crop Sci 14:1715–1722
Lone AB, Unemoto LK, Ferrari EAP et al (2014) The effects of light wavelength and intensity on the germination of pitaya seed genotypes. Aust J Crop Sci 8:1475–1480
Marcos Filho J (2015) Fisiologia de sementes de plantas cultivadas. Abrates, Londrina
Mavi K, Demir I, Matthews S (2010) Mean germination time estimates the relative emergence of seed lots of three cucurbit crops under stress conditions. Seed Sci Technol 38:14–25
Mohamad Thani NS, Mohd Ghazi R, Abdul Wahab IR et al (2020) Optimization of phytoremediation of nickel by Alocasia puber using response surface methodology. Water 12:2707
Morrow RC (2008) LED lighting in horticulture. HortScience 43:1947–1950
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Myo EM, Ge B, Ma J et al (2019) Indole-3-acetic acid production by Streptomyces fradiae NKZ-259 and its formulation to enhance plant growth. BMC Microbiol 19:1–14
Nimac A, Lazarević B, Petek M et al (2018) Effects of salinity and seed priming on germination of sea fennel (Crithmum maritimum L.). Agric Conspec Sci 83:181–185
Olvera-González E, Alaniz-Lumbreras D, Ivanov-Tsonchev R et al (2013) Chlorophyll fluorescence emission of tomato plants as a response to pulsed light based LEDs. Plant Growth Regul 69:117–123
Ouzounis T, Fretté X, Rosenqvist E, Ottosen CO (2014) Effects of LEDs on chlorophyll fluorescence and secondary metabolites in Phalaenopsis. In: II International Orchid Symposium 1078, pp 87–92
Pavlov YL (2019) Random forests. Random for. https://doi.org/10.1201/9780429469275-8
Pedregosa F, Varoquaux G, Gramfort A et al (2011) Scikit-learn: machine learning in {P}ython. J Mach Learn Res 12:2825–2830
Pepe M, Hesami M, Jones AMP (2021a) Machine learning-mediated development and optimization of disinfection protocol and scarification method for ımproved ın vitro germination of cannabis seeds. Plants 10:2397
Pepe M, Hesami M, Small F, Jones AMP (2021b) Comparative analysis of machine learning and evolutionary optimization algorithms for precision micropropagation of Cannabis sativa: prediction and validation of in vitro shoot growth and development based on the optimization of light and carbohydrate sou. Front Plant Sci. https://doi.org/10.3389/fpls.2021.757869
Phat P, Ju H-J, Noh J et al (2017) Effects of hydropriming and explant origin on in vitro culture and frequency of tetraploids in small watermelons. Hortic Environ Biotechnol 58:495–502
Quail PH (2002) Phytochrome photosensory signalling networks. Nat Rev Mol Cell Biol 3:85–93
Ranal MA, de Santana DG (2006) How and why to measure the germination process? Braz J Bot 29:1–11
Ranal MA, de Santana DG, Ferreira WR, Mendes-Rodrigues C (2009) Calculating germination measurements and organizing spreadsheets. Braz J Bot 32:849–855
Salehi M, Farhadi S, Moieni A et al (2021) A hybrid model based on general regression neural network and fruit fly optimization algorithm for forecasting and optimizing paclitaxel biosynthesis in Corylus avellana cell culture. Plant Methods 17:1–13
Samiei S, Rasti P, Ly Vu J, et al (2020) Deep learning-based detection of seedling development. Plant Methods 16:1–11
Selvarani K, Umarani R (2011) Evaluation of seed priming methods to improve seed vigour of onion (Allium cepa cv. aggregatum) and carrot (Daucus carota). J Agric Technol 7:857–867
Sepehri A, Rouhi HR (2017) Effect of hydropriming on morphological and physiological performance of aged groundnut (Arachis hypogaea L.) seeds. علوم گیاهان زراعی ایران 48:43–53
Shahverdi MA, Omidi H, Tabatabaei SJ (2017) Determination of optimum duration and concentration of Stevia (Stevia rebaudiana Bert.) seed priming with Boric acid (H3BO3). Türkiye Tarımsal Araştırmalar Derg 4:24–30
Şimşek Ö, Dalda Şekerci A, Isak MA et al (2024) Optimizing micropropagation and rooting protocols for diverse lavender genotypes: a synergistic approach ıntegrating machine learning techniques. Horticulturae 10:52
Sivakumar G, Heo JW, Kozai T et al (2006) Effect of continuous or intermittent radiation on sweet potato plantlets in vitro. J Hortic Sci Biotechnol 81:546–548
Škrubej U, Rozman Č, Stajnko D (2015) Assessment of germination rate of the tomato seeds using image processing and machine learning. Eur J Hortic Sci 80:68–75
Sorgato JC, Mudolon ED, Guimarães FF et al (2020) Light sources on the germination and initial in vitro establishment of Schomburgkia crispa Lindl., a species of the Brazilian Cerrado. Ciência Rural 51:3, e20190022
Takakı M (2001) New proposal of classification of seeds based on forms of phytochrome instead of photoblastism. Rev Bras Fisiol Veg 13:104–108
Tang D-S, Hamayun M, Khan AL et al (2010) Germination of some important weeds influenced by red light and nitrogenous compounds. Pak J Bot 42:3739–3745
Türkoğlu A, Haliloğlu K, Demirel F et al (2023) Machine learning analysis of the impact of silver nitrate and silver nanoparticles on wheat (Triticum aestivum L.): callus induction, plant regeneration, and DNA methylation. Plants 12:4151
Tzortzakis GN (2009) Effect of pre-sowing treatment on seed germination and seedling vigour in endive and chicory. Hortic Sci 36:117–125
Van Rossum G, Drake FL (2009) Python 3 Reference Manual. CreateSpace, Scotts Valley, CA
Webb GI, Sammut C, Perlich C et al (2011) Leave-one-out cross-validation. Encyclopedia of machine learning. Springer, Boston, pp 600–601
Xu Y, Yang M, Cheng F et al (2020) Effects of LED photoperiods and light qualities on in vitro growth and chlorophyll fluorescence of Cunninghamia lanceolata. BMC Plant Biol 20:1–12
Zulueta-Rodríguez R, Hernández-Montiel LG, Murillo-Amador B et al (2015) Effect of hydropriming and biopriming on seed germination and growth of two Mexican fir tree species in danger of extinction. Forests 6:3109–3122
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Fatma Akın: Research work, Data tabulation, Seyid Amjad Ali: Tabulation of formulas, Visualization, Review, Machine learning analysis, Muhammad Aasim: Supervision, Research designing, Data analysis, Manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Handling Editor: Jose M. Miguel.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aasim, M., Akin, F. & Ali, S.A. Synergizing LED Technology and Hydropriming for Intelligent Modeling and Mathematical Expressions to Optimize Chickpea Germination and Growth Indices. J Plant Growth Regul (2024). https://doi.org/10.1007/s00344-024-11269-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00344-024-11269-z