Abstract
Lithium mining driven by the growing demand for lithium-ion batteries, has environmental consequences linked to soil and water pollution. Nevertheless, research on the environmental impacts of lithium extraction still needs to be improved, highlighting the imperative for additional research. The article addresses the potential impact of the C57 lithiniferous feldspar mine on water quality, specifically focusing on surface, groundwater and spring water samples collected at the mining site and surrounding area in Gonçalo (Guarda, Portugal). The objective is to evaluate the environmental consequences of mining activities, with particular emphasis on mineral leaching. This study aims to evaluate the water quality around the C57 mine and the potential environmental impacts of mining operations. Water samples were collected from different sources, such as surface, underground, and spring waters, and chemical analyses were carried out to determine concentrations of different parameters, which were later compared with national and international reference guidelines. In addition to analysing the water samples, weathering tests were carried out using the Soxhlet extractor method to simulate the leaching of minerals over a shorter period (about 125 days). The concentrations of the analysed elements by atomic absorption spectroscopy (Al, Ca, Cd, Cr, Cu, Fe, K, Li, Mg, Mn, Na, Ni, Pb and Zn) in the weathering solutions were generally low and decreased throughout the testing period, with significant concentrations of aluminium and chromium exceed Canadian environmental quality guidelines for surface waters. The detected lithium concentrations are quite different, ranging from 8.7 to 19.8 μg/L in surface waters, from 6.9 to 74.1 μg/L in groundwater, and from 25.6 to 35.4 μg/L in spring waters, but are all below the US EPA (2021) recommendations threshold of 0.7 mg/L. Based on the findings, the article concludes that there is currently no clear evidence to indicate the environmental impact of mining activities on water quality in the analysed samples. However, weathering tests suggest potential long-term implications regarding the leaching of specific chemical elements, particularly aluminium and chromium.
Similar content being viewed by others
Introduction
In recent decades, population growth and the increasing demand for energy and raw materials by modern societies have emerged as significant factors (Costa et al. 2010; European Union 2021). The exploration of lithium, a valuable but scarce geological resource abundant in Portugal and Spain, has gained special attention within the European Union (EU 2020). The EU's climate-neutrality scenarios for 2050, before the COVID-19 pandemic, project a substantial demand for various raw materials, including lithium. These scenarios also address supply risks across different levels of the supply chain, particularly for electric vehicle batteries and energy storage. By 2030, the EU is projected to require up to 18 times more lithium and five times more cobalt, and by 2050, nearly 60 times more lithium and 15 times more cobalt compared to the current supply to the entire EU economy (EU 2020).
The Iberian Peninsula is home to one of Europe's largest lithium deposit belts, with at least 25 identified and studied locations since the second half of the twentieth century, some of which have been mined in recent decades (Roda-Robles et al. 2016; Dolgopolova et al. 2017). Prioritizing the value of natural endogenous resources like lithium through the development of products, processes, and services is crucial for both the public and private sectors. Additionally, innovative proposals associated with land preservation strategies and sustainability should be implemented. Spain already has a lithium battery factory in operation, and Portugal is expected to establish one soon. Environmental concerns and sustainable development have become top priorities within the EU, necessitating their consideration throughout the mining exploration process (Dunlap and Riquito 2023). According to 2022 data from the United States Geological Survey (USGS 2022), Portugal possesses the highest lithium reserves in the EU.
The choice of mining method for exploiting a mineral deposit depends on its characteristics, as well as considerations of safety, technology, environmental impact, and economics. Geologic conditions also play a vital role in the selection process. Open pit surface mining, which avoids the use of explosives, is employed when mechanically excavating thick deposits in benches or steps. Alternatively, thin deposits may only require a single bench. Open pit mining is typically used for near-surface deposits or those with a low stripping ratio. However, concerns have been raised about the potential environmental implications of this exploration method, particularly regarding the significant environmental impacts of mining activities due to mineral leaching (Toupal et al. 2022). While soils and rocks naturally change over thousands of years, human interventions have accelerated these processes, occurring more frequently and within shorter timeframes. Water, as the most important solvent in nature, plays a crucial role in the weathering of rocks. This weathering process can occur when water acts independently or when it contains dissolved elements, resulting in an acidic character and promoting chemical changes in the rocks within various environments.
The objective of this study is to assess the potential impact of the C57 lithiniferous feldspar mine exploration on water quality. The open pit mine is in Gonçalo village, near Guarda in Portugal (as shown in Fig. 1). Its primary focus is the extraction of lithiniferous feldspars, which are primarily used by the ceramic industry to reduce the melting points of ceramic pastes, thereby lowering energy consumption, and providing environmental benefits. Although it is a small-scale mining operation that mostly outsources its mining process, it produces approximately 12,000 tons of lithiniferous feldspar annually. The mine plays a vital role in the local economy by generating employment opportunities and economic value for the region.
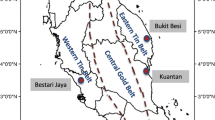
Location of the various lithium mineralization areas in Iberia inside GTMZ (Galicia-Trás-os-Montes Zone) and CIZ (Central-Iberian Zone) (black star C57 Mine Concession). From Roda-Robles et al. (2016)
Site characterization
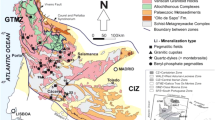
The aplite-pegmatitic vein field of the C57 mine is situated within a large granitic zone in the interior center of Portugal, adjacent to the Serra da Estrela Mountain range. The current lithiniferous vein field is primarily embedded within the Guarda porphyrite monzonite granite (Farinha Ramos 1998, 2007, 2010; Antão 2004; Carvalho and Farinha 2004). The vein outcrops are located on the slope of the granite massif of the Serra da Estrela, spanning an elevation range of 450–850 m over an area of more than 100 km2. These veins exhibit a consistent aplite-pegmatite structure, consisting mainly of quartz, feldspars, and muscovite. Additionally, other mineral phases containing Li, Be, Nb, Ta, and Sn are present, leading to the classification of three vein types in the Gonçalo mining field: lithium veins, stanniferous veins, and mixed veins (Farinha Ramos and Noronha 1995; Farinha Ramos 1998, 2007, 2010; Freitas et al. 2015) (Fig. 2). Lithiniferous veins enriched with lepidolite are found at higher structural levels of the mining field, where the C57 mine is located, while stanniferous veins occur at lower structural levels. Lithium veins exhibit greater Li, Sr, Nb, and Rb enrichment compared to stanniferous and mixed veins.
The granitic rock in the mining area has an average age of 290 million years, and its modal composition varies depending on the degree of weathering (Antão 2004). The raw material used in the Soxhlet extractor test is a residual soil classified as SM (sandy silt with gravel) in the Unified Soil Classification (ASTM D2487-17 2017) and Type VI ('very clayey') sandy gravel according to the Nickless diagram (Nickless and Clayton 1973). The fines in this raw material mainly consist of silt material, typical of poorly evolved soil resulting primarily from the physical alteration of granitic rock materials.
According to the National Water Resources Information System (SNIRH 2022), the C57 mine area is in the Undifferentiated Ancient Massif, which covers a significant portion of the Portuguese territory from a hydrogeological perspective. Within this area are only ten aquifers, none of which fall within the mine's zone of influence. Most of the aquifers are situated in the southern part of the country, with only one, Veiga de Chaves, located in the north. Therefore, it cannot be concluded that there are aquifers with waters possessing clearly defined and stable physical–chemical characteristics from a hydrogeological perspective. It can only be stated that subsurface waters contribute to the main waterways, such as the Zêzere River and the Tagus River.
Sampling
The water sampling process presented challenges due to the absence of water lines near the mining exploration site. The steep slopes in the area cause rapid drainage of precipitation, and the local population has installed a complex system of pipes to transport water for agricultural use, including storage tanks. Surface and underground water samples were collected from the surrounding area of the mining concession, both upstream and downstream of the C57 mine exploitation area, between 2018 and the first months of 2019. The samples were properly identified, collected, and transported to the laboratory in dark and preserved conditions at four degrees Celsius or below.
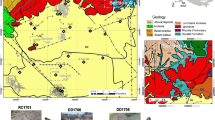
Among the collected samples, WS2 represents groundwater that flows into a surface body of water (WS1 and WS3). The remaining samples (WS4–WS10) are all derived from groundwater, except for samples WS8, WS9, and WS10, which were obtained from water springs located at the top of the mountain, upstream of the C57 mining area (Fig. 3). In the comparative analysis of surface and groundwater quality, a well located in Verdelhos, situated on a tributary of the Zêzere River approximately 10 km southwest of the mining area, was also considered. This well is part of the National Water Resources Information Network and is identified as (224/C36) in Fig. 3.
Location of the water sampling points (WS1–WS10), and the soil sampling point (SHE) for the Soxhlet extractor test. The SNIRH point (224/C36) is located 10 km west of the mine area in a zone with no interference of the mining processes. The light green zone represents the C57 mining concession, near the Gonçalo village (Guarda) in the centre of Portugal (top left image). Google Earth software (Google Inc.)
To simulate the artificial leaching of rocks and minerals over a short period, laboratory weathering studies were conducted using a Soxhlet extractor test. Soil samples were collected in October 2021 from the tailings of the C57 mine (identified as SHE in Fig. 3). The samples were collected using a shovel and stored in appropriately labelled plastic bags. In the laboratory, the samples were mixed in a bucket to ensure a more homogeneous and representative final sample of the soil tailings. A fraction of the resulting sample was taken for soil characterization and preparation for the leaching test.
Materials and methods
Sample preparation followed the LNEC specification E-195 (1966), which involved using a splitter to select a representative amount of the previously dried soil sample collected in the field. The soil tested in the Soxhlet extractor (SHE samples) underwent several tests to determine its properties. Soil density was determined according to Portuguese Standard NP-83 of 1965, and the Atterberg limits were determined according to Portuguese Standard NP-143 of 1969. Granulometric characterization was also performed following LNEC specification E-239 (1970).
To simulate the weathering phenomena of leaching, a glass Soxhlet extractor was used. The mass of each soil fraction to be placed in the glass extractor was determined based on the volume of the Soxhlet extractor and the percentage of each fraction present in the soil, as per the granulometric curve shown in Fig. 4: 382 g of gravel, 512 g of sand, and 262 g of fine particles. Prior to use, the Soxhlet extractor was washed with a 1 M nitric acid solution, followed by rinsing with ultra-pure water to remove any residues. A volume of 1.4 L of ultra-pure water (Milli Q IQ 7003) was added to the Soxhlet extractor, which was placed on a heating blanket to generate water vapor. After cooling in the upper part of the system, the water vapor interacted with the soil sample, allowing the leaching phenomenon to occur. This closed recirculation circuit system was maintained for 125 days, with water samples collected for analysis at intervals of 42 days.
Atomic absorption spectroscopy (model 906, GBC) with flame and electrothermal (GF3000, GBC) atomization techniques were employed to determine the concentration of alkali, alkaline earth, transition, and post-transition metals in the water samples, following Methods 3111 and 3113 (American Public Health Association 1988). UV–vis spectroscopy, following Method 3500 (American Public Health Association 1988), was also utilized. Calibration was performed using standard solutions (Johnson Mathey GmbH) with a concentration of 1000 μg/mL for each element in 5% nitric acid (HNO3), except for aluminum, chromium, and nickel solutions, which utilized 5% hydrochloric acid (HCl). The calibration curves used in the spectroscopic methods exhibited linearity equal to or greater than 0.999.
The pH of the samples was measured using a pH meter (Tritralab TIM900, Radiometer) equipped with a sensitive electrode (PHC3185-67, Radiometer) after calibration with standard solutions of pH 4.0 and 7.0 (Radiometer analytical). Electrical conductivity was determined using a conductivity meter (model 150, Orion) with an appropriate sensor (model 012210, Orion) previously calibrated with a standard solution of 12.85 mS/cm (Radiometer analytical), following Method 2510C (American Public Health Association 1988).
Results and discussion
Granulometric characterization of the soil
The granulometric characterization was performed following LNEC specification E-239 (1970), and the resulting granulometric curve is depicted in Fig. 4. The soil density value was measured as 2.68. The granulometric characterization was used to determine the quantity of soil type required for the Soxhlet extractor test. A correlation was established with the obtained granulometric curve to determine the soil type quantities: 33% gravel size, 44% sand size, and 23% fines (material with a diameter less than 0.074 mm). The sample amount was selected based on the nominal diameter of the largest particles, approximately 38.1 mm.
Water analysis results
The results of water analyses for each sampling point (see Fig. 3) are presented in Table 1, showing the minimum, maximum, and average concentrations of surface, spring, and groundwater. Table 2 provides the minor, major, and mean concentrations of the same chemical parameters, along with quality data from well 224/C36 (SNIRH database for groundwater quality) in Verdelhos village (refer to Fig. 3). The reference guidelines used for comparison are the Canadian government guidelines and the quality guidelines for surface water of the Instituto da Água I.P. (2009).
In comparison to surface water and spring water, groundwater generally exhibited higher concentrations of analysed ions. However, the concentrations of both cations and anions were below the recommended values in the quality guidelines for the protection of aquatic life and human health. Nonetheless, the concentrations of aluminium (ranging from 0.6 to 67.3 μg/L), chromium (ranging from 8.8 to 98.6 μg/L), copper (ranging from 2.8 to 19.8 μg/L), and iron (ranging from 234 to 741 μg/L) were above the long-term reference values of the Canadian water quality guidelines for the protection of aquatic ecosystems. However, they were below the values for ecosystem protection and public health for surface water of the Instituto de Água I.P. (Rodrigues et al. 2019). Despite the high concentration of chromium in the water samples, the oxidation–reduction potential (ORP) of the waters ranged from 0.2 to 0.3 V, and the pH ranged from 5 to 6 (Rodrigues et al. 2019). This indicates that chromium is primarily in its trivalent state, which is not significantly hazardous to human health and ecosystems (Fendorf 1995). However, there is a possibility that the chromium could change to a hexavalent state, which poses a risk to the environment and human life. The pH of the water samples indicates an acidic character, with values ranging from 4.8 to 6.4, which is outside the range defined for the protection of aquatic ecosystems (Table 2). The concentration of lithium detected in these water samples ranged from 6.9 to 74.1 μg/L. According to Neves et al. (2015), in Portugal the maximum concentration of lithium in the public water supply is 191 μg/L, with a median value of 10 μg/L.
Weathering solutions results
The results of the weathering solutions from water samples collected after 42, 84, and 125 days of operating the Soxhlet extractor are presented in Table 3. For most of the chemical elements analysed, the concentrations in the weathering solutions were low and tended to decrease over the testing period. Aluminium exhibited a concentration of 1169 mg/L in the leachate water within the first 42 days of testing, which decreased to 556 mg/L after 84 days and further declined to only 138 mg/L after 125 days. Similarly, potassium and sodium showed comparable trends. In contrast, the concentrations of calcium, chromium, and iron fell below the limit of quantification after 84 days of testing, while lithium and magnesium concentrations fell below the limit of quantification after 125 days of testing.
Additionally, the concentrations obtained in the mining area were, in most cases, lower than those found in surface and groundwaters collected in the area surrounding mining C57. The exceptions were aluminium, sodium, and potassium, which were present in high concentrations in the weathering solutions and had concentrations higher than those found in the surrounding surface and groundwaters. As noted by Ramos et al. (1994) in a study on rare metal mineralization in this region, major elements of aplitopegmatitic lithiniferous veins are characterized by enrichments in Al2O3 (17.55% ± 0.40), MnO (0.16% ± 0.07), CaO (0.11% ± 0.03), and P2O5 (0.94% ± 0.47). Aplitopegmatitic stanniferous veins exhibit enrichments in SiO2 (74.42% ± 1.02), FeO (0.73% ± 0.19), and K2O (3.87% ± 0.85). The mixed veins display transitional characteristics, with contents generally intermediate between the values observed in lithiniferous and stanniferous veins. The intrusion of aplitopegmatitic veins (lithiniferous, stanniferous, or mixed veins) led to the recrystallization of sodium feldspar (albite) and K-feldspar, as well as silica deposition in the regional granites. Additionally, the study highlights the presence of lithium in the soil, with concentrations varying between 1673 ± 908 ppm in stanniferous sills and 5,845 ± 1,832 ppm in lithiniferous sills. During chemical weathering, the mineral composition of rocks is altered. This process is more extensive in areas with abundant water and high temperatures, promoting mineral disintegration mainly through dissolution and hydrolysis processes. When K-feldspar weathers into kaolinite, intermediate minerals such as hydromica, chlorite, and vermiculite may be produced (Eqs. 1–6). Additionally, elements such as potassium and sodium may be released in varying proportions (Berner and Holdren 1977; Chigira and Oyama 2000). When kaolinite hydrolyses, the only products produced are aluminium oxide and silica, as shown in Eq. 7 (Cama et al. 2002).
As a result of this geochemical process, it is expected that elements such as sodium, potassium, and aluminium can be mobilized from the soil into the aqueous solution upon contact with water. Additionally, the initial pH of the ultra-pure water is 7.3, and after the first 42 days, the pH significantly decreases to 5.9. However, in the last two samplings at 84 and 125 days, the pH recovers to 6.6 and 6.7, respectively. This decrease in pH in the weathering solution may be linked to the formation of orthosilicic acid, a weak acid, as represented in chemical reactions (Eqs. 1–7). However, the process of mineral weathering and the release of other ions during this process can affect the pH of water. The acidic character of the water samples may be influenced by the release of certain ions, like the aluminium or iron, as represented in the chemical reaction (Eqs. 8 and 9) during the weathering process. This acidification enhances the leaching power and consequent mobilization of ions from the soil into the aqueous solvent. When comparing the concentrations obtained in the weathering leaching solution with the concentrations of ground and surface waters, it can be observed that, in most cases, the concentration in the latter phases is higher, except for sodium, potassium, aluminium, and lithium.
Conclusions
Based on the limited number of results obtained, there is no clear evidence of the environmental impact of mining activity on water quality for the analyzed surface, groundwater, and spring water samples.
During the 125 days duration of the Soxhlet extractor test conducted under experimental conditions, significant concentrations of potassium, sodium, aluminum, chromium, and lithium were observed in the weathering solution, indicating potential long-term implications. However, only aluminum and chromium exceeded the Canadian environmental quality guidelines for surface water. In comparison to the threshold values set by Instituto da Água I.P., in Portugal for the classification of surface water bodies, there was no non-compliance either because the limits were not exceeded or they were not defined. Nonetheless, adverse effects on water quality downstream of the open pit mining operation may occur for certain chemical elements, particularly aluminum and chromium.
It's important to note that the weathering test conducted using the Soxhlet method employed a reconstructed soil composition, combining different granulometric fractions found in the initial soil (refer to Fig. 4). This approach increased the contact area between water and minerals, intensifying the leaching phenomenon specific to the C57 mine. It should be emphasized that this situation does not reflect natural environmental conditions. Therefore, the concentrations obtained in the solution may be higher than those found in real scenarios of open-pit mining operations. To provide a comparative study, a future investigation will be conducted using raw soil samples taken directly from the mine under environmental conditions that closely resemble those at the C57 mine.
Data availability
All data in the article are available at the electronic addresses provided in the bibliographic references. Author data can be requested via e-mail.
References
Antão A (2004) Comportamento geotécnico do granito da Guarda relacionado com a sua alteração. (Geotechnical behavior of Guarda granite related to its alteration). Ph.D. Thesis, Coimbra University, Portugal.
American Public Health Association (1988) Standard methods for the examination of water and wastewater. 20° Ed. Washington. ISBN-13: 978–0875532356
ASTM International (2017) ASTM D2487–17. Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System). West Conshohocken, PA. https://doi.org/10.1520/D2487-11
Berner RA, Holdren GR (1977) Mechanism of feldspar weathering: Some observational evidence. Geol 5:369–372
Cama J, Metz V, Ganor J (2002) The effect of pH and temperature on kaolinite dissolution rate under acidic conditions. Geochim Cosmochim Acta 66:3913–3926
Carvalho JMF, Farinha JAL (2004) Lithium potentialities in northern Portugal. In: 17th Industrial Minerals International Congress, Barcelona (Spain), pp 1–10.
Chigira M, Oyama T (2000) Mechanism and effect of chemical weathering of sedimentary rocks. Eng Geol 55:3–14
Costa I, Massard G, Agarwal A (2010) Waste management policies for industrial symbiosis development: case studies in European countries. J Clean Prod 18:815–822
Dolgopolova A, Seltmann R, Stanley C, Armstrong R, Noronha F, Ramos V, Guedes A, Simons B, Rollinson G, Andersen J, Reimer W (2017) Mineralogical study of the Gonçalo Li-pegmatite deposit, Portugal. Norsk Geologisk Forening nº2. PEG 2017:23–26
Dunlap A, Riquito M (2023) Social warfare for lithium extraction? Open-pit lithium mining, counterinsurgency tactics and enforcing green extractivism in northern Portugal. Energy Res Soc Sci 95:102912. https://doi.org/10.1016/j.erss.2022.102912
EU (2020) European Commission, Critical materials for strategic technologies and sectors in the EU - a foresight study, 2020
EU European Commission, Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs (2021) 3rd Raw Materials Scoreboard: European innovation partnership on raw materials, Publications Office, 2021, /https://doi.org/10.2873/567799
Farinha Ramos J (1998) Mineralizações de metais raros de Seixo Amarelo - Gonçalo. Contribuições para o seu conhecimento. PhD Dissertation, Universidade de Lisboa.
Farinha Ramos J (2007) Locality Nº5, Seixo-Amarelo – Gonçalo. Rare element aplite-pegmatite field. In: Lima A, Roda-Robles E (eds), Granitic Pegmatites: the state of the art. Field Trip Guidebook, FCUP, Porto.
Farinha Ramos J (2010) Aplitopegmatitos com mineralizações de metais raros de Seixo Amarelo - Gonçalo: o recurso geológico. In: Cotelo Neiva JM, Ribeiro A, Victor L, Noronha F, Ramalho M (eds), Ciências Geológicas: Ensino, Investigação e sua história, Vol II, Cap I, pp 121–130
Farinha Ramos J, Noronha F (1995) Condições de deposição da fase litinífera principal no campo filoniano aplitopegmatítico de Seixo Amarelo- Gonçalo. Mem Mus Labor Miner Geol 4:599–604
Fendorf SE (1995) Surface reactions of chromium in soils and waters. Geoderma 67:55–71
Freitas M, Carolino A, Guedes A, Noronha F (2015) P–T conditions of subsolidus modifications on rare metal enriched pegmatites. An example from Central Portugal. In: Proceedings of SGA, mineral resources in a sustainable world 2, pp 457–460, Nancy
Instituto da Água I.P. (2009) Critérios para a Classificação do estado das massas de águas superficiais - Rios e Albufeiras. https://www.apambiente.pt/dqa/assets/crit%C3%A9rios-classifica%C3%A7%C3%A3o-rios-ealbufeiras.pdf
LNEC Specification E-195 (1966) Solos. Preparação por via seca de amostras para ensaios de identificação. Lisboa. Portugal
LNEC Specification E-239 (1970) Solos. Análise granulométrica por peneiração húmida. Lisboa, Portugal
Neves O, Machete I, Marques JM, da Silva JAL, Simões do Couto F (2015) Lítio em águas engarrafadas e de abastecimento público portuguesas. Com Geol 102:103–106
Nickless EFP, Clayton A (1973) The sand and gravel resources of the country around Hethersett, Norfolk: description of the 1:25 000 resource sheet TG10 (Assessment of British Sand and Gravel Resources nº5). CF73/04
Portuguese Standard NP-143 (1969) Solos. Determinação dos limites de consistência. IPQ, Lisboa
Portuguese Standard NP-83 (1965) Solos. Determinação da densidade das partículas. IPQ, Lisboa
Ramos JF, Ribeiro A, Barriga FJAS (1994) Mineralização de metais raros de Seixo Amarelo – Gonçalo (Guarda) – Breve Nota Introdutória. Bol Minas 31(2):101–115
Roda-Robles E, Pesquera A, Gil-Crespo P, Vieira R, Lima A, Garate-Olave I, Martins T, Torres-Ruiz J (2016) Geology and mineralogy of the Li mineralization in the Central Iberian Zone (Spain and Portugal). Mineral Mag 80(1):103–126
Rodrigues PMSM, Antão AMMC, Rodrigues R (2019) Evaluation of the impact of lithium exploitation at the C57 mine (Gonçalo, Portugal) on water, soil and air quality. EnvironEarth Sci 78(533):1–14
SNIRH (2022) https://snirh.apambiente.pt/index.php?idMain=1&idItem=1.4) accessed on September 9, 2022
Toupal J, Vann DR, Zhu C, Gieré R (2022) Geochemistry of surface waters around four hard-rock lithium deposits in Central Europe. J Geochem Explor 234:106937. https://doi.org/10.1016/j.gexplo.2021.106937
US EPA (2021) National Primary Drinking Water Regulations. https://www.epa.gov/ground-water-and-drinkingwater/national-primary-drinking-water-regulations
USGS (2022) Mineral Commodity Summaries 2022—Lithium. https://pubs.usgs.gov/periodicals/mcs2022/mcs2022-lithium.pdf. Accesssed Feb 2023
Acknowledgements
The author from CGEO/IPG acknowledges the Interior Research Unit of the Polytechnic Institute of Guarda and the support of the Portuguese Foundation for Science and Technology (FCT) in the framework of the Strategic Funding with UIDB/00073/2020 and UIDP/00073/2020 projects of the I&D Unit Geosciences Center (CGEO). Additionally, the author acknowledges the funding provided by the Portuguese Foundation for Science and Technology (FCT) for the R&D unit CIQUP (Project UIDB/00081/2020) and the Associated Laboratory IMS (LA/P/0056/2020).
Funding
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Contributions
Ana Antão and Pedro Rodrigues wrote the manuscript text, and Ricardo Rodrigues do the water chemical analysis. Guilherme Couto make the soil characterization
Corresponding author
Ethics declarations
Competing Interests
The authors have not disclosed any competing interests, namely financial, commercial, legal, or professional relationships with any organization that could influence your research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Antão, A.M.M.C., Rodrigues, P.M.S.M., Rodrigues, R. et al. Laboratory weathering studies to evaluate the water quality impact of a lithium mining in Portugal. Environ Earth Sci 83, 224 (2024). https://doi.org/10.1007/s12665-024-11525-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-024-11525-1