Abstract
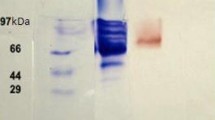
Laccase is an extracellular enzyme that is widely used in the decolonization of textile dyes in waste water. The aim of our study was to isolate, purify, characterize and immobilize the laccase enzyme produced by Trametes versicolor HBB 7328. Purified laccase enzyme was immobilized in polyacrylamide gel to explore its ability in decolonization of textile dyes. Laccase purification process was carried out by fractionation using ammonium sulphate (80%) followed by DEAE Sepharose column (30 × 3 cm) chromatography method. Recovery and fold purification in this step were 27.35 and 16.23%. Purified laccase (named as LAC1) revealed its optimum activity at pH 5.0 and 35 °C temperature, and displayed remarkable stability in the range of 30–40 °C and in the pH range (pH 3.0–7.0). The single bands on SDS-PAGE represent the purity of LAC1 with molecular weight of 60 kDa. Both free and immobilized laccase assessed for their ability to decolorize textile dyes. Free laccase decolorized Methyl red to 72.705%, Reactive orange to 57.851%, Reactive blue to 37.231%, Bromophenol blue to 24.412% however Immobilized laccase decolorized Methyl red to 89.823%, Reactive orange to 63.151%, Reactive blue to 59.548%, Bromophenol blue to 49.421% respectively. This study proposes the role of laccase from Trametes versicolor HBB 7328 in decolonization of textile dyes.
Graphical Abstract









Similar content being viewed by others
References
Maheshwari K, Agrawal M, Gupta AB (2021) Dye pollution in water and wastewater. In: Muthu S S, Khadir A (eds) Novel materials for dye-containing wastewater treatment. Sustainable textiles: production, processing, manufacturing & chemistry. Springer, Singapore, pp 1–25. https://doi.org/10.1007/978-981-16-2892-4_1
Hemashenpagam N, Selvajeyanthi S (2023) Textile dyes and their effect on human beings. In: Ahmad A, Jawaid M, Mohammad Ibrahim MN, Yaqoob A, Alshammari MB (eds) Nanohybrid materials for treatment of textiles dyes. Smart Nanomaterial Technology. Springer, Singapore, pp 41–60.https://doi.org/10.1007/978-981-99-3901-5_3
Katheresan V, Kansedo J, Lau SY (2018) Efficiency of various recent wastewater dye removal methods. J Environ Chem Eng 6:4676–4697. https://doi.org/10.1016/j.jece.2018.06.060
Kumar V, Pallavi P, Sen SK, Raut S (2024) Harnessing the potential of white rot fungi and ligninolytic enzymes for efficient textile dye degradation: a comprehensive review. Water Environ Res 96:e10959. https://doi.org/10.1002/wer.10959
Kunamneni A, Ballesteros A, Plou FJ, Alcalde M (2007) Fungal laccase a versatile enzyme for biotechnological applications. Commun Curr Res Educat Topics Trends Appl Microbiol 1:233–245
Zofair SF, Ahmad S, Hashmi MA, Khan SH, Khan MA, Younus H (2022) Catalytic roles, immobilization and management of recalcitrant environmental pollutants by laccases: significance in sustainable green chemistry: J Environ Manage 309:1146–1176. https://doi.org/10.1016/j.jenvman.2022.114676
Bertrand B, Martínez-Morales F, Tinoco-Valencia R, Rojas S, Acosta-Urdapilleta L, Trejo-Hernández MR (2015) Biochemical and molecular characterization of laccase isoforms produced by the white-rot fungus Trametes versicolor under submerged culture conditions. J Mol Catal B Enzym 122:339–347. https://doi.org/10.1016/j.molcatb.2015.10.009
Giardina P, Faraco V, Pezzella C, Piscitelli A, Vanhulle S, Sannia G (2010) Laccases: a never ending story. Cell Mol Life Sci 67:369–385. https://doi.org/10.1007/s00018009-0169-1
Sitarz AK, Mikkelsen JD, Meyer AS (2016) Structure, functionality and tuning up of laccases for lignocellulose and other industrial applications. Crit Rev Biotechnol 36:70–86. https://doi.org/10.3109/07388551.2014.949617
Brijwani K, Rigdon A, Vadlani PV (2010) Fungal laccases: production, function, and applications in food processing. Enzyme Res 2010:10. https://doi.org/10.4061/2010/149748
Chen L, Zou M, Hong FF (2015) Evaluation of fungal laccase immobilized on natural nanostructured bacterial cellulose. Front Microbiol 6:1245. https://doi.org/10.3389/fmicb.2015.01245
Singh J, Kapoor RK (2019) Immobilization and reusability efficiency of Laccase onto different matrices using different approaches. eIJPPR 9:58–65.
Asgher M, Shahid M, Kamal S, Iqbal HM (2014) Recent trends and valorization of immobilization strategies and ligninolytic enzymes by industrial biotechnology. J Mol Catal B Enzym 101:56–66. https://doi.org/10.1016/j.molcatb.2013.12.016
Liu EK, He WQ, Yan CR (2014) ‘White revolution ‘to ‘white pollution’ agricultural plastic film mulch in China. Environ Res Lett 9:091001. https://doi.org/10.1088/17489326/9/9/091001
Gong T, Zhang X, Liu W (2018) tracing the sources of iodine species in a non saline wastewater. Chemosphere 205:8. https://doi.org/10.1016/j.chemosphere.2018.04.147
Mani S, Chowdhary P, Bharagava R N (2019) Textile wastewater dyes: toxicity profile and treatment approaches. In: Bharagava R, Chowdhary P (eds) Emerging and eco-friendly approaches for waste management. Springer, Singapore, pp 219–244. https://doi.org/10.1007/978-981-10-8669-4_11
Sarkar P, Roy A, Pal S et al (2017) Enrichment and characterization of hydrocarbon degrading bacteria from petroleum refinery waste as potent bio augmentation agent for in situ bioremediation. Bio Resour Technol 242:2. https://doi.org/10.1016/j.biortech.2017.05.010
Kaushik P, Malik A (2009) Fungal dye decolorization: recent advances and future potential. Environ Int 35:127–141. https://doi.org/10.1016/j.envint.2008.05.010
Jeyabalan J, Veluchamy A, Kumar A, Chandrasekar R, Narayanasamy S (2023) A review on the laccase assisted decolorization of dyes: recent trends and research progress. J Taiwan Inst Chem Eng 151:105081. https://doi.org/10.1016/j.jtice.2023.105081
Vasdev K, Kuhad RC (1994) Induction of laccase production in Cyathus bulleri under shaking and static culture conditions. Folia Microbiol 39:30. https://doi.org/10.1007/BF02814322
Kumar VV, Kirupha SD, Periyaraman P, Sivanesan S (2011) Screening and induction of laccase activity in fungal species and its application in dye decolorization. African J Microbiol Res 5:1261–1267. https://doi.org/10.5897/AJMR10.894
Kumar VP, Naik C, Sridhar M (2015) Production, purification and characterization of novel laccase produced by Schizophyllum commune NI-07 with potential for delignification of crop residues. Appl Biochem Microbiol 51:432–441. https://doi.org/10.1134/S0003683815040080
Pointing SB, Jones EB, Vrijmoed LL (2000) Optimization of laccase production by Pycnoporus sanguineus in submerged liquid culture. Mycologia 92:139–144. https://doi.org/10.2307/3761458
Heinzkill M, Bech L, Halkier T, Schneider P, Anke T (1998) Characterization of laccases and peroxidases from wood-rotting fungi (family Coprinaceae). Appl Environ Microbiol 64:1601–1606. https://doi.org/10.1128/aem.64.5.1601-1606.1998
Brazkova M, Mercati A, Hristova I, Lante A, Krastanov A (2016) Isolation, purification and characterization of laccase from the white-rot fungus Trametes Versicolor. Food Sci Eng Technol 62:155–162
Umar A, Ahmed S (2022) Optimization, purification and characterization of laccase from Ganoderma leucocontextum along with its phylogenetic relationship. Sci Rep 12:24–16. https://doi.org/10.1038/s41598-022-06111-z
Umar A, Abid I, Elshikh MS, Dufossé L, Abdel-Azeem AM, Ali I (2023) Agitation role (Dissolved Oxygen) in production of laccase from newly identified Ganoderma multistipitatum sp. nov. and its effect on mycelium morphology. BMC Microbiol 23:280. https://doi.org/10.1186/s12866-023-03009-2
Dong JL, Zhang YZ (2004) Purification and characterization of two laccase isoenzymes from a ligninolytic fungus Trametes gallica. Prep Biochem Biotechnol 34:179–194. https://doi.org/10.1081/PB-120030876
Patel H, Gupte S, Gahlout M, Gupte A (2014) Purification and characterization of an extracellular laccase from solid state culture of Pleurotus ostreatus HP 1.3Biotech 4: 77–84. https://doi.org/10.1007/s13205-013-0129-1
Zheng F, An Q, Meng G, Wu XJ, Dai YC, Si J, Cui BK (2017) A novel laccase from white rot fungus Trametes orientalis: purification, characterization, and application. Int J Biol Macromol 102:758–770. https://doi.org/10.1016/j.ijbiomac.2017.04.089
Guo M, Lu F, Liu M, Li T, Pu J, Wang N, Liang P, Zhang C (2008) Purification of recombinant laccase from Trametes versicolor in Pichia methanolica and its use for the decolorization of anthraquinone dye. Biotech Lett 30:2091–2096. https://doi.org/10.1007/s10529-008-9817-z
Moon-Jeong H, Hong-Gyu S (2005) Purification and characterization of laccase from the white rot fungus Trametes versicolor. J Microbiol 43:555–560
Galhaup C, Wagner H, Hinterstoisser B, Haltrich D (2002) Increased production of laccase by the wood degrading basidiomycete Trametes pubescens. Enzyme Microb Technol 30:529–536. https://doi.org/10.1016/S0141-0229(01)00522-1
Ezike TC, Ezugwu AL, Udeh JO, Eze SO, Chilaka FC (2020) Purification and characterization of new laccase from Trametes polyzona WRF03. Biotechnol Reports 28:00566. https://doi.org/10.1016/j.btre.2020.e00566
Devasia S, Nair A (2022) Purification and characterization of laccase from Arthrographis ksf. Asian J Microbiol Biotechnol Environ Sci 24:528–538. https://doi.org/10.53550/AJMBES.2022.v24i03.0016
More SS, Renuka PS, Malini S (2011) Isolation, purification, and characterization of fungal laccase from Pleurotus sp. Enzyme Res 2011:7. https://doi.org/10.4061/2011/248735
Gökgöz M, Altinok H (2012) Immobilization of laccase on polyacrylamide and polyacrylamide κ carragennan based semi interpenetrating polymer networks. Artif Cells Blood Subst Biotechnol 40:326–330. https://doi.org/10.3109/10731199.2012.658469
Huber D, Pellis A, Daxbacher A, Nyanhongo GS, Guebitz GM (2016) Polymerization of various lignins via immobilized Myceliophthora thermophila Laccase (MtL). Polymers 8:280. https://doi.org/10.3390/polym8080280
Nyanhongo GS, Gomes J, Gübitz GM, Zvauya R, Read J, Steiner W (2002) Decolorization of textile dyes by laccases from a newly isolated strain of Trametes modesta. Water Res 36:1449–1456. https://doi.org/10.1016/S0043-1354(01)00365-7
Ben Younes S, Mechichi T, Sayadi S (2007) Purification and characterization of the laccase secreted by the white rot fungus Perenniporia tephropora and its role in the decolourization of synthetic dyes. J Appl Microbiol 102:1033–1042. https://doi.org/10.1111/j.1365-2672.2006.03152.x
Shraddha SR, Sehgal S, Kamthania M, Kumar A (2011) Laccase: microbial sources, production, purification and potential biotechnological applications. Enzyme Res 2011:217861. https://doi.org/10.4061/2011/217861
Elisashvili V, Kachlishvili E, Penninckx M (2008) Effect of growth substrate, method of fermentation, and nitrogen source on lignocellulose-degrading enzymes production by white-rot basidiomycetes. J Ind Microbiol Biotechnol 35:531–538. https://doi.org/10.1007/s10295-008-0454-2
Wasak A, Drozd R, Grygorcewicz B, Jankowiak D, Rakoczy R (2018) Purification and recovery of laccase produced by submerged cultures of Trametes versicolor by three-phase partitioning as simple and highly efficient technique. Pol J Chem Technol 20:88–95. https://doi.org/10.2478/pjct-2018-0059
Zapata-Castillo P, Villalonga-Santana M (2012) Purification and characterization of laccase from Trametes hirsutaBm2 and its contribution to dye and effluent decolorization. Afr J Biotech 11:3603–3611. https://doi.org/10.5897/AJB11.2050
Zouari-Mechichi H, Mechichi T, Dhouib A, Sayadi S, Martínez AT, Martinez MJ (2006) Laccase purification and characterization from Trametes trogii isolated in Tunisia: decolorization of textile dyes by the purified enzyme. Enzyme Microb Technol 39:141–148. https://doi.org/10.1016/j.enzmictec.2005.11.027
Vaithanomsat P, Sangnam A, Boonpratuang T, Choeyklin R, Promkiam on P, Chuntranuluck S, Kreetachat T (2013) Wood degradation and optimized laccase production by resupinate white rot fungi in northern Thailand. Bio Resources 8:6342–6360. https://doi.org/10.15376/biores.8.4.6342-6360
Minovska V, Winkel hausen E, Kuzmanova S (2005) Lipase immobilized by different techniques on various support materials applied in oil hydrolysis. J Serb Chem Soc 70:609–624. https://doi.org/10.2298/JSC0504609M
Yinghui D, Qiuling W, Shiyu F (2002) Laccase stabilization by covalent binding immobilization on activated polyvinyl alcohol carrier. Lett Appl Microbiol 35:4516. https://doi.org/10.1046/j.1472-765X.2002.01196.x
Al Adhami AJ, Bryjak J, Greb Markiewicz B, Peczyńska zoch W, (2002) Immobilization of wood rotting fungi laccases on modified cellulose and acrylic carriers. Process Biochem 37:1387–1394. https://doi.org/10.1016/S0032-9592(02)00023-7
Forootanfar H, Moezzi A, Aghaie-Khozani M, Mahmoudjanlou Y, Ameri A, Niknejad F, Faramarzi MA (2012) Synthetic dye decolorization by three sources of fungal laccase. Iranian Journal of Environmental Health Science & Engineering 27:1–10. https://doi.org/10.1186/1735-2746-9-27
Kuhar F, Castiglia V, Levin L (2015) Enhancement of laccase production and malachite green decolorization by co culturing Ganoderma lucidum and Trametes versicolor in solid state fermentation. Int Biodeterior Biodegradation 104:238–243. https://doi.org/10.1016/j.ibiod.2015.06.017
Acknowledgements
This work was supported by the Department of Microbiology, Maharshi Dayanand University Rohtak, Haryana, 124001 India and Department of Microbiology, CCS Haryana Agricultural University Hisar, 125004 India.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Nikita Goyat: carried out the experiments; data curation; formal analysis; investigation; methodology; software; validation; visualization; writing original draft. Rajeev Kumar Kapoor: designed the experiment: conceptualization; formal analysis; investigation; resources; super vision; validation; visualization; review. Baljeet Singh Saharan: formal analysis; investigation; super vision; validation; visualization; writing-review and editing. Prexha Kapoor: writing-review and editing; statistical analysis. Kajal Kumari: writing review and editing. Namita Singh: supervision; writing-review and editing.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationship that could have appeared to influence the work reported in this paper.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goyat, N., Kapoor, R.K., Saharan, B.S. et al. Production, Purification and Immobilization of Laccase from Trametes versicolor HBB 7328 for its Role in Decolorization of Textile Dyes. Indian J Microbiol (2024). https://doi.org/10.1007/s12088-024-01260-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12088-024-01260-3