Abstract
Rare earth elements are in high demand in the USA. Bastnaesite, a rare earth fluorocarbonate containing primarily cerium and lanthanum, is one of the most abundant sources of rare earths in the USA. This research was completed using the ore from Mountain Pass, which is the largest rare earth mine in the USA. This research, resulting in a current patent application, was done to find a way to combine flotation with novel collectors and gravity separation techniques to reach an enhanced grade and recovery of rare earth elements while rejecting the gangue minerals, calcite, barite, and silicate minerals. These minerals, particularly calcite, an acid consumer, are well known to be difficult to separate in conventional flotation of bastnaesite ore. Four collectors were examined. They were N,2-dihydroxybenzamide, N-hydroxycyclohexanecarboxamide, N,3- dihydroxy-2-naphthamide, and N-hydroxyoleamide. Through this analysis, it was determined that, to obtain the desired results, flotation would be the rougher stage and gravity separation would be utilized as the cleaner stage. Bench scale flotation tests were conducted on the run of mine ore using conditions that were determined using a previously utilized Stat Ease model for testing and statistical optimization in design of experimentation. The bench tests that produced the most desirable results were then scaled up to a 10 kg float test. A concentrate from this test showed a rare earth oxide grade of 44%, while rejecting 91% of the calcite. This concentrate was used for gravity separation. Through gravity separation, it was found that another 40% of the calcite could be rejected with a final rare earth oxide grade of 47% in the concentrate.
Similar content being viewed by others
1 Introduction
Rare earth elements (REEs) are an essential part of technological growth and development [1,2,3,4]. This study focuses on the beneficiation of bastnaesite, a rare earth fluorocarbonate, from an ore provided by the Mountain Pass mine located in Southern California. Bastnaesite is primarily composed of cerium and lanthanum oxides.
The primary goal of processing this ore is to separate out the rare earth bearing minerals from the gangue minerals. The gangue minerals of this ore body include calcite, dolomite, barite, and silicate minerals. These gangue minerals, particularly calcite, are well known to be problematic in industrial bastnaesite beneficiation via flotation. This study focused on a novel combination of froth flotation and gravity separation as primary methods of separation. For flotation to be optimized, the surface chemistry of the materials that will be treated needs to be understood. The optimization of gravity separation depends on grain size and the amounts of the gangue minerals that remain in the ore body.
Froth flotation is a separation method that changes the surface chemistry of desired minerals, rendering them hydrophobic which allows the desired minerals to attach to air bubbles and float to the surface of the water, forming a froth. Collectors are the primary chemical added into the system to aid in flotation. They are classified into two groups, anionic and cationic, the former is the most used collector type for bastnaesite flotation. Fatty acids and hydroxamates are the primary collectors that have been used or extensively researched for the Mountain Pass bastnaesite mine material [5,6,7,8,9,10,11,12,13,14,15,16,17].
Hydroxamates are collectors that have proven to be more selective to bastnaesite than the gangue minerals. This could be due to chelation, which is a type of bonding that allows two or more bonds to form between a ligand and a separate metal ion [5]. Four new collectors have proven to be selective to bastnaesite in a study completed by Dylan Everly [6, 18]. These collectors were studied using microflotation and bench scale flotation.
Although collectors are the primary reagents used in flotation, there are others which are used to enhance selectivity. Some other reagents include depressants, pH modifiers, and frothers. Depressants are used as an aid to flotation by coating a mineral surface, inhibiting the adsorption of the collector onto that mineral surface. Lignin sulfonate is used in the flotation of bastnaesite to depress barite. Common pH modifiers are potassium hydroxide and soda ash. Soda ash has a dual purpose, as a pH modifier and a depressant, because it is a potential determining ion, which makes the flotation of bastnaesite more effective at a specific pH [5].
Gravity separation is a technique involving the manipulation of particle densities to separate the less dense particles from the denser ones. This study used the ultrafine Falcon centrifugal concentrator as gravity concentrator. This allows effective separation to occur even with reduced particle sizes [19]. In a related research effort, it was determined that, using gravity separation, the REE bearing minerals can be effectively separated from calcite and dolomite [15, 16, 20,21,22].
2 Ore Sub Sampling and Characterization
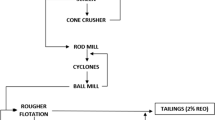
All sample minerals were obtained from the Mountain Pass mine. The samples were cone and quartered to maintain homogeneity. They were then roll crushed. After roll crushing, they were split into 10 kg samples using a Jones Splitter and placed into a rod mill with a capacity of 10 kg and ground to a P80 of 50 microns. The mineralogy of the sample was determined using X-ray fluorescence (XRF) and a mineral liberation analysis (MLA). Samples were sent to Montana Tech for MLA to be completed. MLA was completed on a run of mine ore sample and a gravity concentrated sample. The mineral concentrations of each sample can be seen in Table 1.
For the XRF analysis, a method was used so that the results would be quantitative. The rare earth oxide (REO) compositions were calculated from that of cerium oxide because it is the most consistent throughout the deposit according to data provided from the mine. Cerium oxide makes up 49.1% of the REO content in the deposit. This agreed with the determined XRF data obtained throughout testing. The XRF analysis was used for all the concentrates and tailings from all the test work. The samples were analyzed in the form of fused disks. The disks were made using the Katanax X-300 fluxer. The composition of the disks were 2% sample and 98% lithium borate flux. Table 2 shows the grades of the samples obtained from the XRF. The ore head grade is consistent with Molycorp data, which indicated that there was a head grade of 7–8% REO.
3 Reagents
Four potentially selective collectors were used in this study, and they were provided by Oak Ridge National Laboratory. They are referred to by their number in the remainder of this paper. Collectors 8 and 14 were insoluble in water, so they were dissolved in ethanol and emulsified into the slurry prior to flotation. The summary of all the flotation reagents used can be found in Table 3.
4 Flotation Testing
A microflotation study was completed on both samples using a Partridge-Smith cell. The solution was made up of 0.52 g solids and 52 mL water. For each experiment, the sample was added to the solution along with a specific collector concentration. The pH was changed after the addition of the collector using potassium hydroxide. The slurry was conditioned for 15 min in a 100-mL beaker. After 13 min, a 0.1 mL of frother was added to the slurry. After conditioning was completed, the slurry was placed into the Partridge-Smith cell for flotation. Compressed air was added into the system at a flow rate of 26.6 cm3/min. The concentration of the collector was not varied for any tests involving that collector. The only variable was pH in the range of 9.5–10.5. No additional reagents were used. The experiments were executed in duplicates to obtain enough material to be analyzed by the XRF.
Bench flotation tests were conducted using a Metso Denver D-12 Legacy cell. A total of 333 g of material were added into a 1 L slurry for flotation, forming a slurry concentration of 25 weight percent solids. If heat was required, then the water was heated before it was combined with the ore sample. For the bench flotation study, additional reagents were used. If depressant was needed for the test, the ore, water, and depressant were combined and allowed to condition for 5 min. If soda ash was being used as a pH modifier, then once it was added to the slurry, it was allowed to condition for 3 min. Once the pH was adjusted, then the collector was added and allowed to condition for 10 min. The collectors that needed to be dissolved in ethanol were added to the slurry, then emulsified for 3 min by a Hamilton Beach Commercial HMI200 Immersion Blender. If the pH needed to be modified further, it was done so during the final conditioning stage. Conditioning was done at 900 rpm. Two minutes before flotation began, 0.1 mL of MBIC frother was added. After conditioning, air was allowed into the system and the sample was allowed to float for 2min.
The 10 kg flotation tests were conducted using a flotation cell at Resource Development Inc. The sample used for the tests was removed from the rod mill and placed directly into the flotation cell. The slurry concentration was approximately 25 weight percent solids. If the test required heat, then the slurry was heated using a heating coil, placed directly into the slurry. Once the desired temperature was reached, the depressant was added, if needed. The slurry was then allowed to condition for 5 min. If soda ash was used as the pH modifier, then after it was added, the slurry was allowed to condition for 3 min. After the pH was changed, the collector was added into the solution. If the pH needed to be changed after the collector was added, it was done immediately. The slurry was allowed to condition for 10 min. After 8 min, 0.1 mL of MIBC frother was added. After conditioning was completed, air was allowed to enter the system and it was allowed to float for 2–8 min.
5 Gravity Separation Testing
For the ultrafine Falcon tests, a Sepro Falcon semi-batch concentrator laboratory model L40, with an ultrafine bowl, was used. Water was added to the feed tank in accordance with the required slurry density for the test, 15 weight percent solids. An agitator was used to prevent the solids from settling in the feed tank. Once the solids were added to the water, the Falcon concentrator was turned on to an rpm of 1313. The slurry was then allowed to enter the Falcon at a flow rate of 5 L/min. The concentrate remained in the bowl and was removed and dried for analysis. The tailings were filtered and dried, then were used for another pass in the Falcon concentrator. Three passes were run with the material and the products were analyzed with the XRF and MLA.
6 Results and Discussion
Microflotation was used for the screening of the run of mine ore (ROM) vs. the gravity concentrate. The surface chemistries of both were ascertained prior to testing and it was determined that the only variables would be pH and collector. The collector’s concentrations were determined from previous work completed by Dylan Everly [6, 18]. Three tests were run for each collector and feed combination at 9.5, 10, and 10.5 pH. Figure 1 shows the best results from each collector and feed combination.
The results indicate that the flotation of the gravity concentrate results in a higher REO/CaO ratio, although for most of the tests the REO/CaO ratio is not increased above that of the feed. The tests completed with collector 2 are the only exception. With either feed, flotation using collector 2 increased the REO/CaO ratio significantly. In the case of the flotation of the gravity concentrate, the ratio increased from 0.9 to 2.7.
The REO grade is increased with the aid of these collectors in flotation, but the most significant increase can be seen with collector 2. Although both the ROM ore and the gravity concentrated treated with collector 2 had an increase in grade, collector 2 acted more favorably to the ROM ore sample rather than on the gravity concentrate. The REO grade of the ROM ore treated with collector 2 was 24% while for the gravity concentrate it was 19%. From these results, it was determined that further test work would focus on gravity concentration as a cleaner to flotation.
Bench scale flotation was used to optimize flotation conditions for each collector on the ROM ore sample. These tests added more variables including, collector, collector concentration, depressant concentration, temperature, pH, pH modifier, and continuous pH modification. The conditions evaluated were determined from previous research done by Dylan Everly [6, 18]. From his design of experiments, a minimum of four conditions for each collector were chosen for further study. For collector 2, six conditions were evaluated. Two of the test conditions were ones that Dylan Everly had obtained the best results of each collector with, and the remaining tests were determined by the Design Expert 10 optimization study software developed by Stat-Ease. Figure 2 shows the best tests results obtained from these experiments in a more convenient focused manner than typical grade recovery curve. In Table 4 are the conditions that each of those tests was completed with.
No tests using collector 5 are shown because its use failed to exhibit selectivity to rare earths. Again, the experiments completed using collector 2 exhibited the most selectivity, but collector 8 also showed that it could have high selectivity along with an increased REO grade. Collector 14 did not have high selectivity, but it showed promise in that the REO recovery was high. Test 2.1 stood out as the best flotation experiment with a REO grade of 42% while maintaining a REO recovery of 70%. The selectivity of REO was also extremely high compared with the other gangue minerals. CaO had a recovery of 9%, BaO was 5%, and silicates were also 5% recovered.
Per the request of the funding agency, these bench scale flotation tests were then scaled up to 10 kg of feed material to prove the process on a larger scale. The reagent additions were scaled up at a 1:1 ratio apart from frother. Flotation was conducted for times varying from 2 to 8 min to get an idea of how increased flotation time would affect the results.
Only two of the large-scale flotation tests produced results that were like their corresponding bench scale flotation results. Both tests were completed using collector 2. They are illustrated in Figs. 3 and 4. The test shown in Fig. 3 was completed with the same conditions as bench test 2.2. Even after 8min of flotation, the REO recovery was still slightly lower, at 61%, than what had been seen on the bench scale, which was 85%. The REO grade was not increased from what had been exhibited on the bench scale, both showed grades of 23%. The only redeeming result was that the selectivity had increased from a REO/CaO ratio of 1.25 to 1.5.
Figure 4 illustrates the most promising test result from the large-scale flotation. This test used conditions from bench test 2.1. This test exhibited extremely high selectivity of bastnaesite over the gangue minerals; the REO/CaO ratio is 7. The grade and recovery from this test closely resembled the result obtained from these conditions on the bench scale. After 1min of flotation, the REO grade was 49.9% and the REO recovery was 69.8%. The recovery of calcite was 5.6%, barite was 3.1%, and silicates were 3.4% in the first minute. After 2min of flotation, the REO grade had decreased to 44.4% and recovery had increased to 80.8%. The gangue mineral recoveries were 9.2% for calcite, 5.0% for barite, and 5.6% for silicate minerals. The modal mineral concentrations of each of the concentrates and the ore sample are shown in Table 5.
The concentrate from the best flotation test was then used for a gravity separation test. The conditions for this feed had already been optimized by in parallel research carried out by Alex Norgren for an ultrafine Falcon concentrator [20,21,22]. To be able to scale the Falcon results up to plant scale, a concentrate weight is required. Since the bowl is only able to hold a limited amount of material, multiple passes needed to be made. For this test, 1462.1 g of the flotation concentrate was used. The rare earth oxide (REO) head grade of the feed, as determined by the XRF, was 39.0%, and for calcite, it was 11.3%. The difference in feed grade compared to the flotation product could be due to preferential splitting. Each pass was run in succession, with the tailings from the previous pass being used as the feed for the next one. The flow rate was kept constant between all the passes at 5 L/min. The slurry density was 15 weight percent solids for each pass and the rpms of the Falcon were kept at 1313 for all the passes.
The results of this test are illustrated in Fig. 5, along with the modal mineral concentrations on each pass shown in Table 6 which demonstrates excellent reproducibility and consistency. As the total material recovered increases, the REO grade decreases, while REO recovery increases. As seen, the grade and recovery of CaO increase with increased recovered material, where x is weight percent of the solids recovered and the grade and recovery are in percent. From these equations, we can estimate grades and recoveries within the 37% solids removed and 82% solids recovered range. Since it is undesirable to lower the recovery of REO, a high recovery is required. A REO recovery of 90% or greater was desired from this process, so that the overall recovery of REO would be above 70% total, after accounting for flotation. For this recovery to be met, 77.7% of the solids would need to be recovered. The REO grade is predicted to be 45.2%, with a CaO recovery of 58.2% and a CaO grade of 8.4%. With this process, there is a slight decrease in grade of the calcite, but a significant amount will be removed, while still upgrading the REO grade.
7 Conclusions
This patent pending rare earth separations study determined a process that incorporated both flotation and gravity separation that could be practiced in a continuous manner in an economical way to upgrade the rare earth content of a bastnaesite ore. Importantly, it addresses the effective separation of acid consuming calcite which is well known to be problematic in traditional industrial bastnaesite flotation. Hence, microflotation experiments were conducted using the data from the flotation fundamentals. It was found that for the gravity concentrated ore, the recovery of calcite was decreased, and the grade of the rare earth bearing minerals was also decreased in some cases. Collector 2 showed itself to be the best performing on this scale. From these experiments, it was determined that further study would focus on flotation followed by gravity separation. Bench scale flotation tests were conducted and again collector 2 proved to produce the most promising results for flotation. The best test had a rare earth oxide grade of 42% and a recovery of 70%, while rejecting 90% of the calcite. On the larger-scale test work, only one test proved to be promising, which also utilized collector 2. After a 2min flotation of the rare earth oxide, the grade was 44.4% and the recovery was 81%, while rejecting 91% of the calcite. The concentrate from that large-scale flotation test was used for gravity separation on the ultrafine Falcon concentrator. It was found that the Falcon could reject another 40% of the problematic calcite while still maintaining a rare earth oxide stage recovery of 90%.
References
Gupta NK, Chiranjib K (2016) Introduction. Extractve metallurgy of rare earths. FL, CrC Press, Boca Raton, p 1
USGS, Rare earths, USGS, 2017.
Namibia Rare Earths Inc., How are rare earths used?, 2017. [Online]. Available: http://www.namibiarareearths.com/rare-earths-industry.asp. [Accessed 11 January 2018].
USGS, Risk and reliance: the U.S. economy and mineral resources, USGS, 12 April 2017. [Online]. Available: https://www.usgs.gov/news/risk-and-reliance-us-economy-and-mineral-resources. [Accessed 10 Januray 2018].
Assis S, Montenegro L, Peres A (1996) Utilisation of hydroxamates in minerals froth flotation. Miner Eng 9(1):103–114
D Everly, C Anderson, S Popova, V Bryantsev and B Moyer, Beneficiation of bastnaesite ore with new flotation collector ligands, Aspects Min Miner Sci. 7(2). AMMS. 000657. 2021.
Anderson CD, Taylor P, Anderson CG (2017) Rare earth flotation fundamentals: a review. Am J Eng Res 6(11):12
C D Anderson, Improved understanding of rare earth surface chemistry and its application to froth flotation, Kroll Institute for Extractive Metallurgy, Colorado School of Mines, PhD Thesis, 2015.
P. Keller, Surface interactions and locked cycle flotation of novel collectors on bastnäsite ore, Kroll Institute for Extractive Metallurgy, Colorado School of Mines, PhD Thesis, 2020.
Owens CL, Nash GR, Hadler K, Fitzpatrick RS, Anderson CG, Wall F (2018) Zeta potentials of the rare earth element bearing bastnäsite series of fluorocarbonate minerals. Adv Colloid Interface Sci 256:152–162
R. Chapleski Jr., A. Chowdhury, A. Wanhala, V. Bocharova, S. Roy, P Keller, D. Everly, S. Jansone-Popova, A. Kisliuk, R. Sacci, A. Stack, C G. Anderson, B. Doughty, B., V. Bryantsev (2020) A molecular-scale approach to rare-earth beneficiation: thinking small to avoid large losses ISCIENCE https://doi.org/10.1016/j.isci.2020.101435
Pradip, Fuerstenau D (1991) The role of inorganic and organic reagents in the flotation separation of rare-earth ores. Int J Miner Process 32(1–2):1–22
Pradip, Fuerstenau D (2013) Design and development of novel flotation reagents for the beneficiation of Mountain Pass rare-earth ore. Min Metall Process 30(1):1–9
Pradip, Fuerstenau D (1983) The adsorption of hydroxamate collectors on semi-soluble minerals, part I: adsorption on barite, calcite and bastnaesite. Colloids Surf 8:103–119
Schriner D, Anderson C (2015) Centrifugal concentration of rare earth minerals from calcitic gangue. J Mater Eng 4:69–77
D. Schriner, Advanced beneficiation of bastnaesite ore through centrifugal concentration and froth flotation, Kroll Institute for Extractive Metallurgy, Colorado School of Mines, MSc Thesis, 2015
N. Williams, Bastnaesite beneficiation by froth flotation and gravity separation, Kroll Institute for Extractive Metallurgy, Colorado School of Mines, MSc Thesis, 2018.
D. Everly, Surface chemistry of novel collectors and their application to froth flotation of rare earth minerals, MSc Thesis, Golden, CO, 2018.
Sepro, Falcon UF gravity concentrators, Sepro, 2018. [Online]. Available: http://seprosystems.com/products/gravity-concentrators/_falcon-uf-gravity-concentrators/. [Accessed 30 January 2018].
A. Norgren, Gravity separations, Thesis, Colorado School of Mines, Golden, CO, 2018.
Norgren A, Anderson C (2021) Ultra-fine centrifugal concentration of bastnaesite ore. Metals 11:12
Norgren A, Anderson, C (2021) Recovery of rare earth oxides from flotation concentrates of bastnaesite ore by ultra-fine centrifugal concentration. Metals 11:15
Acknowledgements
We would like to thank the Critical Materials Institute and the Kroll Institute of Extractive Metallurgy at the Colorado School of Mines which made this research possible. We would also like to thank Resource Development Inc. for allowing us to use their 10 kg flotation cell. Finally, we would like to thank Oak Ridge National Laboratory for providing the collectors.
Funding
This work was supported by the Critical Materials Institute, an Energy Innovation Hub funded by the US Department of Energy, Office of Energy Efficiency and Renewable Energy, Advanced Materials and Manufacturing Technologies Office. The research was funded through the Ames National Laboratory, which is operated for the US DOE by Iowa State University under contract # DE-AC02-07CH11358.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declarations
The authors have acknowledged the funding source for this research. On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Williams, N., Anderson, C. Bastnaesite Beneficiation by Froth Flotation and Gravity Separation. Mining, Metallurgy & Exploration (2024). https://doi.org/10.1007/s42461-024-00971-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42461-024-00971-x