Abstract
The goal of this study is to synthesize nanocomposite PVA/ Co0.2Zn0.2Cd0.6Fe2O4 films with varying contents of Co0.2Zn0.2Cd0.6Fe2O4 nanoparticles (x = 0, 0.2, 0.4, 0.6, 0.8) to improve the structural, optical, and mechanical characteristics of the examined materials. Casting technique is used to prepare PVA/ Co0.2Zn0.2Cd0.6Fe2O4 films. X-ray diffraction, FTIR, UV–Vis spectroscopy, and Vickers Hardness were used to examine the effect of magnetic nanoparticle content on the structural, optical, and mechanical characteristics of PVA nanocomposite films. The refractive index rises from 1.4967 for pure PVA to 7.6511 for PVA + 0.8Co0.2Zn0.2Cd0.6Fe2O4, allowing it to be employed in a variety of applications such as solar cell construction. Because of the presence of ferrite in the glass matrix, the average hardness values enhanced and increased from 90.7 to 114.2 MPa. The stress exponent (n) varies from 1.12 to 3.33, with grain boundary sliding as the deformation mechanism for x = 0, viscous glid for x = 0.2, 0.4, and 0.6, and dislocation climb for x = 0.8 nanocomposite thin films.
Similar content being viewed by others
1 Introduction
Polyvinyl alcohol (PVA), a polar organic polymer, has sparked a lot of attention in recent years due to its extremely transparent, mechanical flexibility, low cost, non-toxicity, high biocompatibility, and good storage qualities, as well as strong chemical and thermal stabilities [1,2,3]. The novel properties of PVA-based materials have made them suitable for a wide range of applications, particularly optoelectronic technology [4]. Recent advances in polymer technology have changed the area of materials science, increasing the usage of polymer materials in several industrial domains such as packaging materials, electrical engineering, communications, automobiles, aviation, and medical equipment [5]. PVA is widely recognized for its exceptional thermal stability, remarkable chemical stability, high dielectric strength, hydrophilic nature, lack of toxicity, strong storage capacity, and physical properties that are reliant on dopants [6].
Furthermore, it has been employed as a polymer host matrix for nanoparticles to produce nanocomposites [7, 8]. Because the physical properties of polymers are altered by filling, the physical characteristics of PVA can be enhanced by adding fillers to the matrix [9]. Nanomaterials play an important role in polymeric materials by maximizing desirable properties for industrial applications [10]. Because of the growing number of possible technological applications, PVA nanocomposite films doped with ferrite nanoparticles were deemed promising materials for experimental and theoretical investigation. The properties of polymer nanocomposites are determined by the nanoparticles’ chemistry, shape, size, concentration, interfacial bonding, and dispersion within the polymer matrix [11]. The inclusion of a little amount of dopant material can greatly improve the optical, electrical, and thermal properties of polymeric materials, allowing the development of new composite materials of high quality for the device sector [12]. The synthesis and structural characteristics of Cd0.5Zn0.5Fe2O4 nanoparticles, as well as their effect on the structure and optical properties of polyvinyl alcohol films, have been investigated [5]. The refractive index rose (from 1.409 for pure PVA to 14.449 for PVA-9% Cd0.5Zn0.5Fe2O4). As the quantities of Cd0.5Zn0.5Fe2O4 nanoparticles in the polymer matrix grew, so did their optical dielectric characteristics and optical conductivity. The increased characteristics of PVA-Cd0.5Zn0.5Fe2O4 nanocomposite films may be useful in a variety of optical and electrical applications [5]. Polymeric nanocomposite of PVA/CoFe2O4 was also prepared, and it was discovered that Cobalt ferrite nanoparticles dispersed on the surface of the polymer prevent oxygen and heat from accessing the core regions of the polymer. This technique increases the fire resistance of nanocomposite [13]. Spinel ferrite has gained popularity due to its functional qualities and employment in a variety of applications such as magneto-optical systems, magnetic recording devices, sensors, microwave shielding, and so on [14,15,16]. The flash auto combustion method was used to prepare Co0.2Zn0.2Cd0.6Fe2O4 [17]. The aim of this research is to prepare nanocomposite PVA/Co0.2Zn0.2Cd0.6Fe2O4 films with varying amounts of Co0.2Zn0.2Cd0.6Fe2O4 nanoparticles (x = 0, 0.2, 0.4, 0.6, 0.8) to improve structural, optical, and mechanical characteristics. The investigation was completed using a range of methodologies, including XRD, FTIR, UV–Vis spectroscopy, and Vickers Hardness. The findings showed that PVA-Co0.2Zn0.2Cd0.6Fe2O4 nanocomposites might be employed in solar cell applications.
2 Experimental Details
2.1 Pure PVA Thin Film
The casting technique is used to synthesize pure PVA thin film by combining 1 g of PVA powder with 40 ml of distilled water at 60 °C and mixing for 1 h at room temperature with magnetic stirring until the powder has completely dissolved and changed into a clear solution. The solution is placed into a glass petri dish and allowed to dry for three days at room temperature. Finally, the dried film on the petri dish is scraped away and its thickness was found to equal 0.015 cm.
2.2 PVA/ Co0.2Zn0.2Cd0.6Fe2O4 Films
To begin, the Co0.2Zn0.2Cd0.6Fe2O4 ferrite nanoparticle is chosen because of its strong magnetic characteristics and prepared using the flash auto combustion technique [17]. PVA/(Co0.2Zn0.2Cd0.6Fe2O4 NPs) thin films are gained by combining different contents of (Co0.2Zn0.2Cd0.6Fe2O4 NPs) (0.2, 0.4, 0.6, 0.8) with the previous solution (pure PVA) and agitating the mixture with a magnetic stirrer until an aqueous suspension is formed. The finished mixture is placed in a glass petri dish and allowed to cure for three days at room temperature before the dried films are scraped off. Figure 1 displays the color gradient of samples with increasing Co0.2Zn0.2Cd0.6Fe2O4 concentrations. The prepared samples’ film thicknesses ranged from 0.015 to 0.019 cm.
2.3 Measurements
A Philips model (PW-1729) diffractometer is used to assess the XRD pattern and crystalline structure of the prepared thin films, and the polymeric thin films are subjected to a Cu-K radiation source (λ = 1.540598Å) with \(2\theta\) in the range of 4–80°. Infrared spectra were recorded at room temperature using a PERKIN-ELMER-1430, with infrared spectra ranging from 200 to 4000 cm−1 (at Tanta University, Central lab.). The optical property changes are examined using an Ultraviolet-Visible spectrometer (V-630 UV–Vis) with a fixed band pass of 1.5 nm in the range of 200–1100 nm. For measuring the hardness of materials, Digital Vickers microhardness tester model (FM-7) is used. The surface is subjected to standard pressure for a fixed interval of time by means of a pyramid-shaped diamond as shown in Fig. 2. The indenter is a square pyramid whose opposite sides meet at a 136-degree angle at the top. Diamond pressure is made on the material surface using load 10 gf, and the imprint size is measured microscopically. The number of Vickers (HV) is calculated using,
where (F) is the load applied (Kgf), d is the mean of two diagonals d1 and d2 (mm).
3 Results and Discussion
3.1 X-Ray Structural Studies
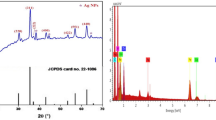
The XRD diffraction patterns of pure PVA and synthesized composite PVA/Co0.2Zn0.2Cd0.6Fe2O4 films with varying ferrite content are shown in Fig. 3. The study of the diffraction pattern by predicting the reflection planes (2 2 0), (3 1 1), (4 2 2), (5 1 1), and (4 4 0) assures the formation of the cubic spinel structure [17]. These peaks demonstrate that all samples belong to the Fd3m phase group [17]. The broad XRD line reveals that the materials are nano size [17].
XRD pattern of pure PVA reveals its semi-crystalline structure, with two major peaks [18]. The first broad peak around \(2\theta =19.5^\circ\) corresponds to the PVA crystalline phase and is indexed to (002), resulting from strong intermolecular hydrogen bonding between the PVA chains. This peak broadens and decreases in intensity as the ferrite content increases up to x = 0.4 in the PVA matrix due to the breakage of the PVA matrix’s hydrogen bond [19], where it increases at x = 0.6, as confirmed by the calculation of the crystalline fraction (XC) of the polymeric thin films using the Hermans-weidinger method. The other peak appeared at\(2\theta =41^\circ\), indicating amorphous nature and was indexed to (100). To calculate the XC, the XRD spectra of the samples must be deconvoluted to identify the area of the amorphous and crystalline peaks [20]. The following equation was used to determine the XC:
where AC and AT are the crystalline peak area and total amorphous and crystalline peak area, respectively. Because of the increase in ferrite in the polymeric thin film, the degree of crystallinity of the composite falls up to x = 0.4, as shown in Table 1. The sample x = 0.6 has the highest crystalline fraction due to aggregation. Comparing the crystallite size value with the pure ferrite (52.3 nm) [17], it was found that the value of crystallite size has lower value in the PVA/ Co0.2Zn0.2Cd0.6Fe2O4 films ranged from (21.5–25.4 nm) as given in Table 1. This behavior can be referred to the effect of polymer on the decreasing of magnetic crystalline agglomeration leading to the decrease of its value. The lattice parameter was found to be higher than the pure ferrite value (8.474Å) [17] which may be attributed to the effect of polymer on the increasing of cation – cation and anion – cation bond length leading to the slight increase in the lattice parameter.
3.2 FTIR Analysis
The FTIR measurements of the PVA and nanocomposite samples were performed in the wavelength range of 200–4000 cm−1, as shown in Fig. 4. The spinel ferrite’s FTIR spectra contain two primary distinctive absorption zones that correspond to the inherent stretching vibrations of the oxygen bonds with metal cations at postures A and B. According to Fig. 4, the distinctive absorption bands about 470 cm−1 and 345 cm−1 correspond to the tetrahedral and octahedral stretching vibrations of the Fe+3–O−2 bond, respectively as given in Table 2. M. Rahimi et al. [21] and Sindhu et al. [22] obtained similar results. All PVA/Co0.2Zn0.2Cd0.6Fe2O4 nanocomposites exhibit a C–O band of acetyl group stretching vibrations at about 1523 cm−1. The OH stretching vibration of the PVA main chain is coupled with a pronounced wide band at around 3430 cm−1 [23]. The CH bond’s symmetric and asymmetric stretching vibrations are joined by bands at 2923 cm−1 and 2850 cm−1. The observed bands at 791 cm−1 and 671 cm−1 correspond to the polymer’s C–C chains [23]. PVA/Co0.2Zn0.2Cd0.6Fe2O4 nanocomposites show a distinctive band at ~ 1081 cm−1, due to the Fe–O–C bond [21]. The absorption band at ~ 1660 cm−1 corresponds to the –OH bending vibration [24]. The introduction of ferrite nanoparticles as a filler causes changes in the FTIR spectra of PVA, including a shift in some bands and variations in their intensities. These modifications were caused by nanoparticle crosslinking and excellent dispersion throughout the polymer matrix [25]. The tetrahedral absorption band shifted to higher frequency in comparison with the sample x = 0.2 as given in Table 2. This variation of absorption band frequency is correlated to the change in the bondlenght Fe+3–O−2 bond. The primary characteristic bands ʋ1 and ʋ2 fall within the frequency range stated in the earlier study for ferrite spinel phase [17]. There was a noticeable change in the absorption band frequency from pure ferrite, which might be attributed to the influence of polymer on the bond length of Fe+3–O−2 as a result of crosslinking interaction with the polymer matrix.
3.3 Ultraviolet and Visible Spectroscopy (UV–Vis)
UV–Vis spectroscopy can be extremely useful for studying the interaction between radiation and electrons. The optical transition spectrum gives fundamental information about the optical bandgap of a substance. It is one of the most essential ways for picking an innovative material for optical applications. UV–Vis absorption spectra for pure PVA and PVA/ Co0.2Zn0.2Cd0.6Fe2O4 composite thin films vs. the wavelength of the incident light (λ) in the range 200–1100 nm at room temperature are shown in Fig. 5. Increasing the ferrite content increased the optical absorbance of the PVA films. The primary absorption peak for pure PVA is located about 280 nm in this spectrum. It is obvious that the absorption of the pure PVA is characterized by a sharp absorption edge, which indicates the semi-crystalline nature of the PVA film [26,27,28], and the absorption spectra are redshifted as the ferrite concentration in the PVA matrix increases. This could be because the particle size surfaces area increases as particle size decreases, and the transparency of the samples decreases as filler content increases, resulting in an increase in absorbance%. As shown, an absorption band \(\pi -{\pi }^{*}\) transition at 280 nm is seen, which is connected to the semicrystalline structure of the PVA matrix [5, 29,30,31]. Another absorption band appeared at ferrite content x = 0.6 and x = 0.8 at 333 nm due to the presence of ferrite nanoparticles in the polymer matrix which is related to d-d transition between Cd2+, Zn2+ and Fe3+ [5]. Doping nanoparticles with polymer resulted in numerous absorption centers, leading to an overall increase in absorbance. The absorption spectra of pure PVA decrease in the 200–300 nm region and stabilize at higher wavelengths, suggesting that the free-standing PVA film is transparent across the whole visible wavelength range of more than 300 nm [32].
According to Tauc’s relation [5, 33,34,35], the direct and indirect band gap can be estimated from the absorption edge using the absorption spectra:
where α is the absorption coefficient and \(h\nu\) represent the energy of photon, B is constant, Eg represent the band gap and m is variable value can be equal \(1/2\) or 2 for direct and indirect transitions, respectively. The values of optical band gap can be calculated using extrapolated linear component of photon energy \(h\nu\) various \({\left(\alpha h\nu \right)}^{m}\) as shown in Fig. 6. As seen in Fig. 7, the optical band gaps, both direct and indirect, decreased with increasing ferrite content in the polymer matrix. This tendency is consistent with prior work [28]. This decrease could be attributed to the presence of ferrite. By increasing ferrite concentration, more localized energy levels between the valence and conduction bands are created, resulting in a drop in optical Eg.
Urbach’s energy (EU) is linked to local states that have broadened into the band gap as a result of impurities, non-crystallinity, and defects. The disordering degree of the polymer films can be demonstrated by calculating the Urbach energy (EU), using the following equation [36]:
where \({\alpha }_{o}\) denotes a constant. EU is calculated by graphing ln(α) vs. \(h\nu\). This energy can be determined from Fig. 8 by taking the reciprocal of the slope in curve’s linear Sect. [37]. As shown in Table 3, the overall trend of EU increasing with increasing ferrite content in the PVA matrix. As a result, EU validates the degree of disorder in polymeric thin films after the addition of ferrite. The sample x = 0.2 ferrite content has small EU value due to the disorder and amorphous character which are present in this sample. It is commonly known that the disordered character of the material leading to the tail in the valence and conduction bands is connected with an increase in EU. EU values vary inversely with Eg because a rise in Urbach energy corresponds to an increase in the breadth of the localized state in the optical band gap [38].
Optical density (OD) measures the refraction of electromagnetic light within a medium, providing essential information on light transmission within materials. The value of OD as a function of α and l for material is given by [39]:
where \(l\) is the thickness. Figure 9 displays OD as a function of the wavelength (λ) for various PVA-Co0.2Zn0.2Cd0.6Fe2O4 nanocomposites. The value of OD passes through 2 stages: the first is in the wavelength range 200 to roughly 250 nm, where the values of OD are decreases rapidly as the incident wavelength increases. OD values reach a consistent value for the remainder of the examined wavelength during the second stage above 250 nm. When comparing the Abs. and OD curves, both exhibit similar behavior with wavelength and exhibit a step-change at the same wavelength equal to 333 nm.
In terms of optoelectronic applications, refractive index (n) is considered as one of the most important optical parameters. The refractive index is a measure of the reduction of the speed of light in a material [40]. Figure 10 displays “n” of PVA / ferrite nanocomposite films for different ferrite content. The refractive index “n” is seen to increase with increasing filler content in the polymer matrix and decrease with increasing wavelength. The increase in packing density generated by the Co0.2Zn0.2Cd0.6Fe2O4 content can explain this. As shown in Table 3, the refractive index value increase from 1.4967 for pure PVA to 7.6511 for PVA + 0.8 Co0.2Zn0.2Cd0.6Fe2O4. Filters, polymer lenses, optical communications, optoelectronic, reflecting and antireflective coatings, and solar cell fabrication can all benefit from the as-prepared nanocomposite films [5].
The extinction coefficient (k) of a substance describes how strongly it absorbs or reflects radiation or light at a certain wavelength. k is linked to the absorption coefficient by the following Eqs. [41, 42]:
where λ is the wavelength. As demonstrated in Fig. 11, the extinction coefficient increases as the ferrite concentration of the PVA matrix increases. Because the extinction coefficient is directly proportional to the absorption coefficient, this is connected to the rise in the absorption coefficient [5]. It is obvious from Fig. 11 that increasing the ferrite concentration of nanoparticles increases k. This might be because the incident photon has enough energy to excite electrons from the ground state to the excited state, causing k to fall by increasing photon energy and vice versa, causing k to rise by increasing wavelength.
The spectrophotometer cannot be used to detect dielectric parameters, but they may be computed from refractive index and extinction coefficient data using the following Eqs. [43,44,45,46]:
where “\(\varepsilon _r\)” represents the real and “\({\varepsilon }_{i}\)” imaginary components of the dielectric constant. Figure 12 depicts the photon energy dependences of \({\varepsilon }_{r}\) and \({\varepsilon }_{i}\). Both \({\varepsilon }_{r}\) and \({\varepsilon }_{i}\) rise when the ferrite content in the PVA matrix increases, as seen in Fig. 6. Furthermore, the rise in the optical dielectric constant with increasing photon energy is generated by the interaction of photons and electrons, which results in the formation of induced polarization [26].
Figure 13 depicts the loss factor, tan(δ), versus the photon energy (hυ) for PVA-ferrite nanocomposites. \(Tan\delta = \frac{{\epsilon }_{i}}{{\epsilon }_{r}}\) may be used to compute the loss factor. The alteration of the host polymer’s crosslinking ions by the Co0.2Zn0.2Cd0.6Fe2O4 doping filler is shown by the change in dielectric constant components.
Two functions may be used to represent energy loss: the volume energy loss function (VELF) and the surface energy loss function (SELF). The VELF represents the energy lost by fast electrons within the bulk material, whereas the SELF represents energy loss on the material surface. Both VELF and SELF are functions of the dielectric constant components (\({\varepsilon }_{i}\) and\({ \varepsilon }_{r}\)) and may be computed using the following equations [23]:
The VELF and SELF values provide information about the total energy loss. Figure 14a, b show the VELF and SELF vs. photon energy (hv) for PVA- Co0.2Zn0.2Cd0.6Fe2O4 nanocomposites. The VELF and SELF values fall considerably as the energy value increases, as does the ferrite content in the PVA matrix.
The optical conductivity of a material is defined as its optical response to charge carrier diffusion produced by excitation generated by incident photon energy [47, 48]. According to the following Eqs. [47, 49], optical conductivity is exactly proportional to the refractive index and absorption coefficient of the materials:
where C is the vacuum speed of light. Figure 15 depicts \({\sigma }_{opt}\) vs. \(\text{h}{\upnu }\) (eV), In general, it is obvious that optical conductivity rises with incident photon energy. There were two zones that were observed. The photon energy influencing the sample in the low photon energy area (< 5 eV) is inadequate to excite the electrons in the polymer films; hence, optical conductivity seems to be energy independent in this region. However, in the high photon energy area (> 5 eV), the photon energy is sufficient to excite electrons from the valance band to the conduction band, resulting in an increase in charge carriers. This causes an increase in optical conductivity as well. The presence of a localized tail state in the band gap facilitates the transition from the valence band to the closest level states [50, 51]. The contribution of hopping conduction electrons to overall conductivity causes the optical conductivity to grow as the ferrite percentage increases. This is due to the creation of charge transfer between the PVA molecules and the ferrite nanoparticles [52]. The increase in nanoparticle concentration produces a rise in the density of localized states in the band structure, which enhances the absorption coefficient and optical conductivity [33, 42]. The optical conductivity increases dramatically in the vicinity of the absorption edge, as seen in the figure. This is due to an excess of free charge carriers induced by incoming photon energy that is sufficient to overcome the band gap of the polymer film [42].
3.4 Hardness Indentation and Micro-Creep Dependence
Figure 16 shows the creep behavior for five nanocomposite thin films using Vickers hardness test for an applied load 10 gf and a dwell time 5–100 Sect. [53]. It can be seen that as the indentation time increases, the indentation length increases. Figure 17 shows the variation of Vickers hardness as a function of indentation time [54]. From this figure, the hardness decreases with increasing indentation time. The average hardness values are shown in Table 4, which increases from 90.7 to 114.2 MPa as the ferrite concentration in the PVA matrix increases. The enhancement of hardness values can be attributed to the reduction of crystallite size as shown by X-ray analysis. The stress exponent calculated from the following equation and listed in Table 4 according to [55] and [56] is
The stress exponent is used to determine deformation mechanisms. Here HV is the Vickers hardness value, d is the diagonal length of indentation, and \(\dot{\text{d}}\)is the rate of change of the diagonal indentation length. The stress exponent n is calculated from the slope of straight line obtained by plotting \(\dot{\text{d}}\) versus HV on a double logarithmic scale as shown in Fig. 18 [57]. The stress exponent indicates the deformation mechanism at room temperature. As shown in Table 4. The stress exponent (S) ranges from 1.12 to 3.33. Stress exponent can be used to identify the mechanisms controlling deformation. Grain boundary sliding is the deformation mechanism for x = 0, viscous glid for x = 0.2, 0.4, 0.6 and dislocation climb for x = 0.8 nanocomposite thin films [58, 59].
This study and others [60] found that HV decreases as dwell time increases. Therefore, the increase in strain with the indentation time under constant load is known as creep. Strain is considered here as a partial change in area and is given by:
where ∆S = S − So, and So are taken as the area at minimum indentation time [61]. Measuring creep using this method is called “micro-creep”. The term micro creep derives from applying the stress to a very small area comparable to the area of a grain, and is observed under a built-in microscope. Plotting strain versus indentation time yields a typical creep curve as shown in Fig. 19. In the first stage of the curve, a rapid increase in strain with time is recorded from zero to 20 s. The second region shows a slowly increasing area where strain increases slowly for all samples. Since the hardness test is actually a compression test, there is no sample failure and therefore it is clearly impossible to record the third phase of the curve, unlike what happens in a normal creep test. We can conclude that the factors affecting the creep resistance are crystalline factor and crystallite size. The sample x = 0.6 and 0.8 have high creep resistance which means the inverse relation between crystallite size and creep resistance.
4 Conclusions
Pure PVA thin films and nanocomposites of (PVA/Co0.2Zn0.2Cd0.6Fe2O4) are successfully prepared using the casting procedure. Pure PVA has a semi-crystalline structure with two main peaks, as shown by its XRD pattern. In the PVA/Co0.2Zn0.2Cd0.6Fe2O4 films, the value of crystallite size has a lower value, ranging from (21.5 to 25.4 nm). This can be related to the influence of polymer on the lowering magnetic crystalline agglomeration, which leads to the decrease in its value. The absorption band frequency of pure ferrite changed noticeably from FTIR, which could be related to the polymer’s effect on the bond length of of Fe+3 - O−2 as a result of crosslinking interaction with the polymer matrix. The PVA films’ optical absorbance increased as the ferrite content rose. The as-prepared nanocomposite films can be advantageous for solar cell manufacturing because the refractive index “n” is observed to increase with increasing filler content in the polymer matrix and decrease with increasing wavelength. As the ferrite content in the PVA matrix rises, so do the dielectric constant’s real and imaginary components. The density of localized states in the band structure increases as the number of nanoparticles increases, improving the optical conductivity and absorption coefficient. The decrease in crystallite size is responsible for the increase in hardness values, which rise from 90.7 to 114.2 MPa as the ferrite content in the PVA matrix increases. The samples x = 0.6 and 0.8 exhibit high creep resistance.
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
References
A. Gamal El-Shamy, The optical anatomy of new polyvinyl alcohol/zinc peroxide (PVA/ZnO) nanocomposite films for promising optical limiting applications. Prog. Org. Coat. 150, 105981 (2021)
M. Abdolrahimi, M. Seifi, M.H. Ramezanzadeh, Study the effect of acetic acid on structural, optical and mechanical properties of PVA/chitosan/MWCNT films. Chin. J. Phys. 56(1), 221–230 (2018)
M. Aslam, M.A. Kalyar, Z.A. Raza, Polyvinyl alcohol: a review of research status and use of polyvinyl alcohol-based nanocomposites. Polym. Eng. Sci. 58(12), 2119–2132 (2018)
J. Selvi, V. Parthasarathy, S. Mahalakshmi, R. Anbarasan, M.O. Daramola, P.S. Kumar, Optical, electrical, mechanical, and thermal properties and non-isothermal decomposition behavior of poly (vinylalcohol)–ZnO nanocomposites. Iran. Polym. J. 29, 411–422 (2020)
T.S. Soliman, A.S. Abouhaswa, Synthesis and structural of Cd0.5Zn0.5Fe2O4 nanoparticles and its influence on the structure and optical properties of polyvinyl alcohol films. J. Mater. Sci. 31, 9666–9674 (2020)
T. Rebar, O.G. Abdulwahid, S.B. Abdullah, S.A. Aziz, F. Hussein, Fahmi, M.Y. Muhammad, Yahya, The study of structural and optical properties of PVA:PbO2 based solid polymer nanocomposites. J. Mater. Sci. 27, 12112–12118 (2016). https://doi.org/10.1007/s10854-016-5363-y
T.A. Hamdalla, T.A. Hanafy, A.E. Bekheet, Infuence of erbium ions on the optical and structural properties of polyvinyl alcohol. J. Spectrosc. 2015, 204867 (2015). https://doi.org/10.1155/2015/204867
H.M. Shanshool, M. Yahaya, W.M.M. Yunus, I.Y. Abdullah, Investigation of energy band gap in polymer/ZnO nanocomposites. J. Mater. Sci. Mater. Electron. 27, 9804–9811 (2016). https://doi.org/10.1007/s10854-016-5046-8
J.K. Rao, A. Raizada, D. Ganguly, M.M. Mankad, S.V. Satyanarayana, G.M. Madhu, Enhanced mechanical properties of polyvinyl alcohol composite films containing copper oxide nanoparticles as filler. Polym. Bull. 72, 2033–2047 (2015)
R. Bavandpour, H.K. Maleh, M. Asif, V.K. Gupta, N. Atar, M. Abbasghorbani, Liquid phase determination of adrenaline uses a voltammetric sensor employing CuFe2O4 nanoparticles and room temperature ionic liquids. J. Mol. Liq. 213, 369–373 (2016)
F. Shahbaa, O.G. Bdewi, B.K. Abdullah, A.R. Aziz, Ayad, Mutar, Synthesis, structural and optical characterization of MgO Nanocrystalline embedded in PVA Matrix. J. Inorg. Organomet. Polym. 26, 326–334 (2016). https://doi.org/10.1007/s10904-015-0321-3
O.G. Abdullah, S.B. Aziz, M.A. Rasheed, Structural and optical characterization of PVA:KMnO4 based solid polymer electrolyte. Results Phys. 6, 1103–1108 (2016)
M. Karbasi, F. Maghazeii, D. Ghanbari, Magnetic investigation of microwave synthesized and thermal stable polyvinyl alcohol-cobalt Ferrite nanocomposites. J. Nanostruct. 9(2), 365–375 (2019)
S. Joshi, M. Kumar, S. Chhoker, G. Srivastava, M. Jewariya, V.N. Singh, Structural, magnetic, dielectric and optical properties of nickel ferrite nanoparticles synthesized by co-precipitation method. J. Mol. Struct. 1076, 55–62 (2014). https://doi.org/10.1016/j.molstruc.2014.07.048
A.V. Ravindra, M. Chandrika, C. Rajesh, P. Kollu, S. Ju, S.D. Ramarao, Simple synthesis, structural and optical properties of cobalt ferrite nanoparticles. Eur. Phys. J. Plus. 134, 1–10 (2019). https://doi.org/10.1140/epjp/i2019-12690-2
K. Kalimuthu, S.C. Rangasamy, M. Rakkiyasamy, Synthesis and structural studies of nanocrystalline Cd0.3Zn0.7Fe2O4. S Afr. J. Chem. 67, 91–93 (2014)
M. Mostafa, B.I. Salem, Studying structural, molecular, morphological and electrical properties of Co0.2 Zn0.8 Fe2O4 doped with Cadmium. Mater. Sci. Eng. B 286, 116043 (2022)
X. Jiang, N. Xiang, H. Zhang, Y. Sun, Z. Lin, L. Hou, Preparation and characterization of poly(vinyl alcohol)/sodium alginate hydrogel with high toughness and electric conductivity. Carbohydr. Polym. 186, 377–383 (2018)
M. Rashad, Tuning optical properties of polyvinyl alcohol doped with different metal oxide nanoparticles. Opt. Mater. 105, 109857 (2020)
M.A. Morsi, S.A. El-Khodary, A. Rajeh, Enhancement of the optical, thermal and electrical properties of PEO/PAM:Li Polymer electrolyte films doped with ag nanoparticles. Phys. B Condens. Matter. 539, 88–96 (2018)
M. Rahimi, P. Kameli, M. Ranjbar, H. Salamati, The effect of polyvinyl alcohol (PVA) coating on structural, magnetic properties and spin dynamics of Ni0.3Zn0.7Fe2O4 ferrite nanoparticles. J. Magn. Magn. Mater. 347, 139–145 (2013)
S. Sindhu, S. Jegadesan, A. Parthiban, S. Valiyaveettil, Synthesis and characterization of ferrite nanocomposite spheres from hydroxylated polymers. J. Magn. Magn. Mater. 296(2), 104–113 (2006)
M. Alaa, M. Abd-Elnaiem, T.A. Rashad, N.M. Hanafy, Shaalan, Improvement of Optical properties of Functionalized polyvinyl alcohol-zinc oxide hybrid nanocomposites for wide UV Optoelectronic Applications. J. Inorg. Organomet. Polym. Mater. 33, 2429–2444 (2023)
Z. Wang, Y. Xie, P. Wang, Y. Ma, S. Jin, X. Liu, Microwave anneal effect on magnetic properties of Ni0.6Zn0.4Fe2O4 nano-particles prepared by conventional hydrothermal method. J. Magn. Magn. Mater. 323(23), 3121–3125 (2011)
A.J. Mohammed, H. Sameer, Al-nesrawy, Nano ferrite incorporated poly (Vinyl Pyrrolidone (PVP) /Poly (Vinyl Alcohol (PVA) blend: preparation and investigation of structural, Morphological and Optical Properties. NeuroQuantology 20(3), 251–258 (2022)
A.A. Ebnalwaled, A. Thabet, Controlling the optical constants of PVC nanocomposite films for optoelectronic applications. Synth. Met. 220, 374–383 (2016). https://doi.org/10.1016/j.synthmet.2016.07.006
A. El-Khodary, Vibrational, thermal, optical and magnetic investigations of PVA films filled with FeCl3 and CoCl2. Phys. B Condens. Matter. 404, 1287–1294 (2009). https://doi.org/10.1016/J.PHYSB.2008.11.238
T.S. Soliman, S.A. Vshivkov, Effect of Fe nanoparticles on the structure and optical properties of polyvinyl alcohol nanocomposite films. J. Non-Cryst Solids. 519, 119452 (2019)
S. Choudhary, Characterization of amorphous silica nanofiller effect on the structural, morphological, optical, thermal, dielectric and electrical properties of PVA–PVP blend based Polymer nanocomposites for their flexible nanodielectric applications. J. Mater. Sci. 29, 10517–10534 (2018). https://doi.org/10.1007/s10854-018-9116-y
S. Mallakpour, Z. Khani, Fabrication of poly(vinyl alcohol) nanocomposites having different contents of modifed SiO by vitamin B1 as biosafe and novel coupling agent to improve mechanical and thermal properties. Polym. Compos. 39, E1589–E1597 (2018). https://doi.org/10.1002/pc.24517
S. Mallakpour, H.Y. Nazari, The influence of bovine serum albumin modifed silica on the physicochemical properties of poly(vinyl alcohol) nanocomposites synthesized by ultrasonication technique. Ultrason. Sonochem. 41, 1–10 (2018). https://doi.org/10.1016/j.ultsonch.2017.09.017
B. Shujahadeen, O.G. Aziz, M.A. Abdullah, K. Brza, Auday, D.A. Azawy, Tahir, Effect of Carbon Nano-dots (CNDs) on Structural and Optical properties of PMMA Polymer Composite. Results Phys. 15, 102776 (2019). https://doi.org/10.1016/j.rinp.2019.102776
H. Ahmed, H.M. Abduljalil, A. Hashim, Structural, optical and electronic properties of novel (PVA–MgO)/SiC nanocomposites films for humidity sensors. Trans. Electr. Electron. Mater. 20, 218–232 (2019). https://doi.org/10.1007/s42341-019-00111-z
F.M. Ali, Structural and optical characterization of [(PVA:PVP)Cu2+] composite films for promising semiconducting polymer devices. J. Mol. Struct. 1189, 352–359 (2019). https://doi.org/10.1016/J.MOLSTRUC.2019.04.014
J. Tauc, Optical properties of amorphous semiconductors. Amorphous Liquid Semiconductors 1, 159–220 (1974)
O.G. Abdullah, D.M. Salh, A.H. Mohamad, G.M. Jamal, H.T. Ahmed, B.S. Mustafa, M.H. Suhail, Linear and nonlinear optical characterization of dye–polymer composite films based on methylcellulose incorporated with varying content of methylene blue. J. Electron. Mater. 51, 675–683 (2022)
N.F. Mott, The electrical conductivity of transition metals. Proc. R. Soc. London. Ser. A-Mathematical Phys. Sci. 153(880), 699–717 (1936)
H. Azhin, S.R. Mohamad, O.G. Saeed, Abdullah, Synthesis of very-fine PbS nanoparticles dispersed homogeneously in MC matrix: effect of concentration on the structural and optical properties of host polymer. Mater. Res. Express. 6, 115332 (2019)
A.M. Abd–Elnaiem, M.A. Abdelraheem, M.A. Abdel–Rahim, S. Moustafa, Substituting silver for Tellurium in Selenium–Tellurium thin films for improving the optical characteristics. J. Inorg. Organomet. Polym. Mater. 32, 2009–2021 (2022)
S.B. Aziz, A.Q. Hassan, S.J. Mohammed, W.O. Karim, M.F.Z. Kadir, H.A. Tajuddin, N.N.M.Y. Chan, Structural and optical characteristics of PVA:C-Dot composites: tuning the absorption of ultra violet (UV) region. Nanomaterials 9, 216 (2019). https://doi.org/10.3390/nano9020216
N.J. Mohamed, Z.S. Rasheed, A.S. Hassan, Improvement Optical Properties of PVA/TiC2 and PVA/ZnO Nanocomposites. Al- Mustansiriyah J. Sci. 29, 118 (2019)
M.B. Mohamed, M.H. Abdel-Kader, Effect of excess oxygen content within different nano-oxide additives on the structural and optical properties of PVA/PEG blend. Appl. Phys. Mater. Sci. Process. 125, 1–11 (2019). https://doi.org/10.1007/s00339-019-2492-1
J.L. Wilson, P. Poddar, N.A. Frey, H. Srikanth, K. Mohomed, J.P. Harmon, S. Kotha, J. Wachsmuth, Synthesis and magnetic properties of polymer nanocomposites with embedded iron nanoparticles. J. Appl. Phys. 95, 1439–1443 (2004). https://doi.org/10.1063/1.1637705
A. Sonal, S. Aggarwal, Optical investigation of soda lime glass with buried silver nanoparticles synthesised by ion implantation. J. Non-Cryst Solids. 485, 57–65 (2018). https://doi.org/10.1016/j.jnoncrysol.2018.01.038
S. Dadashi, R. Poursalehi, H. Delavari, Structural and optical properties of pure Iron and Iron oxide nanoparticles prepared via pulsed nd:YAG laser ablation in liquid. Procedia Mater. Sci. 11, 722–726 (2015). https://doi.org/10.1016/j.mspro.2015.11.052
I.S. Yahia, A.A.M. Farag, M. Cavas, F. Yakuphanoglu, Effects of stabilizer ratio on the optical constants and optical dispersion parameters of ZnO nano-fiber thin films. Superlattice Microstruct. 53, 63–75 (2013). https://doi.org/10.1016/j.spmi.2012.09.008
T.A. Taha, A. Saleh, Dynamic mechanical and optical characterization of PVC/fGO Polymer nanocomposites. Appl. Phys. Mater. Sci. Process. 124, 1–12 (2018). https://doi.org/10.1007/s00339-018-2026-2
P. Krishnan, K. Gayathri, S. Gunasekaran, G. Anbalagan, Optical, spectral and thermal properties of organic nonlinear optical single crystal: 2,3-Dimethoxy-10oxostrychnidinium hydrogen oxalate dihydrate. Optik 125, 3852–3859 (2014). https://doi.org/10.1016/J.IJLEO.2014.01.174
M.M. Abutalib, I.S. Yahia, Analysis of the linear/nonlinear optical properties of basic fuchsin dye/FTO films: controlling the laser power of red/green lasers. Optik 179, 145–153 (2019). https://doi.org/10.1016/J.IJLEO.2018.10.081
T.A. Taha, N. Hendawy, S. El-Rabaie, A. Esmat, M.K. El-Mansy, Effect of NiO NPs doping on the structure and optical properties of PVC Polymer films. Polym. Bull. 76, 4769–4784 (2019). https://doi.org/10.1007/s00289-018-2633-2
T.A. Taha, Optical properties of PVC/Al2O3 nanocomposite films. Polym. Bull. 76, 903–918 (2019). https://doi.org/10.1007/s00289-018-2417-8
O.G. Abdullah, S.B. Aziz, K.M. Omer, Y.M. Salih, Reducing the optical band gap of polyvinyl alcohol (PVA) based nanocomposite. J. Mater. Sci. 26, 5303–5309 (2015). https://doi.org/10.1007/s1085f4-015-3067-3
M. Ali, O.M. Hemeda, A.M.A. Henaish, Tailoring changes in the physical, structural and linear optical properties of SrCuTi2Fe14O27 W-type hexaferrite-doped PVA polymeric composite films. J. Mater. Sci. 32, 16038–16051 (2021). https://doi.org/10.1007/s10854-021-06153-5
A. Jain, M. Banerjee, G.S. Mukherjee, Effect of UV irradiation on optical properties of pristine and MnCl2 doped PVA films. Mater. Today Proc. 47, 697–703 (2020). https://doi.org/10.1016/j.matpr.2020.12.558
M.H. Suhail, Structural and optical properties for PVA- PEG-MnCl2 composites. Iraqi J. Phys. 15, 99–113 (2019). https://doi.org/10.30723/ijp.v15i32.160
T. Shibutani, Q. Yu, M. Shiratori, A study of deformation mechanism during nanoindentation creep in tin-based solder balls. J. Electron. Packag Trans. ASME. 129, 71–75 (2007). https://doi.org/10.1115/1.2429712
Z.H. Cao, P.Y. Li, X.K. Meng, Nanoindentation creep behaviors of amorphous, tetragonal, and bcc Ta films. Mater. Sci. Eng. A 516, 253–258 (2009). https://doi.org/10.1016/j.msea.2009.03.019
A.E. Hmmad, A.A. Ibrahiem, Enhancing the microstructure and tensile creep resistance of Sn-3.0Ag-0.5Cu Solder alloy by refining nano-sized ZnO particles. Microelectron. Reliab. 75, 187–194 (2017). https://doi.org/10.1016/j.microrel.2017.07.034
O. Sahin, O. Uzun, U. Kölemen, N. Uçar, Stress exponent investigation of β-Sn single crystal by depth-sensing indentation tests. Phys. B Condens. Matter. 39(1–2), 87–90 (2007). https://doi.org/10.1016/j.physb.2007.03.015
A. Mustafa kamal, A.R. El-bediwi, A.H. Lashin, El-Zarka, Copper effects in mechanical properties of rapidly solidified Sn-Pb-Sb babbitt bearing alloys. Mater. Sci. Eng. A 530, 327–332 (2011)
R.M. Shalaby, Indium, chromium and nickel-modified eutectic Sn–0.7 wt% Cu lead-free solder rapidly solidified from molten state. J. Mater. Sci. Mater. Electron. 26, 6625–6632 (2015)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). All Authors declare that they have no funding sources for this research.
Author information
Authors and Affiliations
Contributions
M.M.: Conceptualization, Resources, Data analysis, Visualization, Writing–Review and Editing. N.A. : Investigation, Formal Analysis, Visualization, Writing., O.H.: Investigation, Visualization, Writing–Original Draft Preparation, Supervision, R.S.: Visualization, Writing–Review and Editing B.S.: Investigation, Data Analysis, Visualization, Writing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
This article does not contain any studies with human or animal subjects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mostafa, M., Abdelhakim, N.A., Hemeda, O.M. et al. Incorporation of Magnetic Filler (Co0.2Zn0.2Cd0.6Fe2O4) in PVA Matrix and Studying the Structural, Optical and Mechanical Properties of Nanocomposite Films. J Inorg Organomet Polym (2024). https://doi.org/10.1007/s10904-024-03045-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10904-024-03045-z