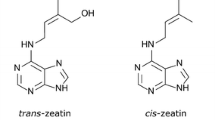
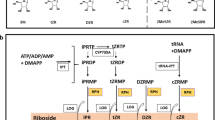
Abstract
The review examines the main stages of cytokinin biosynthesis and metabolism with an emphasis on the important role of cytokinin oxidase/dehydrogenase (CKO/CKX) in cytokinin degradation. In this context, arguments are made for the crucial importance of this enzyme in maintaining a balanced level of cytokinins in plants. The role of CKX genes encoding cytokinin oxidase/dehydrogenase in determining plant resistance to abiotic stress factors and their yield was analyzed. The molecular genetic ways of regulating the activity of CKX genes are characterized. The results of research on the regulation of CKO/CKX activity in increasing the resistance to abiotic stress and crop yield are summarized and the biotechnological ways of realizing such opportunities are described. Prospects for finding substances that inhibit CKO/CKX activity to create preparations for agriculture are outlined separately. Prospective chemical inhibitors of CKO/CKX and their effects on cultivated plants are considered.
Similar content being viewed by others
REFERENCES
Akhtar, S.S., Mekureyaw, M.F., Pandey, C., and Roitsch, T., Role of cytokinins for interactions of plants with microbial pathogens and pest insects, Front. Plant Sci., vol. 10, p. 1777. https://doi.org/10.3389/fpls.2019.01777
Andreas, P., Kisiala, A., Emery, R.J.N., et al., Cytokinins are abundant and widespread among insect species, Plants, 2020, vol. 9, p. 208. https://doi.org/10.3390/plants9020208
Aremu, A.O., Masondo, N.A., Sunmonu, T.O., et al., A novel inhibitor of cytokinin degradation (INCYDE) influences the biochemical parameters and photosynthetic apparatus in NaCl-stressed tomato plants, Planta, 2014, vol. 240, pp. 877–889. https://doi.org/10.1007/s00425-014-2126-y
Arkhipov, D.V., Lomin, S.N., Myakushina, Y.A., Savelieva, E.M., Osolodkin, D.I., and Romanov, G.A., Modeling of protein–protein interactions in cytokinin signal transduction, Int. J. Mol. Sci., 2019, vol. 20, pp. 2096–2118. https://doi.org/10.3390/ijms20092096
Artner, C. and Benkova, E., Ethylene and cytokinin: partners in root growth regulation, Mol. Plant, 2019, vol. 12, pp. 1312–1314. https://doi.org/10.1016/j.molp.2019.09.003
Ashikari, M., Sakakibara, H., Lin, S., et al., Cytokinin oxidase regulates rice grain production, Science, 2005, vol. 309, pp. 741–745. https://doi.org/10.1126/science.1113373
Astot, C., Dolezal, K., Nordstrom, A., et al., An alternative cytokinin biosynthesis pathway, Proc. Natl. Acad. Sci. U. S. A., 2000, vol. 97, pp. 14778–14783. https://doi.org/10.1073/pnas.260504097
Bae, E., Bingman, C.A., Bitto, E., et al., Crystal structure of Arabidopsis thaliana cytokinin dehydrogenase, Proteins: Struct. Funct. Bioinf., 2007, vol. 70, pp. 303–306. https://doi.org/10.1002/prot.21678
Bartrina, I., Otto, E., Strnad, M., et al., Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus, seed yield in Arabidopsis thaliana, Plant Cell, 2011, vol. 23, pp. 69–80. https://doi.org/10.1105/tpc.110.079079
Bilyeu, K.D., Cole, J.L., Esparza, T.J., et al., Molecular and biochemical characterization of a cytokinin oxidase from maize, Plant Physiol., 2001, vol. 125, pp. 378–386. https://doi.org/10.1104/pp.125.1.378
Blume, R., Yemets, A., Korkhovyi, V., Radchuk, V., et al., Genome-wide identification and analysis of the cytokinin oxidase/dehydrogenase (ckx) gene family in finger millet (Eleusine coracana), Front. Genet., 2022, vol. 13, p. 963789. https://doi.org/10.3389/fgene.2022.963789
Brugière, N., Shuping, J., Hanke, S., et al., Cytokinin oxidase gene expression in maize is localized to the vasculature, and is induced by cytokinins, abscisic acid, and abiotic stress, Plant Physiol., 2003, vol. 132, pp. 1228–1240. https://doi.org/10.1104/pp.102.017707
Brugière, N., Bohn, J., Humbert, S., et al., A member of the maize isopentenyl transferase gene family, Zea mays isopentenyl transferase 2 (ZmIPT2), encodes a cytokinin biosynthetic enzyme expressed during kernel development, Plant Mol. Biol., 2008, vol. 67, pp. 215–229. https://doi.org/10.1007/s11103-008-9312-x
Chen, L., Zhao, J., Song, J., and Jameson, P.E., Cytokinin dehydrogenase: A genetic target for yield improvement in wheat, Plant Biotechnol. J., 2020, vol. 18, pp. 614–630. https://doi.org/10.1111/pbi.13305
Czajkowska, B.I., Finlay, C.M., Jones, G., and Brown, T.A., Diversity of a cytokinin dehydrogenase gene in wild and cultivated barley, PLoS One, 2019, vol. 14, p. e0225899. https://doi.org/10.1371/journal.pone.225899
Dabravolski, S.A. and Isayenkov, S.V., Evolution of the cytokinin dehydrogenase (CKX) domain. J. Mol. Evol., 2021, vol. 89, pp. 665–677. https://doi.org/10.1007/s00239-021-10035-z
Debi, B.R., Taketa, S., and Ichii, M., Cytokinin inhibits lateral root initiation but stimulates lateral root elongation in rice (Oryza sativa), J. Plant Physiol., 2005, vol. 162, no. 5, pp. 507–515. https://doi.org/10.1016/j.jplph.2004.08.007
Frébort, I., Šebela, M., Galuszka, P., et al., Cytokinin oxidase/cytokinin dehydrogenase assay: Optimized procedures and applications, Anal. Biochem., 2002, vol. 306, pp. 1–7. https://doi.org/10.1006/abio.2002.5670
Frébort, I., Kowalska, M., Hluska, T., et al., Evolution of cytokinin biosynthesis and degradation, J. Exp. Bot., 2011, vol. 62, pp. 2431–2452. https://doi.org/10.1093/jxb/err004
Frébortová, J., Galuszka, P., Werner, T., et al., Functional expression and purification of cytokinin dehydrogenase from Arabidopsis thaliana (AtCKX2) in Saccharomyces cerevisiae, Biol. Plant., 2007, vol. 51, pp. 673–682. https://doi.org/10.1007/s10535-007-0141-6
Galuszka, P., Frébort, I., Šebela, M., et al., Cytokinin oxidase or dehydrogenease? Mechanism of cytokinin degradation in cereals, Eur. J. Biochem., 2001, vol. 268, pp. 450–461. https://doi.org/10.1046/j.1432-1033.2001.01910.x
Galuszka, P., Popelková, H., Werner, T., et al., Biochemical characterization of cytokinin oxidases/dehydrogenases from Arabidopsis thaliana expressed in Nicotiana tabacum L., J. Plant Growth Regul., 2007, vol. 26, pp. 255–267. https://doi.org/10.1007/s00344-007-9008-5
Gasparis, S., Przyborowski, M., Kała, M., and Nadolska-Orczyk, A., Knockout of the HvCKX1 or HvCKX3 gene in barley (Hordeum vulgare L.) by RNA-Guided Cas9 nuclease affects the regulation of cytokinin metabolism and root morphology, Cells, 2019, vol. 8, p. 782. https://doi.org/10.3390/cells8080782
Gruhn, N. and Heyl, A., Updates on the model and the evolution of cytokinin signaling, Curr. Opin. Plant Biol., 2013, vol. 16, pp. 569–574. https://doi.org/10.1016/j.pbi.2013.09.001
Hinsch, J., Vrabka, J., Oeser, B., et al., De novo biosynthesis of cytokinins in the biotrophic fungus Claviceps purpurea, Environ. Microbiol., 2015, vol. 17, pp. 2935–2951. https://doi.org/10.1111/1462-2920.12838
Holubová, K., Hensel, G., Vojta, P., et al., Modification of barley plant productivity through regulation of cytokinin content by reverse-genetics approaches, Front. Plant Sci., 2018, vol. 871, p. 1676. https://doi.org/10.3389/fpls.2018.01676
Hwang, I. and Sheen, J., Two-component circuitry in Arabidopsis cytokinin signal transduction, Nature, 2001, vol. 413, pp. 383–389. https://doi.org/10.1038/35096500
Hwang, I., Sheen, J., and Müller, B., Cytokinin signaling networks, Annu. Rev. Plant Biol., 2012, vol. 63, pp. 353–380. https://doi.org/10.1146/annurev-arplant-042811-105503
Jabłoński, B., Ogonowska, H., Szala, K., et al., Silencing of TaCKX1 mediates expression of other TaCKX genes to increase yield parameters in wheat, Int. J. Mol. Sci., 2020, vol. 21, p. 4809. https://doi.org/10.3390/ijms21134809
Jabłoński, B., Szala, K., Przyborowski, M., Bajguz, A., Chmur, M., Gasparis, S., Orczyk, W., and Nadolska-Orczyk, A., TaCKX2.2 genes coordinate expression of other TaCKX family members, regulate phytohormone content and yield-related traits of wheat, Int. J. Mol. Sci., 2021, vol. 16, no. 8, p. 4142. https://doi.org/10.3390/ijms22084142
Jones, R.J. and Schreiber, B.M.N., Role and function of cytokinin oxidase in plants, Plant Growth Regul., 1997, vol. 23, pp. 123–134. https://doi.org/10.1023/A:1005913311266
Kakimoto, T., Biosynthesis of cytokinins, J. Plant Res., 2003, vol. 116, pp. 233–239. https://doi.org/10.1007/s10265-003-0095-5
Khablak, S.G. and Abdullaeva, Y.A., Features of the structure and development of root systems in plants of mutant lines ahk2-5 and ahk3-7 Arabidopsis thaliana (L.) Heynh, Bull. Odessa Natl. Univ., Ser. Biol., 2012, vol. 17, pp. 58–68.
Kieber, J.J., Cytokinin signaling: two-components and more, Trends Plant Sci., 2008, vol. 13, pp. 85–92. https://doi.org/10.1016/j.tplants.2007.11.005
Kieber, J.J. and Schaller, G.E., Cytokinin signaling in plant development, Development, 2018, vol. 145, p. 149344. https://doi.org/10.1242/dev.149344
Kopečný, D., Briozzo, P., Popelková, H., et al., Phenyl- and benzylurea cytokinins as competitive inhibitors of cytokinin oxidase/dehydrogenase: A structural study, Biochimie, 2010, vol. 92, pp. 1052–1062. https://doi.org/10.1016/j.biochi.2010.05.006
Kosakivska, I.V., Vedenicheva, N.P., Babenko, L.M., et al., Exogenous phytohormones in the regulation of growth and development of cereals under abiotic stresses, Mol. Biol. Rep., 2022, vol. 49, pp. 617–628. https://doi.org/10.1007/s11033-021-06802-2
Krall, L., Raschke, M., Zenk, M.H., and Baron, C., The Tzs protein from Agrobacterium tumefaciens C58 produces zeatin riboside 5’-phosphate from 4-hydroxy-3-methyl-2-(E)-butenyl diphosphate and AMP, FEBS Lett., 2002, vol. 527, pp. 315–318. https://doi.org/10.1016/S0014-5793(02)03258-1
Li, S., An, Y., Hailati, S., et al., Overexpression of the cytokinin oxidase/dehydrogenase (CKX) from Medicago sativa enhanced salt stress tolerance of Arabidopsis, J. Plant Biol., 2019, vol. 62, pp. 374–386. https://doi.org/10.1007/s12374-019-0141-z
Li, S.M., Zheng, H.X., Zhang, X.S., et al., Cytokinins as central regulators during plant growth and stress response, Plant Cell Rep., 2021, vol. 40, pp. 271–282. https://doi.org/10.1007/s00299-020-02612-1
Mandal, S., Ghoraiet, M., et al., Cytokinins: A genetic target for increasing yield potential in the CRISPR era, Front. Genet., 2022, vol. 13. https://doi.org/10.3389/fgene.2022.883930
Niemann, M.C.E., Weber, H., Hluska, T., et al., The cytokinin oxidase/dehydrogenase CKX1 is a membrane-bound protein requiring homooligomerization in the endoplasmic reticulum for its cellular activity, Plant Physiol., 2018, vol. 176, pp. 2024–2039. https://doi.org/10.1104/pp.17.00925
Nisler, J., Kopečný, D., Končitíková, R., et al., Novel thidiazuron-derived inhibitors of cytokinin oxidase/dehydrogenase, Plant Mol. Biol., 2016, vol. 92, nos. 1–2, pp. 235–248. https://doi.org/10.1007/s11103-016-0509-0
Nisler, J., Kopečný, D., Pěkná, Z., et al., Diphenylureaderived cytokinin oxidase/dehydrogenase inhibitors for biotechnology and agriculture, J. Exp. Bot., 2020, vol. 72, pp. 355–370. https://doi.org/10.1093/jxb/eraa437
O’Keefe, D., Song, J., and Jameson, P.E., Isopentenyl transferase and cytokinin oxidase/dehydrogenase gene family members are differentially expressed during pod and seed development in rapid-cycling Brassica, J. Plant Growth Regul., 2011, vol. 30, pp. 92–99. https://doi.org/10.1007/s00344-010-9171-y
Ongaro, V. and Leyser, O., Hormonal control of shoot branching, J. Exp. Bot., 2008, vol. 59, pp. 67–74. https://doi.org/10.1093/jxb/erm134
Panda, B.B., Sekhar, S., Dash, S.K., et al., Biochemical and molecular characterisation of exogenous cytokinin application on grain filling in rice, BMC Plant Biol., 2018, vol. 18, p. 89. https://doi.org/10.1186/s12870-018-1279-4
Pils, B. and Heyl, A., Unraveling the evolution of cytokinin signaling, Plant Physiol., 2009, vol. 151, pp. 782–791. https://doi.org/10.1104/pp.109.139188
Radchuk, V., Radchuk, R., Pirko, Y., Vankova, R., Gaudinova, A., Korkhovoy, V., Yemets, A., Weber, H., Weschke, W., and Blume, Ya.B., A somaclonal line SE7 of finger millet (Eleusine coracana) exhibits modified cytokinin homeostasis and increased grain yield, J. Exp. Bot., 2012, vol. 63, pp. 5497–5506. https://doi.org/10.1093/jxb/ers200
Ramireddy, E., Chang, L., and Schmülling, T., Cytokinin as a mediator for regulating root system architecture in response to environmental cues, Plant Signal. Behav., 2014, vol. 9, no. 1. https://doi.org/10.4161/psb.27771
Ren, B., Liang, Y., Deng, Y., et al., Genome-wide comparative analysis of type-A Arabidopsis response regulator genes by overexpression studies reveals their diverse roles and regulatory mechanisms in cytokinin signaling, Cell Res., vol. 19, pp. 1178–1190. https://doi.org/10.1038/cr.2009.88
Rong, X., Sang, Y., Wang, L., et al., Type-B ARRs control carpel regeneration through mediating AGAMOUS expression in Arabidopsis, Plant Cell Physiol., 2018, vol. 59, pp. 761–769. https://doi.org/10.1093/pcp/pcx187
Schmülling, T., New insights into the functions of cytokinins in plant development, J. Plant Growth Regul., 2002, vol. 21, pp. 40–49.
Schmülling, T., Werner, T., Riefler, M., et al., Structure and function of cytokinin oxidase/dehydrogenase genes of maize, rice, Arabidopsis and other species, J. Plant Res., 2003, vol. 116, pp. 241–252. https://doi.org/10.1007/s10265-003-0096-4
Shimizu-Sato, S., Tanaka, M., and Mori, H., Auxincytokinin interactions in the control of shoot branching, Plant Mol. Biol., 2009, vol. 69, p. 429. https://doi.org/10.1007/s11103-008-9416-3
Todorova, D., Vaseva-Gemisheva, I., Petrov, P., et al., Cytokinin oxidase/dehydrogenase (CKX) activity in wild and ethylene-insensitive mutant eti5 type of Arabidopsis thaliana (L.) Heynh plants and the effect of cytokinin N1-(2-chloro-4-pyridyl)-N2-phenylurea on enzymatic activity and leaf morphology, Acta Physiol. Plant., 2006, vol. 28, pp. 613–617. https://doi.org/10.1007/s11738-006-0057-3
van Voorthuizen, M.J., Nisler, J., Jiancheng Song, Lukáš Spíchal, and Jameson, P.E., Targeting cytokinin homeostasis in rapid cycling Brassica rapa with plant growth regulators INCYDE and TD-K, Plants, 2021, vol. 10, no. 1, p. 39. https://doi.org/10.3390/plants10010039
Vaseva, I., Todorova, D., Malbeck, J., et al., Response of cytokinin pool and cytokinin oxidase/dehydrogenase activity to abscisic acid exhibits organ specificity in peas, Acta Physiol. Plant., 2008, vol. 30, pp. 151–155. https://doi.org/10.1007/s11738-007-0103-9
Vaseva-Gemisheva, I., Lee, D., Karanov, E., et al., Response of Pisum sativum cytokinin oxidase/dehydrogenase expression and specific activity to drought stress and herbicide treatments, Plant Growth Regul., 2005, vol. 46, pp. 199–208. https://doi.org/10.1007/s10725-005-0143-3
Wang, X., Ding, J., Lin, S., et al., Evolution and roles of cytokinin genes in angiosperms 2: Do ancient CKXs play housekeeping roles while non-ancient CKXs play regulatory roles?, Hortic. Res., 2020, vol. 7, p. 29. https://doi.org/10.1038/s41438-020-0246-z
Werner, T., Motyka, V., Strand, M., and Schmülling, T., Regulation of plant growth by cytokinin, Proc. Natl. Acad. Sci. U. S. A., 2001, vol. 98, no. 18, pp. 10487–10492. https://doi.org/10.1073/pnas.171304098
Werner, T., Motyka, V., Laucou, V., et al., Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity, Plant Cell, 2003, vol. 15, no. 11, pp. 2532–2550. https://doi.org/10.1105/tpc.014928
Werner, T., Nehnevajova, E., Kollmer, I., et al., Rootspecific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco, Plant Cell, 2010, vol. 22, pp. 3905–3920. https://doi.org/10.1105/tpc.109.072694
Ye, C., Wu, S., Kong, F., et al., Identification and characterization of an isopentenyltransferase (IPT) gene in soybean (Glycine max L.), Plant Sci., 2006 vol. 170, pp. 542–550. https://doi.org/10.1016/j.plantsci.2005.10.008
Yeh, S.-Y., Chen, H.-W., Ng, C.-Y., et al., Down-regulation of cytokinin oxidase 2 expression increases tiller number and improves rice yield, Rice, 2015, vol. 8, p. 36. https://doi.org/10.1186/s12284-015-0070-5
Zatloukal, M., Gemrotová, M., Doležal, K., et al., Novel potent inhibitors of A. thaliana cytokinin oxidase/dehydrogenase, Bioorg. Med. Chem., 2008, vol. 16, no. 20, pp. 9268–9275. https://doi.org/10.1016/j.bmc.2008.09.008
Zubko, E., Adams, C.J., Machaekova, I., et al., Activation tagging identifies a gene from Petunia hybrida responsible for the production of active cytokinins in plants, Plant J., 2002, vol. 29, pp. 797–808. https://doi.org/10.1046/j.1365-313X.2002.01256.x
Funding
The study was supported by the budget project of the National Academy of Sciences of Ukraine (state registration number 0120U100937, 2020-24).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human and animal subjects.
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Allerton Press remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Khablak, S.H., Spivak, S.I., Pastukhova, N.L. et al. Cytokinin Oxidase/Dehydrogenase as an Important Target for Increasing Plant Productivity. Cytol. Genet. 58, 115–125 (2024). https://doi.org/10.3103/S0095452724020051
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452724020051