Abstract
Purpose
The proximate localization of MTAP, which encodes methylthioadenosine phosphorylase, and CDKN2A/B on Chromosome 9q21 has allowed the loss of MTAP expression as a surrogate for homozygous deletion of CDKN2A/B. This study aimed to determine whether MTAP status correlates with clinical outcomes and 11C-methionine uptake in astrocytomas with IDH mutations.
Methods
We conducted immunohistochemistry for MTAP in 30 patients with astrocytoma, IDH-mutant who underwent 11C-methionine positron emission tomography scans prior to surgical resection. The tumor-to-normal (T/N) ratio of 11C-methionine uptake was calculated using the mean standardized uptake value (SUV) for tumor and normal brain tissues. Cox regression analysis was used for multivariate survival analysis.
Results
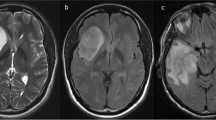
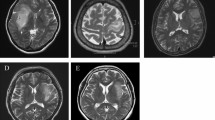
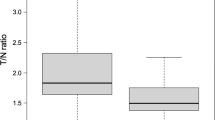
Among IDH-mutant astrocytomas, 26.7% (8/30) exhibited the loss of cytoplasmic MTAP expression, whereas 73.3% (22/30) tumors retained MTAP expression. The median progression-free survival (PFS) was significantly shorter in patients with MTAP loss than those with MTAP retention (1.88 years vs. 6.80 years, p = 0.003). The median overall survival (OS) was also shorter in patients with MTAP loss than in MTAP-retaining counterparts (5.23 years vs. 10.69 years, p = 0.019). Multivariate analysis identified MTAP status (hazard ratio (HR), 0.081) and extent of resection (HR, 0.104) as independent prognostic factors for PFS. Astrocytomas lacking cytoplasmic MTAP expression showed a significantly higher median T/N ratio for 11C-methionine uptake than tumors retaining MTAP (2.12 vs. 1.65, p = 0.012).
Conclusion
Our study revealed that the loss of MTAP expression correlates with poor prognosis and an elevated T/N ratio of 11C-methionine uptake in astrocytoma, IDH-mutant.



Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251. https://doi.org/10.1093/neuonc/noab106
Appay R, Dehais C, Maurage CA, Alentorn A, Carpentier C, Colin C, Ducray F, Escande F, Idbaih A, Kamoun A, Marie Y, Mokhtari K, Tabouret E, Trabelsi N, Uro-Coste E, Delattre JY, Figarella-Branger D, Network P (2019) CDKN2A homozygous deletion is a strong adverse prognosis factor in diffuse malignant IDH-mutant gliomas. Neuro Oncol 21:1519–1528. https://doi.org/10.1093/neuonc/noz124
Shirahata M, Ono T, Stichel D, Schrimpf D, Reuss DE, Sahm F, Koelsche C, Wefers A, Reinhardt A, Huang K, Sievers P, Shimizu H, Nanjo H, Kobayashi Y, Miyake Y, Suzuki T, Adachi JI, Mishima K, Sasaki A, Nishikawa R, Bewerunge-Hudler M, Ryzhova M, Absalyamova O, Golanov A, Sinn P, Platten M, Jungk C, Winkler F, Wick A, Hanggi D, Unterberg A, Pfister SM, Jones DTW, van den Bent M, Hegi M, French P, Baumert BG, Stupp R, Gorlia T, Weller M, Capper D, Korshunov A, Herold-Mende C, Wick W, Louis DN, von Deimling A (2018) Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol 136:153–166. https://doi.org/10.1007/s00401-018-1849-4
Jeuken J, Cornelissen S, Boots-Sprenger S, Gijsen S, Wesseling P (2006) Multiplex ligation-dependent probe amplification: a diagnostic tool for simultaneous identification of different genetic markers in glial tumors. J Mol Diagn 8:433–443. https://doi.org/10.2353/jmoldx.2006.060012
Chapel DB, Schulte JJ, Berg K, Churg A, Dacic S, Fitzpatrick C, Galateau-Salle F, Hiroshima K, Krausz T, Le Stang N, McGregor S, Nabeshima K, Husain AN (2020) MTAP immunohistochemistry is an accurate and reproducible surrogate for CDKN2A fluorescence in situ hybridization in diagnosis of malignant pleural mesothelioma. Mod Pathol 33:245–254. https://doi.org/10.1038/s41379-019-0310-0
Hida T, Hamasaki M, Matsumoto S, Sato A, Tsujimura T, Kawahara K, Iwasaki A, Okamoto T, Oda Y, Honda H, Nabeshima K (2017) Immunohistochemical detection of MTAP and BAP1 protein loss for mesothelioma diagnosis: comparison with 9p21 FISH and BAP1 immunohistochemistry. Lung Cancer 104:98–105. https://doi.org/10.1016/j.lungcan.2016.12.017
Kinoshita Y, Hamasaki M, Yoshimura M, Matsumoto S, Sato A, Tsujimura T, Ueda H, Makihata S, Kato F, Iwasaki A, Nabeshima K (2018) A combination of MTAP and BAP1 immunohistochemistry is effective for distinguishing sarcomatoid mesothelioma from fibrous pleuritis. Lung Cancer 125:198–204. https://doi.org/10.1016/j.lungcan.2018.09.019
Hustinx SR, Leoni LM, Yeo CJ, Brown PN, Goggins M, Kern SE, Hruban RH, Maitra A (2005) Concordant loss of MTAP and p16/CDKN2A expression in pancreatic intraepithelial neoplasia: evidence of homozygous deletion in a noninvasive precursor lesion. Mod Pathol 18:959–963. https://doi.org/10.1038/modpathol.3800377
Hustinx SR, Hruban RH, Leoni LM, Iacobuzio-Donahue C, Cameron JL, Yeo CJ, Brown PN, Argani P, Ashfaq R, Fukushima N, Goggins M, Kern SE, Maitra A (2005) Homozygous deletion of the MTAP gene in invasive adenocarcinoma of the pancreas and in periampullary cancer: a potential new target for therapy. Cancer Biol Ther 4:83–86. https://doi.org/10.4161/cbt.4.1.1380
Powell EL, Leoni LM, Canto MI, Forastiere AA, Iocobuzio-Donahue CA, Wang JS, Maitra A, Montgomery E (2005) Concordant loss of MTAP and p16/CDKN2A expression in gastroesophageal carcinogenesis: evidence of homozygous deletion in esophageal noninvasive precursor lesions and therapeutic implications. Am J Surg Pathol 29:1497–1504. https://doi.org/10.1097/01.pas.0000170349.47680.e8
Su CY, Chang YC, Chan YC, Lin TC, Huang MS, Yang CJ, Hsiao M (2014) MTAP is an independent prognosis marker and the concordant loss of MTAP and p16 expression predicts short survival in non-small cell lung cancer patients. Eur J Surg Oncol 40:1143–1150. https://doi.org/10.1016/j.ejso.2014.04.017
de Oliveira SF, Ganzinelli M, Chila R, Serino L, Maciel ME, Urban Cde A, de Lima RS, Cavalli IJ, Generali D, Broggini M, Damia G, Ribeiro EM (2016) Characterization of MTAP gene expression in breast cancer patients and cell lines. PLoS One 11:e0145647. https://doi.org/10.1371/journal.pone.0145647
Bataille F, Rogler G, Modes K, Poser I, Schuierer M, Dietmaier W, Ruemmele P, Muhlbauer M, Wallner S, Hellerbrand C, Bosserhoff AK (2005) Strong expression of methylthioadenosine phosphorylase (MTAP) in human colon carcinoma cells is regulated by TCF1/[beta]-catenin. Lab Invest 85:124–136. https://doi.org/10.1038/labinvest.3700192
Olopade OI, Pomykala HM, Hagos F, Sveen LW, Espinosa R, 3rd, Dreyling MH, Gursky S, Stadler WM, Le Beau MM, Bohlander SK (1995) Construction of a 2.8-megabase yeast artificial chromosome contig and cloning of the human methylthioadenosine phosphorylase gene from the tumor suppressor region on 9p21. Proc Natl Acad Sci U S A 92: 6489-6493.https://doi.org/10.1073/pnas.92.14.6489
Satomi K, Ohno M, Matsushita Y, Takahashi M, Miyakita Y, Narita Y, Ichimura K, Yoshida A (2021) Utility of methylthioadenosine phosphorylase immunohistochemical deficiency as a surrogate for CDKN2A homozygous deletion in the assessment of adult-type infiltrating astrocytoma. Mod Pathol 34:688–700. https://doi.org/10.1038/s41379-020-00701-w
Nariai T, Tanaka Y, Wakimoto H, Aoyagi M, Tamaki M, Ishiwata K, Senda M, Ishii K, Hirakawa K, Ohno K (2005) Usefulness of L-[methyl-11C] methionine-positron emission tomography as a biological monitoring tool in the treatment of glioma. J Neurosurg 103:498–507. https://doi.org/10.3171/jns.2005.103.3.0498
Nojiri T, Nariai T, Aoyagi M, Senda M, Ishii K, Ishiwata K, Ohno K (2009) Contributions of biological tumor parameters to the incorporation rate of L: -[methyl-(11)C] methionine into astrocytomas and oligodendrogliomas. J Neurooncol 93:233–241. https://doi.org/10.1007/s11060-008-9767-2
Ogishima T, Tamura K, Kobayashi D, Inaji M, Hayashi S, Tamura R, Nariai T, Ishii K, Maehara T (2017) ATRX status correlates with 11 C-methionine uptake in WHO grade II and III gliomas with IDH1 mutations. Brain Tumor Pathol 34:20–27. https://doi.org/10.1007/s10014-017-0280-1
Nakano T, Tamura K, Tanaka Y, Inaji M, Hayashi S, Kobayashi D, Nariai T, Toyohara J, Ishii K, Maehara T (2018) Usefulness of (11)C-methionine positron emission tomography for monitoring of treatment response and recurrence in a glioblastoma patient on bevacizumab therapy: A case report. Case Rep Oncol 11:442–449. https://doi.org/10.1159/000490457
Shimizu Y, Suzuki M, Akiyama O, Ogino I, Matsushita Y, Satomi K, Yanagisawa S, Ohno M, Takahashi M, Miyakita Y, Narita Y, Ichimura K, Kondo A (2023) Utility of real-time polymerase chain reaction for the assessment of CDKN2A homozygous deletion in adult-type IDH-mutant astrocytoma. Brain Tumor Pathol 40:93–100. https://doi.org/10.1007/s10014-023-00450-z
Takeuchi K, Choi YL, Togashi Y, Soda M, Hatano S, Inamura K, Takada S, Ueno T, Yamashita Y, Satoh Y, Okumura S, Nakagawa K, Ishikawa Y, Mano H (2009) KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res 15:3143–3149. https://doi.org/10.1158/1078-0432.Ccr-08-3248
Kanda Y (2013) Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant 48:452–458. https://doi.org/10.1038/bmt.2012.244
Menezes WP, Silva VAO, Gomes INF, Rosa MN, Spina MLC, Carloni AC, Alves ALV, Melendez M, Almeida GC, Silva LSD, Clara C, da Cunha IW, Hajj GNM, Jones C, Bidinotto LT, Reis RM (2020) Loss of 5'-methylthioadenosine phosphorylase (MTAP) is frequent in high-grade gliomas; nevertheless, it is not associated with higher tumor aggressiveness. Cells 9. https://doi.org/10.3390/cells9020492
Hansen LJ, Sun R, Yang R, Singh SX, Chen LH, Pirozzi CJ, Moure CJ, Hemphill C, Carpenter AB, Healy P, Ruger RC, Chen CJ, Greer PK, Zhao F, Spasojevic I, Grenier C, Huang Z, Murphy SK, McLendon RE, Friedman HS, Friedman AH, Herndon JE 2nd, Sampson JH, Keir ST, Bigner DD, Yan H, He Y (2019) MTAP loss promotes stemness in glioblastoma and confers unique susceptibility to purine starvation. Cancer Res 79:3383–3394. https://doi.org/10.1158/0008-5472.CAN-18-1010
Li Z, Jin Y, Zou Q, Shi X, Wu Q, Lin Z, He Q, Huang G, Qi S (2021) Integrated genomic and transcriptomic analysis suggests KRT18 mutation and MTAP are key genetic alterations related to the prognosis between astrocytoma and glioblastoma. Ann Transl Med 9:713. https://doi.org/10.21037/atm-21-1317
Serrano M, Hannon GJ, Beach D (1993) A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 366:704–707. https://doi.org/10.1038/366704a0
Sherr CJ (1996) Cancer cell cycles. Science 274:1672–1677. https://doi.org/10.1126/science.274.5293.1672
Stott FJ, Bates S, James MC, McConnell BB, Starborg M, Brookes S, Palmero I, Ryan K, Hara E, Vousden KH, Peters G (1998) The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. EMBO J 17:5001–5014. https://doi.org/10.1093/emboj/17.17.5001
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N (2012) The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2:401–404. https://doi.org/10.1158/2159-8290.CD-12-0095
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6:11. https://doi.org/10.1126/scisignal.2004088
Ceccarelli M, Barthel FP, Malta TM, Sabedot TS, Salama SR, Murray BA, Morozova O, Newton Y, Radenbaugh A, Pagnotta SM, Anjum S, Wang J, Manyam G, Zoppoli P, Ling S, Rao AA, Grifford M, Cherniack AD, Zhang H, Poisson L, Carlotti CG Jr, Tirapelli DP, Rao A, Mikkelsen T, Lau CC, Yung WK, Rabadan R, Huse J, Brat DJ, Lehman NL, Barnholtz-Sloan JS, Zheng S, Hess K, Rao G, Meyerson M, Beroukhim R, Cooper L, Akbani R, Wrensch M, Haussler D, Aldape KD, Laird PW, Gutmann DH, Network TR, Noushmehr H, Iavarone A, Verhaak RG (2016) Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell 164:550–563. https://doi.org/10.1016/j.cell.2015.12.028
Varn FS, Johnson KC, Martinek J, Huse JT, Nasrallah MP, Wesseling P, Cooper LAD, Malta TM, Wade TE, Sabedot TS, Brat D, Gould PV, Woehrer A, Aldape K, Ismail A, Sivajothi SK, Barthel FP, Kim H, Kocakavuk E, Ahmed N, White K, Datta I, Moon HE, Pollock S, Goldfarb C, Lee GH, Garofano L, Anderson KJ, Nehar-Belaid D, Barnholtz-Sloan JS, Bakas S, Byrne AT, D’Angelo F, Gan HK, Khasraw M, Migliozzi S, Ormond DR, Paek SH, Van Meir EG, Walenkamp AME, Watts C, Weiss T, Weller M, Palucka K, Stead LF, Poisson LM, Noushmehr H, Iavarone A, Verhaak RGW, Consortium G (2022) Glioma progression is shaped by genetic evolution and microenvironment interactions. Cell 185:2184-2199 e2116. https://doi.org/10.1016/j.cell.2022.04.038
Zaragoza R (2020) Transport of amino acids across the blood-brain barrier. Front Physiol 11:973. https://doi.org/10.3389/fphys.2020.00973
Nawashiro H, Otani N, Uozumi Y, Ooigawa H, Toyooka T, Suzuki T, Katoh H, Tsuzuki N, Ohnuki A, Shima K, Shinomiya N, Matsuo H, Kanai Y (2005) High expression of L-type amino acid transporter 1 in infiltrating glioma cells. Brain Tumor Pathol 22:89–91. https://doi.org/10.1007/s10014-005-0188-z
Singhal T, Narayanan TK, Jacobs MP, Bal C, Mantil JC (2012) 11C-methionine PET for grading and prognostication in gliomas: a comparison study with 18F-FDG PET and contrast enhancement on MRI. J Nucl Med 53:1709–1715. https://doi.org/10.2967/jnumed.111.102533
Grosu AL, Weber WA, Riedel E, Jeremic B, Nieder C, Franz M, Gumprecht H, Jaeger R, Schwaiger M, Molls M (2005) L-(methyl-11C) methionine positron emission tomography for target delineation in resected high-grade gliomas before radiotherapy. Int J Radiat Oncol Biol Phys 63:64–74. https://doi.org/10.1016/j.ijrobp.2005.01.045
Herholz K, Holzer T, Bauer B, Schroder R, Voges J, Ernestus RI, Mendoza G, Weber-Luxenburger G, Lottgen J, Thiel A, Wienhard K, Heiss WD (1998) 11C-methionine PET for differential diagnosis of low-grade gliomas. Neurology 50:1316–1322. https://doi.org/10.1212/wnl.50.5.1316
Matsuo M, Miwa K, Tanaka O, Shinoda J, Nishibori H, Tsuge Y, Yano H, Iwama T, Hayashi S, Hoshi H, Yamada J, Kanematsu M, Aoyama H (2012) Impact of [11C]methionine positron emission tomography for target definition of glioblastoma multiforme in radiation therapy planning. Int J Radiat Oncol Biol Phys 82:83–89. https://doi.org/10.1016/j.ijrobp.2010.09.020
Palanichamy K, Thirumoorthy K, Kanji S, Gordon N, Singh R, Jacob JR, Sebastian N, Litzenberg KT, Patel D, Bassett E, Ramasubramanian B, Lautenschlaeger T, Fischer SM, Ray-Chaudhury A, Chakravarti A (2016) Methionine and kynurenine activate oncogenic kinases in glioblastoma, and methionine deprivation compromises proliferation. Clin Cancer Res 22:3513–3523. https://doi.org/10.1158/1078-0432.CCR-15-2308
Ishiwata K, Kubota K, Murakami M, Kubota R, Sasaki T, Ishii S, Senda M (1993) Re-evaluation of amino acid PET studies: can the protein synthesis rates in brain and tumor tissues be measured in vivo? J Nucl Med 34:1936–1943
Acknowledgements
We would like to thank Dr. Kaishi Satomi (Kyorin University) for invaluable advice on MTAP immunohistochemistry, Dr. Hiroyuki Sato and Dr. Akihiro Hirakawa (Tokyo Medical and Dental University) for invaluable advice on the statistical analysis, Dr. Koichi Ichimura (Juntendo University) for CDKN2A analysis and Editage (www.edtage.jp) for English language editing.
Funding
This work was supported by JSPS KAKENHI Grant Number 20K09321.
Author information
Authors and Affiliations
Contributions
The study concept and design were conceived by Toshihiro Yamamura, Kaoru Tamura, Daisuke Kobayashi, Motoki Inaji, Hiroaki Wakimoto and Taketoshi Maehara. Material preparation and data collection were performed by Toshihiro Yamamura, Kaoru Tamura, Daisuke Kobayashi, Motoki Inaji, Yuka Toyama, Juri Kiyokawa, Shoko Hara, Yoji Tanaka, Tadashi Nariai, Kazuhide Shimizu, Kenji Ishii, and Taketoshi Maehara. Analysis was performed by Toshihiro Yamamura, Kaoru Tamura, Daisuke Kobayashi, Motoki Inaji, Hiroaki Wakimoto, Juri Kiyokawa, Shoko Hara, Yoji Tanaka, Tadashi Nariai, Kazuhide Shimizu, and Taketoshi Maehara. The first draft of the manuscript was written by Toshihiro Yamamura and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Medical Ethics Committee of the University Hospital of Tokyo Medical and Dental University in Tokyo, Japan (Protocol #M2000-1307).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11060_2024_4661_MOESM1_ESM.jpg
Supplementary file1. Supplementary Fig.1 Chromosome 9 ideogram with an enlarged view of the 9q21 region showing the proximity of CDKN2A and MTAP in the genome (JPG 218 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yamamura, T., Tamura, K., Kobayashi, D. et al. Loss of methylthioadenosine phosphorylase immunoreactivity correlates with poor prognosis and elevated uptake of 11C-methionine in IDH-mutant astrocytoma. J Neurooncol (2024). https://doi.org/10.1007/s11060-024-04661-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11060-024-04661-y