Abstract
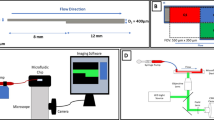
Blood flow disorders are often the result of the non-physiological narrowing of blood arteries caused by atherosclerosis and thrombus. The blood then proceeds through rising-peak-decreasing phases as it passes through the narrow area. Although abnormally high shear is known to activate platelets, the shear process that platelets undergo in small arteries is complex. Thus, understanding how each shear phase affects platelet activation can be used to improve antiplatelet therapy and decrease the risk of side effects like bleeding. Blood samples were sheared (68.8 ms,5200 s−1) in vitro by the microfluidic technique, and platelet activation levels (P-selectin and integrin αIIbβ3) and von Willebrand factor (vWF) binding to platelets were analyzed by flow cytometry. Post-stenosis platelet aggregation was dynamically detected using microfluidic technology. We studied TXA2, P2Y12-ADP, and integrin αIIbβ3-fibrinogen receptor pathways by adding antiplatelet drugs, such as acetylsalicylic acid (ASA, an active ingredient of aspirin that inhibits platelet metabolism), ticagrelor (hinders platelet activation), and tirofiban (blocks integrin αIIbβ3 receptor) in vitro, respectively, to determine platelet activation function mediated by transient non-physiological high shear rates. We demonstrated that platelets can be activated under transient pathological high shear rates. The shear rise and fall phases influenced shear-induced platelet activation by regulating the binding of vWF to platelets. The degree of platelet activation and aggregation increased with multiple shear rise and fall phases. ASA did not inhibit shear-mediated platelet activation, but ticagrelor and tirofiban effectively inhibited shear-mediated platelet activation. Our data demonstrated that the shear rise and fall phases play an important role in shear-mediated platelet activation and promote platelet activation and aggregation in a vWF-dependent manner. Blocking integrin αIIbβ3 receptor and hindering P2Y12-ADP were beneficial to reducing shear-mediated platelet activation.






Similar content being viewed by others
References
Meijden. PEJvd, Heemskerk JWM (2019) Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol 16(3):166–179. https://doi.org/10.1038/s41569-018-0110-0
Dib PRB, Quirino-Teixeira AC, Merij LB, Pinheiro MBM, Rozini SV, Andrade FB, Hottz ED (2020) Innate immune receptors in platelets and platelet‐leukocyte interactions. J Leukoc Biol 108(4):1157–1182. https://doi.org/10.1002/jlb.4mr0620-701r
Ding J, Chen Z, Niu S, Zhang J, Mondal NK, Griffith BP, Wu ZJ (2015) Quantification of Shear-Induced platelet activation: high shear stresses for short exposure time. Artif Organs 39(7):576–583. https://doi.org/10.1111/aor.12438
Chen Z, Koenig SC, Slaughter MS, Griffith BP, Wu ZJ (2018) Quantitative characterization of Shear-Induced platelet receptor shedding: glycoprotein ibalpha, glycoprotein VI, and glycoprotein IIb/IIIa. ASAIO J 64(6):773–778. https://doi.org/10.1097/MAT.0000000000000722
Al-Tamimi M, Tan CW, Qiao J, Pennings GJ, Javadzadegan A, Yong AS, Arthur JF, Davis AK, Jing J, Mu FT, Hamilton JR, Jackson SP, Ludwig A, Berndt MC, Ward CM, Kritharides L, Andrews RK, Gardiner EE (2012) Pathologic shear triggers shedding of vascular receptors: a novel mechanism for down-regulation of platelet glycoprotein VI in stenosed coronary vessels. Blood 119(18):4311–4320. https://doi.org/10.1182/blood-2011-10-386607
Maxwell MJ, Westein E, Nesbitt WS, Giuliano S, Dopheide SM, Jackson SP (2007) Identification of a 2-stage platelet aggregation process mediating shear-dependent thrombus formation. Blood 109(2):566–576. https://doi.org/10.1182/blood-2006-07-028282
Hanke J, Ranke C, Perego E, Koster S (2019) Human blood platelets contract in perpendicular direction to shear flow. Soft Matter 15(9):2009–2019. https://doi.org/10.1039/c8sm02136h
Sang Y, Roest M, De Laat B, De Groot PG, Huskens D (2021) Interplay between platelets and coagulation. Blood Rev 46100733. https://doi.org/10.1016/j.blre.2020.100733
Mariscal A, Zamora C, Magallares B, Salman-Monte TC, Ortiz MA, Diaz-Torne C, Castellvi I, Corominas H, Vidal S (2021) Phenotypic and functional consequences of PLT binding to Monocytes and its association with clinical features in SLE. Int J Mol Sci 22(9). https://doi.org/10.3390/ijms22094719
Pluta K, Porebska K, Urbanowicz T, Gasecka A, Olasinska-Wisniewska A, Targonski R, Krasinska A, Filipiak KJ, Jemielity M, Krasinski Z (2022) Platelet-leucocyte aggregates as novel biomarkers in Cardiovascular diseases. Biology (Basel) 11(2). https://doi.org/10.3390/biology11020224
Bester J, Pretorius E (2016) Effects of IL-1beta, IL-6 and IL-8 on erythrocytes, platelets and clot viscoelasticity. Sci Rep 632188. https://doi.org/10.1038/srep32188
Slepian MJ, Sheriff J, Hutchinson M, Tran P, Bajaj N, Garcia JGN, Scott Saavedra S, Bluestein D (2017) Shear-mediated platelet activation in the free flow: perspectives on the emerging spectrum of cell mechanobiological mechanisms mediating cardiovascular implant thrombosis. J Biomech 5020–5025. https://doi.org/10.1016/j.jbiomech.2016.11.016
Gidaro A, Delitala AP, Manetti R, Caccia S, Soloski MJ, Lambertenghi Deliliers G, Castro D, Donadoni M, Bartoli A, Sanna G, Bergamaschini L, Castelli R (2023) Platelet microvesicles, inflammation, and coagulation markers: a pilot study. Hematol Rep 15(4):684–695. https://doi.org/10.3390/hematolrep15040069
Lui M, Gardiner EE, Arthur JF, Pinar I, Lee WM, Ryan K, Carberry J, Andrews RK (2019) Novel stenotic microchannels to study Thrombus formation in Shear gradients: influence of Shear forces and Human platelet-related factors. Int J Mol Sci 20(12). https://doi.org/10.3390/ijms20122967
Bark DL Jr. DNK (2010) Wall shear over high degree stenoses pertinent to atherothrombosis. J Biomech 43(15):2970–2977. https://doi.org/10.1016/j.jbiomech.2010.07.011
Kamada H, Imai Y, Nakamura M, Ishikawa T, Yamaguchi T (2017) Shear-induced platelet aggregation and distribution of thrombogenesis at stenotic vessels. Microcirculation 24(4). https://doi.org/10.1111/micc.12355
Jesty J, Yin W, Perrotta P, Bluestein D (2003) Platelet activation in a circulating flow loop: combined effects of shear stress and exposure time. Platelets 14(3):143–149. https://doi.org/10.1080/0953710031000092839
Rahman SM, Hlady V (2021) Microfluidic assay of antiplatelet agents for inhibition of shear-induced platelet adhesion and activation. Lab Chip 21(1):174–183. https://doi.org/10.1039/d0lc00756k
Zhang T, Liu L, Huang X, Gao X, Chen D, Huan X, He C, Li Y (2022) Application of microfluidic chip technology to study the inhibitory effect of tetramethylpyrazine on platelet aggregation, activation, and phosphatidylserine exposure mediated by pathological high shear rate. Blood Coagul Fibrinolysis 001–14. https://doi.org/10.1097/MBC.0000000000001179
Frojmovic MM (1998) Platelet aggregation in flow: Differential roles for adhesive receptors and ligands. Am Heart J 135:S119–S131 ((5 Pt 2 Su))
Rahman SM, Hlady V (2019) Downstream platelet adhesion and activation under highly elevated upstream shear forces. Acta Biomater 91(0):135–143. https://doi.org/10.1016/j.actbio.2019.04.028
Chen Z, Mondal NK, Ding J, Koenig SC, Slaughter MS, Wu ZJ (2016) Paradoxical effect of Nonphysiological Shear Stress on platelets and von Willebrand Factor. Artif Organs 40(7):659–668. https://doi.org/10.1111/aor.12606
Chen Z, Zhang J, Li T, Tran D, Griffith BP, Wu ZJ (2020) The impact of shear stress on device-induced platelet hemostatic dysfunction relevant to thrombosis and bleeding in mechanically assisted circulation. Artif Organs 44(5):1–13. https://doi.org/10.1111/aor.13609
Shida Y, Swystun LL, Brown C, Mewburn J, Nesbitt K, Danisment O, Riches JJ, Hough C, Lillicrap D (2019) Shear stress and platelet-induced tensile forces regulate ADAMTS13-localization within the platelet thrombus. Res Pract Thromb Haemost 3(2):254–260. https://doi.org/10.1002/rth2.12196
Bark DL Jr., Para AN, Ku DN (2012) Correlation of thrombosis growth rate to pathological wall shear rate during platelet accumulation. Biotechnol Bioeng 109(10):2642–2650. https://doi.org/10.1002/bit.24537
Liu ZL, Bresette C, Aidun CK, Ku DN (2022) SIPA in 10 milliseconds: VWF tentacles agglomerate and capture platelets under high shear. Blood Adv 6(8):2453–2465. https://doi.org/10.1182/bloodadvances.2021005692
Schneider SW, Nuschele S, Wixforth A, Gorzelanny C, Alexander-Katz A, Netz RR, Schneider MF (2007) Shear-induced unfolding triggers adhesion of Von Willebrand factor fibers. Proc Natl Acad Sci U S A 104(19):7899–7903. https://doi.org/10.1073/pnas.0608422104
Koo B-K, Kang J, Park KW, Rhee T-M, Yang H-M, Won K-B, Rha S-W, Bae J-W, Lee NH, Hur S-H, Yoon J, Park T-H, Kim BS, Lim SW, Cho YH, Jeon DW, Kim S-H, Han J-K, Shin E-S, Kim H-S, Koo B-K, Kang J, Park KW, Rhee T-M, Lee H, Yang H-M, Won K-B, Rha S-W, Bae J-W, Lee NH, Hur S-H, Yoon J, Park T-H, Kim BS, Lim SW, Cho YH, Jeon DW, Kim S-H, Han J-K, Shin E-S, Kim H-S, Han K-R, Moon K-W, Oh SK, Kim U, Rhee M-Y, Kim D-I, Kim S-Y, Lee S-Y, Lee SU, Kim S-W, Kim SY, Jeon H-K, Cha KS, Jo S-H, Ryu JK, Suh I-W, Choi H-H, Woo S-I, Chae I-H, Shin W-Y, Kim D-K, Oh JH, Jeong MH, Kim YH (2021) Aspirin versus clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention (HOST-EXAM): an investigator-initiated, prospective, randomised, open-label, multicentre trial. Lancet 397(10293):2487–2496. https://doi.org/10.1016/s0140-6736(21)01063-1
Luckie M, Khattar RS, Fraser D (2009) Non-cardiac surgery and antiplatelet therapy following coronary artery stenting. Heart 95(16):1303–1308. https://doi.org/10.1136/hrt.2008.161273
Funding
National Natural Science Foundation of China (11702047); Joint project of Chongqing Health Commission and Science and Technology Bureau (2023GDRC008); Graduate Innovation Fund of Yongchuan Hospital affiliated to Chongqing Medical University (YJSCX202204).
Author information
Authors and Affiliations
Contributions
Xuemei Gao contributed to the study design, data collection, statistical analysis, data interpretation, and manuscript preparation. Tiancong Zhang contributed to data collection. Xiaojing Huang contributed to statistical analysis, and Xuanrong Huan contributed to data collection and statistical analysis. Yuan Li contributed to the manuscript preparation. All authors read and approved the final manuscript.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, X., Zhang, T., Huang, X. et al. Impact of rise and fall phases of shear on platelet activation and aggregation using microfluidics. J Thromb Thrombolysis 57, 576–586 (2024). https://doi.org/10.1007/s11239-024-02968-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-024-02968-1