Abstract
Ecological theory predicts niche partitioning between high-level predators living in sympatry as a mechanism to minimise the selective pressure of competition. Accordingly, male Australian fur seals Arctocephalus pusillus doriferus and New Zealand fur seals A. forsteri that live in sympatry should exhibit partitioning in their broad niches (in habitat and trophic dimensions) in order to coexist. However, at the northern end of their distributions in Australia, both are recolonising their historic range after a long absence due to over-exploitation, and their small population sizes suggest competition should be weak and may allow overlap in niche space. We found some niche overlap, yet clear partitioning in diet trophic level (δ15N values from vibrissae), spatial niche space (horizontal and vertical telemetry data) and circadian activity patterns (timing of dives) between males of each species, suggesting competition may remain an active driver of niche partitioning amongst individuals even in small, peripheral populations. Consistent with individual specialisation theory, broad niches of populations were associated with high levels of individual specialisation for both species, despite putative low competition. Specialists in isotopic space were not necessarily specialists in spatial niche space, further emphasising their diverse individual strategies for niche partitioning. Males of each species displayed distinct foraging modes, with Australian fur seals primarily benthic and New Zealand fur seals primarily epipelagic, though unexpectedly high individual specialisation for New Zealand fur seals might suggest marginal populations provide exceptions to the pattern generally observed amongst other fur seals.
Similar content being viewed by others
Introduction
Understanding the factors that limit species’ distributions is a key theme in ecology. An important factor that limits the distribution of many plants and animals is interrelations amongst species which determine food supply, threat of predation, disease and competition (Krebs 2001). In the case of competition, two species living in a community can compete for resources to a point where one species compromises the fitness of another, but can coexist by partitioning resources or risk competitive exclusion (MacArthur and Levins 1967; Pacala and Roughgarden 1982; Luiselli 2006). Interspecific competition is ubiquitous in plants and animals, though particularly prevalent at higher trophic levels and/or amongst larger animals where available resources may be more limited (Connell 1983; Schoener 1983). Many populations of large carnivores are currently recovering and expanding their range due to persistent conservation efforts (Wabakken et al. 2001; Chapron et al. 2014; Gompper et al. 2015; Martinez Cano et al. 2016). During such recoveries, the interrelations with species in the existing community and with other recovering carnivores are often unknown, but can involve interspecies competition with detrimental impacts to some species, including human conflict (Gompper 2002; Thornton et al. 2004; Kilgo et al. 2010; Reddy et al. 2019; Engebretsen et al. 2021; Franchini et al. 2021). Therefore, determining factors that mitigate competition and mechanisms for coexistence remain important in ecology and will support conservation management.
Niche theory suggests it is possible for competing species to coexist if they occupy different niches (Hardin 1960; MacArthur and Levins 1967). Within a species, similar individuals manage to coexist by partitioning resources, with individuals that have contrasting morphology, physiological capacity, energy requirements or social status typically adopting different strategies to exploit available resources (Svanbäck and Bolnick 2007). Individuals can also use a subset of the population’s resources for reasons unrelated to sex, age and morphological variation, i.e. inter-individual variation (Bolnick et al. 2003; Araújo et al. 2011), with more specialised individuals using a smaller subset and more generalised individuals using a larger subset of the population resources. The level of inter-individual variation can be positively related to population density—a proxy for intraspecies competition (Svanbäck and Persson 2004; Svanbäck and Bolnick 2005, 2007; Araújo et al. 2008; Tinker et al. 2012; Newsome et al. 2015). At the edge of a species’ geographic range, population size is small and thereby intraspecies competition tends to be low, reducing pressures associated with population density, but here interspecies competition with a novel community can be an important factor setting range limits (Hersteinsson and Macdonald 1992; Case and Taper 2000; Case et al. 2005; Pigot and Tobias 2013).
By progressing the study of how species coexist, particularly at expanding margins of their range, we can better assess and predict the interrelations between species as they recover and move into new communities. There are now well-established methods for quantifying ecological niche size and partitioning, including variance and ellipse-based metrics, and spatial, resource and temporal dimensions (Pielou 1972; Petraitis 1979; Bearhop et al. 2004; Peres-Neto et al. 2006; Jackson et al. 2011; Swanson et al. 2015; Frey et al. 2017) have been used to demonstrate that individuals can coexist by partitioning parts of their niche space, resources and time (Luiselli 2006; Navarro et al. 2013; Dehnhard et al. 2020). These niche dimensions have often been assessed in isolation, but with the proliferation of stable isotope analyses and telemetry more studies are demonstrating the importance of a multifaceted approach to understanding niche partition (Kleynhans et al. 2011; Matich and Heithaus 2014; Baylis et al. 2015; Giménez et al. 2018; Riverón et al. 2021; Schwarz et al. 2021). There have also been advances in measuring intra- and interspecific variability in resource and space use (Bolnick et al. 2002; Araújo et al. 2007; Zaccarelli et al. 2013; Carneiro et al. 2017; Bonnet-Lebrun et al. 2018) that require serial sampling individuals to determine individual specialisation (Newsome et al. 2010; Eerkens et al. 2016). Animals can be monitored over long periods of time by combining telemetry with sampling tissues that accumulate isotopes, with both approaches capable of quantifying individual specialisation (Bearhop et al. 2006; Newsome et al. 2009; Elorriaga-Verplancken et al. 2013; Kernaléguen et al. 2016; Bonnet-Lebrun et al. 2018). Commonly analysed isotopes include nitrogen, as an indicator of trophic position of prey, and carbon, as an indicator of geographic origin of prey (Kelly 2000; McCutchan et al. 2003). In marine systems, carbon isotopes can reflect nearshore vs. offshore foraging and prey originating from benthic vs. epipelagic environments (Michener and Kaufman 2007; Newsome et al. 2010). Therefore, the tools are now available to provide detailed assessments of how previously exploited large predators coexist as they recover and expand their range.
Otariids, fur seals and sea lions, were universally overharvested for their fur from the eighteenth to twentieth century, with extinction of many populations and dramatic range reductions (Bonner 1989; Gerber and Hilborn 2001). With persistent conservation efforts, many species have been recovering in recent decades and reoccupying parts of their historic ranges (Wickens and York 1997; Gerber and Hilborn 2001; Kirkman et al. 2013; Crespo 2021; Salton et al. 2021). There are many incidences of two otariid species living in sympatry during such recoveries (Majluf and Trillmich 1981; Lyons et al. 2000; Wege et al. 2016; Elorriaga-Verplancken et al. 2021), and whilst this seems to be possible by niche partitioning (Robinson 2002; Franco-Trecu et al. 2012; Páez-Rosas et al. 2012; Jeglinski et al. 2013; Pablo‐Rodríguez et al. 2016; Hoskins et al. 2017) different levels of individual specialisations in diet and foraging amongst species may also play a role (Franco-Trecu 2014; Kernaléguen et al. 2015a, 2015b; Riverón et al. 2021). During population recovery, some sympatric species have displayed disparate population growth rates and range expansion, which could be attributed to interrelations between the similar species (Wickens and York 1997; Villegas-Amtmann et al. 2013; Franco-Trecu 2014; Elorriaga-Verplancken et al. 2021).
Here, we investigate how two otariids, the Australian fur seal, Arctocephalus pusillus doriferus, and the New Zealand fur seal, A. forsteri (also known as long-nosed fur seal, Shaughnessy and Goldsworthy 2015), coexist in sympatry at an expanding margin of both species’ range. These species have recently re-established seasonal occupation of their north-eastern range margins (Warneke 1975; Irvine et al. 1997; Shaughnessy et al. 2001; Burleigh et al. 2008; Salton et al. 2021) following broader population recovery and range expansion (Arnould et al. 2003; Shaughnessy et al. 2015; McIntosh et al. 2018). Their populations at the margins remain small and predominantly consists of juveniles and sub-adult males (Burleigh et al. 2008), though both breed on Montague Island (36° 14′ S, 150° 13′ E), in small numbers (McIntosh et al. 2018). The two species are typically considered ‘generalists’ due to their broad diets (Page et al. 2005a; Kliska et al. 2022), but in some areas, Australian fur seals do exhibit individual specialisations in diet and foraging (Kernaléguen et al. 2012, 2016; Knox et al. 2018). The two species have apparently distinct foraging modes, with Australian fur seals primarily foraging during benthic dives over the continental shelf (Knox et al. 2017; Salton et al. 2019) and New Zealand fur seals foraging during pelagic dives on and off the continental shelf (Page et al. 2005b, 2006; Salton et al. 2021). From two independent studies of diet and foraging behaviour of males in this part of their range (Hardy et al. 2017; Salton et al. 2021), the two species are known to partition parts of their diets and foraging behaviour, though the discrete analyses and absence of any measurement of the extent of overlap in these niche dimensions left uncertainty over what are the actual mechanisms for coexistence. Given the small population sizes of both species, we expect intraspecies competition to be low and, accordingly, relaxation of niche partitioning and greater overlap between species or niche partitioning to be re-enforced at range margins either by interspecies interactions or because individuals are not plastic enough in foraging behaviour to relax constraints imposed at the range core when inhabiting the range margin.
To understand the mechanisms for coexistence in a situation with purported low intraspecies competition, we aim to (1) estimate niche sizes, in isotopic and spatial dimensions, and the degree of partitioning between species at a population level, and (2) the degree of individual specialisation at the intra-population level and how it relates to their population niche size. Then, (3) we assess the relationship between-individual specialisation in isotopic space and individual specialisation in spatial dimensions, and the importance of intrinsic differences in body size.
Methods
Ethics statement
All research protocols were conducted under Office of Environment and Heritage Animal Ethics Committee Approval (100322/03) and Macquarie University Ethics Committee Approval 2011/054. Capture and handling methods are detailed in Salton et al. (2021), and included sedation by intra-muscular injection of zoletil using a pneumatic dart-gun, then restraint using a catch net with the animal maintained under sedation with a mix of oxygen and isoflurane delivered via a potable vaporiser. Whilst sedated, standard body length was measured using standard methods (±1 cm, Kirkwood et al. 2006), and the telemetry device (see below) was glued to the dorsal midline of each seal with a quick-setting epoxy (Araldite® K-268, Huntsman Advanced Materials; Quick Set Epoxy Resin 850-940, RS components, Australia). Devices remained on the seals until they fell off, once their fur weakened towards the annual moult. Access to the study site at Jervis Bay was under the guidance and support of the Australian Navy, New South Wales National Parks and Wildlife Service, Jervis Bay Marine Park and the Beecroft Ranger Station. Access to the study site at Montague Island was under the guidance and support of New South Wales National Parks and Wildlife Service.
Study species, study site and data collection
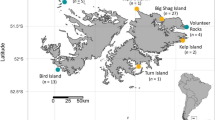
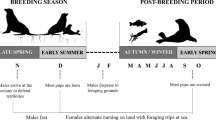
The data were collected during the male’s inter-breeding period between 25-May and 22-Aug in 2011 to 2014, inclusive, when they are free of immediate reproductive constraints and, therefore, have no requirement to attend a specific terrestrial site and so can range widely. The breeding period for Australian fur seals is between late October and late December and for New Zealand fur seals between early November and early January (Crawley and Wilson 1976; Warneke and Shaughnessy 1985). Males move away from areas used during their inter-breeding period towards breeding colonies at the approach of breeding seasons, and it is assumed the reverse occurs at the end of breeding, consistent with the seasonal pattern of attendance at these inter-breeding areas (Shaughnessy et al. 2001; Burleigh et al. 2008) and resighted seals marked with flipper tags at colonies (Warneke 1975). Male fur seals were captured at two study sites, Jervis Bay (35° 3′ S, 150° 50′ E) and Montague Island (36° 14′ S, 150° 13′ E) on the southeast coast of Australia (Fig. 1). This coastline has a narrow continental shelf (17–72 km width) with the shelf break between 130 and 170 m (Geoscience Australia, data.gov.au, 2017-06-24). The populations of both fur seal species have recently been growing in this north-eastern region of both species’ range after near extirpation from over harvesting, and at the time of this study, the populations remained small (<150 seals and <20 pups, compared to >10,000 seals and >1500 pups at large colonies; Warneke 1975; Burleigh et al. 2008; Kirkwood et al. 2010; McIntosh et al. 2018).
Utilisation distributions a 95% b 50% and box–whisker plots of spatial niche parameters for male Australian fur seals (A. pusillus doriferus, AuFS, red; N = 10) and New Zealand fur seals (A. forsteri, NZFS, yellow; N = 35 and 39 for dive and location data, respectively) from Jervis Bay and Montague Island (sites combined). Continental shelf (<500 m depth) is light blue. Inset map in panel a shows approximate range of each species. In panels c and d, boxes represent 1st and 3rd quartiles and median as a thick line, and whiskers are ×1.5 inter quartile range. Panel c is cropped between 100 and 200 km for clarity (16 points for NZFS not visible). Notches in the boxes indicate 95% confidence interval around the median and overlap in notches between groups suggests the medians are not significantly different
Movements of males were recorded with Mk10-AF Fastloc-GPS devices (Wildlife Computers; 105 × 60 × 20 mm, 240 g) at Jervis Bay and CTD-SRDL-9000 (Conductivity-Temperature-Depth Satellite Relay Data Logger, Sea Mammal Research Unit, St Andrews, UK; 120 × 72 × 60 mm, 545 g) at Montague Island. Both devices collected Argos satellite-derived locations (collected at irregular time intervals, with a median fix rate of 1 fix per 1.1 h), and Mk10 devices also recorded GPS locations (collected at 2 min intervals, with a median fix rate of 1 fix per 1.5 h), both of which were transmitted via the Argos satellite network (Collecte Localisation Satellites, Saint-Agne, France). Dive data were collected with both devices (but not Mk10-AF in 2011), with depth (±0.5 m) sampled every 5 s when the device was wet. Single dives were defined by a minimum depth of 5 m and minimum duration of 10 s, and if these criteria were exceeded, the maximum depth of the dive was recorded.
To account for potential inter-annual variability in resource use (Rodríguez-Malagón et al. 2021), we sampled individual vibrissae from both species across each year of the study. The longest whisker was sampled (plucked) from each seal whilst a tracking device was being attached. One whisker was sampled from a dead seal incidentally in 21 November 2012. In the laboratory, vibrissae were hand-washed in 100% ethanol and cleaned in an ultrasonic bath of distilled water for 5 min. Vibrissae were then dried, measured and cut into 3 mm-long consecutive sections starting from the proximal (facial) end, following Cherel et al. (2009). The first 10 sections were sampled from all individuals. Vibrissae growth rate estimates for Australian fur seal males are 0.17 ± 0.04 mm d−1 (Kernaléguen et al. 2015b), and whilst they are not known for male New Zealand fur seals, we assume it is similar based on growth rate estimates of other male fur seals: Arctocephalus australis 0.13 mm d−1, Arctocephalus gazelle 0.14 ± 0.02 mm d−1, and Arctocephalus tropicalis 0.14 ± 0.04 mm d−1 (Kernaléguen et al. 2012; Vales et al. 2015). Hence, a 3 mm section corresponded to approximately 18 days (Kernaléguen et al. 2015b). The δ13C and δ15N values of each whisker section were determined by a PDZ Europa ANCA-GSL elemental analyser interfaced to a PDZ Europa 20–20 isotope ratio mass spectrometer (Sercon, Cheshire, UK) at the University of California Davis (UC-Davis) Stable Isotope Facility. Results are presented in the conventional δ notation relative to Vienna PeeDee Belemnite marine fossil limestone and atmospheric N2 for δ13C and δ15N, respectively. Replicate measurements of internal laboratory standards indicate measurement errors of <0.19%0 and <0.26%0 for δ13C and δ15N values, respectively.
Data processing
All data processing, analysis and figure development were conducted in R v4.1.1 (R Core Team 2020).
Locations were subjected to standard quality-control checks, including removal of erroneous and duplicated locations, removal of locations after a tag fell off a seal, and reclassification of Argos Z-class locations to B-class (n = 86/56,978 locations). A continuous-time correlated random walk state-space model (Jonsen et al. 2020) was then fitted to the quality-controlled locations using the ‘fit_ssm’ function in the ‘foieGras’ R package (Jonsen and Patterson 2020). This approach accounted for observation errors in the Argos location data, and provided location estimates with standard errors at regular 3 h time intervals along each individual’s track (Jonsen et al. 2013). Foraging ‘distance to land’ was used as an index of horizontal movement behaviour. To calculate this index, SSM-estimated locations were projected using Albers equal-area based on the extent of the seal’s movements, determined using https://projectionwizard.org/, then distance to the Australian coastline (GEODATA Coast 100 K 2004, Geosciences Australia) was calculated using the ‘gDistance’ function in the ‘rgeos’ R package (Bivand and Rundel 2021). Locations within 100 m of land were assumed to be indicative of the seal being on land or not foraging and removed.
To best represent the foraging behaviour of animals at the expanding range margins, we analysed only the 10 most recent whisker sections to represent an individual’s isotopic niche and the first 10 weeks of tracking data to represent their spatial niche. This avoided details of their seasonal migrations that may influence the stable isotope values preceding the period at the range margin (Online Resource, Fig. S1; Kernaléguen et al. 2015b; Salton et al. 2021). Based on the whisker growth rate estimates (presented above), the isotope data corresponded to diet approximately 180 days prior to sampling (i.e. approximately the first 6 months of the year). Each whisker section represented a unique sample of δ13C and δ15N values per individual. In terms of their movements at sea, unlike females, these male fur seals are liberated from the reproductive constraint of returning to a central place to feed pups, and so the vast majority of their movements at sea are likely to be driven by their own foraging needs, and these needs are motivated by selective pressure to attain a large, competitive body size to enable access to females. Thus, for movement data, distance to land and maximum dive depth were averaged per week for each individual, and these weekly averaged values represented individual samples of movement behaviour.
Niche partitioning and individual specialisation
Species differences in the two isotope variables (δ13C and δ15N) and two spatial variables (distance to land and dive depth) were tested using linear mixed models. For each of the four variables, a linear mixed model was fitted with species a fixed categorical effect and sample nested in individual identity as a random effect, using the ‘lme’ function in the ‘nlme’ R package (Pinheiro et al. 2021). All models included a temporal autocorrelation (corAR1 of form ~1|ID) to account for serial sampling of individuals. When there were model convergence issues (i.e. δ15N), these were corrected by removing the nested sample component of the random effect. Akaike Information Criterion (AIC) and analysis of variance tests were used to compare the model with fixed effects to the null model, with P < 0.05 indicative of a significant difference from the null model, following the protocol outlined by Zuur et al. (2009). We tested the interactions between species and year and species and deployment site to account for these possible spatial and temporal variations in the isotopes (Rodríguez-Malagón et al. 2021). A weak species:year interaction effect for carbon isotope (conditional R2 = 0.14, P = 0.014) was apparent, but no year effect for each species. Given their absence or that they were weak, we did not include them in subsequent models.
Distance to land and dive depth were log-transformed to account for these indexes being highly positively skewed, and the model estimates are presented back-transformed with their confidence interval (alternatively, isotope estimates are presented with their modelled standard error).
The 95% and 50% spatial utilisation distribution (UD) probabilities were calculated for the inter-breeding period. Smoothing parameters for the UD were calculated using the plug-in bandwidth selector function ‘Hpi’ and associated ‘kde’ function in the ‘ks’ R package (Duong 2021), and the Australian coastline was used as a habitat grid to ensure realistic UD probabilities over water. UDs were calculated for each individual and then standardised to produce a population level 95% and 50% UD for AuFS and NZFS. Percentage UD overlap was calculated using the equation [(areaab/UDa) × (areaab/UDb)]0.5, where areaab is the area of overlap in the home ranges of species a and b, and UDa and UDb refer to the UD of species a and b, respectively (Atwood et al. 2003; Hoskins et al. 2017).
To test for partitioning in the circadian pattern of dive behaviour, we assessed whether dive frequency and dive depth differed with three diel periods: day, twilight and night. Solar position was calculated using solar azimuth and elevation based on location, local date and time (Australian eastern standard time: UTC + 10 h), using the ‘solarpos’ function in the ‘maptools’ R package (Bivand and Lewin-Koh 2021). From solar position, a categorical variable for diel period was defined with three levels: positive values of solar elevation angle identified ‘day’; values between zero and −12° below the horizon identified nautical ‘twilight’; and values below −12° identified ‘night’. Generalised linear mixed models were fitted to assess whether dive frequency was explained by diel period, for each species separately, using the ‘lmer’ function in the ‘lme4’ R package (Bates et al. 2015) with a random effect for individual (intercept only, to elevate convergence issues with the models) and a Poisson error distribution with a log link function. Linear mixed models were fitted to assess whether dive depth (log-transformed) was explained by diel period, for each species separately, using the ‘lmer’ function in the ‘lme4’ R package (Bates et al. 2015) with a random effect for individual (intercept only, to elevate convergence issues with the models). AIC and analysis of variance were again used to compare the model with fixed effects to the null model, with P < 0.05 indicative of a significant difference from the null model.
Isotopic and spatial niche size and partitioning between species were estimated using Bayesian ellipse-based metrics calculated in the ‘SIBER’ R package (Jackson et al. 2011). SIBER applies a ‘typical’ individual approach to calculate the core niche of a population, and incorporates uncertainties relating to sampling biases and small sample sizes, including robust comparison amongst datasets of different sample size (Jackson et al. 2011; Syväranta et al. 2013). We used the 40% Bayesian standard ellipse area (SEAb) to represent the most reliable population-level niche, with the variance estimated through 104 posteriori draws, and a 95% SEAb to capture individual variation and enable more accurate cross-study comparisons. Repeated sample measurements per individual were not independent, yet the small sample size of individual Australian fur seals produced highly variable niche estimates for that population, albeit with consistent niche size compared to the whole dataset (Sup 1). Independent sampling is a required assumption for use of Bayesian SEAb (Jackson et al. 2011), but incorporating a large number of individuals as in this case was preferable to other methods of assessing isotope niche. SEAb results should nevertheless be interpreted in combination with results from mixed effect models. Overlap of isotopic and spatial niches was calculated per species based on the posterior distributions of the fitted ellipses using the ‘baysianOverlap’ function (n = 360, draws = 50).
The degree of individual specialisation in male AuFS and NZFS for each of the four niche parameters was measured and compared using Roughgarden’s WIC/TNW index for continuous data (Bolnick et al. 2002). The approach considers the total niche width (TNW), or variance in total niche parameter for all individuals, to be a sum of the within-individual component (WIC) and the between-individual component (BIC). The WIC is the average of individual niche widths, for example, the variance in isotopes within each individual’s whisker, and the BIC is the variance in mean parameter estimates (e.g. isotope values) amongst individuals. The ‘WTcMC’ function in the ‘RInSp’ R package (Zaccarelli et al. 2013) was used to calculate the specialisation index (SI) for each population, weighting each individual equally to account for slight variances in the number of samples per individual. The SI varied between 0 (specialist) and 1 (generalist), and we applied Monte Carlo resampling (using 1000 replicates) to test the null hypothesis that all individuals were sampled equally from a generalist population. Relationships between the SI for the four niche parameters and with individual body length were tested using linear models, separately for each species, with t-statistics used to assess the fitted linear model, with P < 0.05 indicative of a significant relationship. A lack of relationship between the SI of each niche parameter and body size ensured the measure of individual specialisation aligned with the definition by Bolnick et al. (2002).
Results
We analysed 9 vibrissae from male Australian fur seals (AuFS), with 10 3 mm segments from each vibrissae, and 35 vibrissae from male New Zealand fur seals (NZFS), with 8, 9 or 10 3 mm segments from each vibrissae. Location and dive recording data were retrieved from 10 male AuFS and 35 male NZFS, and location data with no dive recording data were retrieved from an additional four male NZFS. Location and dive data were recorded for 15–259 days (mean ± SE 131.9 ± 15.5 days and 101.4 ± 10.7 days per individual, respectively), which was equivalent to 15 ± 1.2 weeks with location and dive data, 635 ± 53 locations (from SSM, at 3 h intervals) and 1151 ± 221 dives per individual. Based on body length of the seals, male AuFS were larger than male NZFS (body length mean ± SE 192 ± 7.9 cm, N = 9 individual, vs. 137 ± 5.7, N = 39 individuals, respectively; Wilcoxon rank sum test W = 339, P < 0.001).
Isotopic and spatial niche
The two species had broad, segregated isotopic niches of similar size. There were significant differences in δ15N and δ13C values between male AuFS and NZFS, with AuFS having higher δ15N values (AuFS mean 16.4 ± 0.2; NZFS mean 15.2 ± 0.2) and higher but ecologically similar δ13C values (AuFS mean −15.4 ± 0.2; NZFS mean 15.2 ± 0.2) (models were significantly different to the null model, δ15N ΔAIC = 17.65 χ2 = 19.65 P < 0.001; δ13C ΔAIC = 3.16 χ2 = 5.16 P = 0.023; Table 1). Australian fur seals had a narrower range of δ15N values (trophic levels; AuFS 15.91–17.55; NZFS 13.92–16.59) and wider range of δ13C (nutritional sources; AuFS −15.98 to −14.74; NZFS −16.21 to −15.25) compared to New Zealand fur seals (Fig. 2; Table 1). Bayesian estimation of the isotopic niche space of the two species shows similar sized isotopic niches, based on the 40% SEAb and 95% SEAb, yet trophic niche (40% SEAb) overlap was negligible at ~5%, suggesting strong resource partitioning between the two pinniped populations. Based on the 40% SEAb, partitioning of their iso-niche space was primarily in δ15N values that relate to trophic level (Fig. 2; Table 1).
Isotopic and spatial niche bi-plots (left) and posterior density plots (right) from Bayesian standard ellipse area (SEAb; solid lines 40%, dashed line 95%; density plots are of 40% SEAb) of male Australian fur seals (Arctocephalus pusillus doriferus, red; N = 9) and New Zealand fur seals (A. forsteri, yellow; N = 35). In isotope bi-plot, points represent isotope values from the ten most recent whisker samples from each individual. For clarity, a sample of 50 modelled ellipses (40% SEAb) per species are shown. Bi-plots represent the size and overlap of the niche space, and density plots compare size (similar niche size have more overlap) and variance amongst 40% SEAb estimates (height-width of density plot)
Male AuFS remained close to the coast over the continental shelf, including over the shelf edge, whilst NZFS travelled across the continental shelf (coastal and over the shelf edge) and well off the shelf over deep water. Consequently, male NZFS had a much larger 95% utilisation distribution than AuFS (Table 1), and the percentage overlap or 95% UD shared with the other species was ~80% for AuFS and ~10% for NZFS. However, the 50% UD for both species was predominantly over the continental shelf, of similar size, and showed approximately 50% species overlap (Fig. 1; Table 1). Accordingly, the mean distance that an individual travelled from land per week was highly positively skewed for male AuFS and NZFS, and not significantly different between the two species (distance to land not significantly different to the null model, ΔAIC = 1.6 χ2 = 0.32 P = 0.574; Table 1). The two species also shared the vertical dimension of their spatial niche, but on average male AuFS dived deeper than NZFS (dive depth significantly different to the null model, ΔAIC = 7.9 χ2 = 9.89 P = 0.002; Fig. 1; Table 1). The movement behaviour of AuFS (i.e. predominantly deep dives over the continental shelf) was consistent with a benthic foraging mode, and the movement behaviour of NZFS (shallow dives over the shelf and deep water) was consistent with epipelagic foraging mode. However, four male NZFS with weekly average maximum depth >100 m also remained close to land (<20 km) during those weeks, suggesting benthic foraging; this was the case for all weeks recorded for one of these four NZFS, suggesting it used a benthic foraging mode during the inter-breeding period.
With horizontal and vertical movements combined, overall NZFS had a much larger spatial niche space (40 and 95% SEAb; Table 1). Whilst they had overlapping spatial niche space, AuFS shared more of their spatial niche space with NZFS, but due to more extensive horizontal movements, NZFS had more space segregated from AuFS space. At the same time, whilst the larger spatial niche space for NZFS derived from horizontal movement, partitioning in spatial niche space between species was primarily due to segregation in vertical use of the water column, as Australian fur seals dived deeper and New Zealand fur seals dived shallower (Fig. 2).
The two species also had different circadian patterns in dive frequency, with NZFS diving significantly more at night and AuFS diving similarly between night and day, but significantly less during twilight (Online Resource; Fig. S2, Table S1). Neither species had a diel pattern in dive depth (Online Resource; Fig. S2, Table S1).
Individual specialisation
The individual specialisation index (SI) of δ13C values, δ15N values and dive depth for AuFS and NZFS indicated these male fur seals were specialists in each of these niche dimensions (P < 0.001; Table 2). However, there was high variability in the SI amongst individuals for each species (Fig. 3), with some individuals tending towards the generalist end of the spectrum but most individuals at the specialist end of the spectrum. For distance to land, AuFS were generalists and NZFS were specialists, though both species had high variability in the SI amongst individuals with their values spread across the SI spectrum (Fig. 3). There were a relatively large number of highly specialised male NZFS for ‘distance to land’; 12 individuals with SI values <0.05. These individuals include some who travelled off the continental shelf into deep water during each week, and other individuals who only moved between islands and the coastline (i.e. remained very close to land).
Density plot of specialisation index (SI) in δ13C and δ15N values and spatial parameters for each individual male Australian fur seals (Arctocephalus pusillus doriferus, AuFS, red) and New Zealand fur seals (A. forsteri, NZFS, yellow). Sample size for isotopic data N = 9 AuFS and N = 35 NZFS and for spatial data N = 10 AuFS and N = 35 and 39 for dive and location NZFS data, respectively. Vertical dotted lines show the population-level SI (from Table 1)
There were no correlations between an individual’s SI in any dimension and its body length (Online Resource; Table S2); all P > 0.05. An individual’s SI in one dimension (e.g. δ13C) was not related to its SI in another dimension (e.g. δ15N).
Discussion
Our results indicate that male Australian and New Zealand fur seals that are reoccupying the north-eastern extent of their respective ranges share a broad ecological niche space but have significant partitioning in isotopic and spatial dimensions of their niche, despite expectations of ecological release from high intraspecies competition. Given their broad niches, it was not surprising that males of both species showed high levels of individual specialisation in isotopic and spatial niche space, particularly given their increased intraspecies competition over recent decades. Highly specialised individuals in isotopic space were not necessarily highly specialised in spatial niche space, further emphasising their diverse strategies for niche partitioning. There was support for a link between foraging mode and individual specialisation, as for other fur seals, though unexpectedly high specialisation for epipelagic NZFS males suggests exceptions may be apparent amongst marginal populations of a species’ distribution.
Niche partitioning
As populations increase in size so can intraspecies competition for favourable food resources (i.e. rate of energy gain), which should drive individuals to broaden their niche (diet and/or foraging behaviour) to maintain optimal foraging (MacArthur and Pianka 1966; Roughgarden 1972; Bolnick 2001; Svanbäck and Bolnick 2007). Amongst marine predators, increased intraspecies competition has been associated with broader dietary niche and foraging niche attributed to the need to access different prey, prey at deeper depths and greater distances from their colony (Lewis et al. 2001; Kuhn et al. 2014; Ratcliffe et al. 2018). Along the same lines, subantarctic fur seals in a large population that has reached carrying capacity had a wider niche than those from a smaller population that is still increasing (Kernaléguen et al. 2015a). In contrast, at their range margin where population sizes are still small, these male fur seals continued to display a broad dietary niche (δ15N values) and spatial niche (horizontal and vertical behaviour), and this is consistent with an earlier dietary analysis of fur seal scats (Hardy et al. 2017). Whilst sample size was small, particularly for Australian fur seals, SIBER is robust to both this and unbalanced samples between populations (Jackson et al. 2011). Ideally, n > 20 would shrink confidence intervals as per Syvaranta et al. (2013), but whilst our sample size is small, the use of serially growing tissue for each individual would mitigate this by increasing the accuracy of each individuals’ niche values. The results presented here are also consistent with earlier dietary analyses and the size and consistency of the difference in niche space between species supports this analysis of niche space. Perhaps more discrete spatial niches of both individuals and the species as a whole might emerge if we could limit analyses to foraging areas and exclude travel behaviour. Individuals may expand their foraging niche in response to high intra- or high interspecific competition or decreased availability of food resources (Chiaradia et al. 2003; Moleón et al. 2009; Prati et al. 2021) and these factors typify a species’ range margin (MacArthur 1984; Case et al. 2005; Guo et al. 2005). Therefore, individuals may need to maintain a broad niche when moving between their range core and margins to mitigate different types of competition (intra and interspecies) and variable abundance of favourable prey throughout their distribution.
Interspecific competition was expected at this range margin, with two congeneric species living in sympatry. Elsewhere, large, established populations of two other otariids, South American fur seals and southern sea lions, show significant partitioning in their isotope niche space (Riverón et al. 2021). However, at the range margins populations are small so interspecific competition should be low thereby allowing these species to share the most favourable resources (in terms of energy gain) and overlap niche space. These male fur seals did indeed overlap in the prey source of primary productivity (δ13C values), trophic level of their prey (δ15N values; Kelly 2000; Davenport and Bax 2002) and spatial niche space, consistent with males of both species being high order predators that frequently return to land to rest and digest, and have foraging habitat at a range of depths (Page et al. 2005a; Hardy et al. 2017; Knox et al. 2017; Salton et al. 2021). Although the two species had overlapping niches, they had clear partitioning in their dietary niche and dive behaviour, with AuFS typically feeding on higher trophic level prey or on prey with different δ15N at the base of the food web in their feeding location than NZFS (based on δ15N values; Davenport and Bax 2002) and generally diving deeper than NZFS. Similar means of niche partitioning (different dietary composition and foraging behaviour) were found between sympatric female AuFS and NZFS at a breeding colony (Hoskins et al. 2017) and between sympatric male AuFS and NZFS at a New Zealand fur seal breeding colony (Page et al. 2005a). Unlike small populations at range margins, at breeding colonies this partitioning is expected because the larger populations suggest that absolute competition (intra and interspecific competition combine) should be higher (Shaughnessy et al. 2015; McIntosh et al. 2018). It is possible that competition in the core of their range drove niche partitioning ancestrally, and neither species is plastic enough in foraging to relax feeding constraints when seasonally present at the range margin, even in the absence of resource limitations. This idea could be explored more completely if similar individuals from populations at the range centre and range margin were sampled concurrently and then both intra and interpopulation comparisons could be considered in addition to intra and interspecies comparisons.
Individual specialisation
Niche expansion can occur when all individuals of a population exploit a wider niche or via increased between-individual variation in niche dimensions. The latter is termed the Niche Variation Hypothesis (Van Valen 1965) and has supporting quantitative evidence from numerous taxa (Bolnick et al. 2007). Consistent with this hypothesis, fur seal populations that feed only on a few prey species are often made up of generalist individuals and populations with a broad dietary niche often have high levels of individual specialisation (Kernaléguen et al. 2015a; Riverón et al. 2021), including Australian fur seals (Kernaléguen et al. 2015b; this study) and New Zealand fur seals (this study). In addition to the Niche Variation Hypothesis, the level of individual specialisation in a population can be positively related to population density (Svanbäck and Persson 2004; Svanbäck and Bolnick 2005, 2007; Tinker et al. 2008), presumably because smaller populations have less intraspecies competition driving niche expansion, which appears to be the case for some fur seals (Franco-Trecu 2014; Kernaléguen et al. 2015a). Therefore, individuals at range margins, within small populations, may have lower individual specialisation than conspecifics at the range core. In contrast to this, the level of individual specialisation in δ13C values and δ15N values amongst male AuFS at this range margin (0.40 and 0.36, respectively) was higher (more specialised) compared to male AuFS in the core of the species’ range (0.93 and 0.56, respectively; Kernaléguen et al. 2015b). Some of this disparity could be associated with the shorter temporal scale used to measure individual specialisation in our study (10 whisker segments, rather than whole vibrissae), which often exaggerates the apparent level of individual specialisation (Araújo et al. 2007; Novak and Tinker 2015; Kernaléguen et al. 2016), though niche size and overlap were similar for the 10 segment and whole whisker datasets (Online Resource; Fig. S1). Alternatively, this disparity may provide support for the hypothesis that behavioural differences exist between dispersers and residents, with dispersers having higher heterogeneity in behaviour that supports population expansion into novel environments (Cote et al. 2010).
The level of specialisation in niche dimension varied amongst individuals, suggesting disproportionate effects of the drivers of specialisation on individuals. Accordingly, we tested whether the level of individual specialisation in one niche dimension was linearly related to specialisation in other niche dimensions, and found this was not the case for any of the four niche dimensions. Therefore, a seal may show a highly specialised dietary niche (δ15N values) but forage across a range of habitats to access their prey (less specialised spatial niche). Alternatively, a seal may have principally foraged epipelagically in inshore habitat (specialised spatial niche) on a broad range of prey (less specialised dietary niche). This suggests that individuals respond to the drivers of specialisation in different ways, potentially specialising in various niche dimensions but not necessarily all of them. This emphasises the behavioural plasticity of individuals to selection pressures and highlights the importance of considering multiple niche dimensions when assessing ecological drivers and consequences of individual specialisation.
Whilst species-specific foraging modes were apparent (i.e. benthic verses pelagic), both species were specialists in isotopic and spatial niche space based on Monte Carlo resampling tests for a null, generalist population. Benthic environments typically have a high diversity of prey, with each prey species having relatively low abundance, compared to the low diversity of pelagic species that are highly abundant (Gray 1997). Therefore, the benthic environment offers greater opportunity and motivation (e.g. to alleviate competition for limited resources) for predators to specialise on particular prey, whereas the pelagic environment has less potential and perhaps motivation for individuals to diverge from the average population diet. Empirical evidence shows pelagic foraging fur seals using offshore habitats have narrow isotopic niches, with generalist individuals and low specialisation, whilst benthic foraging fur seals using inshore habitats have a broader population isotopic niche with many specialist individuals (Riverón et al. 2021). Male AuFS on the range margin are consistent with this prediction from other fur seals, displaying benthic inshore foraging and consisting of mostly individual niche specialists. However, male NZFS movement behaviour was typical of epipelagic foraging, and they also had high individual specialisation. These male NZFS exploited predominantly inshore but also offshore habitats, and some male NZFS remained close to the coast displaying an apparent benthic foraging mode. Ecological diversification often occurs in marine mammals that forage in inshore areas (Wolf et al. 2008; Chilvers and Wilkinson 2009; Aurioles-Gamboa et al. 2013), perhaps due to the greater diversity of isotopic pathways in coastal environments (Ray 1991) and greater habitat complexity (Sequeira et al. 2018). Given these populations are small, perhaps there is some interspecies competition release that creates space for some male NZFS to exploit the benthic and inshore habitats, thereby increasing potential for inter-individual diversification. This may change as populations increase, and male AuFS come to dominate the inshore environment and NZFS forage more epipelagically further from the coast (Page et al. 2006).
Ecological implications
As species expand their range into new habitat, they must compete for resources with the native community, which already compete amongst themselves. The size of a community can influence the level of niche overlap, with increasing number of species associated with less overlap (Pianka 1974), and if the community is sufficiently large it can prevent newly introduced species from becoming established (Case 1990). This has implications for the success of biological invasions (MacArthur 1984; Freed and Cann 2014), and potentially the recovery and range expansion associated with conservation efforts of a native species. Given the smaller populations of both species at this expanding range margin, there was potential for high niche overlap associated with competition release. Somewhat contradictory, the niche overlap and individual specialisation between and within these male fur seals suggests there is available niche for each of these species and potential for further mitigation of inter and intraspecies competition, and therefore, potential for population growth and range expansion. Indeed, prior to this study both populations of fur seals in Australia had positive population trajectories (Shaughnessy et al. 2015; McIntosh et al. 2018). Ongoing assessments of niche partitioning and individual specialisation within and between these sympatric and congeneric species at this range margin will further develop ecological understanding of the mechanisms for successful population growth and range expansion and should consider the role of a rapidly warming environment. These assessments would benefit from concurrent sampling of individuals within the core parts of their species range to better quantify the mechanisms operating throughout different parts of a species range.
Individual specialisation and behavioural plasticity provide opportunities for a population to adapt to environmental change (Brent 1978; Bolnick et al. 2003; Tuomainen and Candolin 2011; Edelaar and Bolnick 2019). Accordingly, the high individual specialisation amongst these male fur seals may contribute to their successful re-occupation of this margin of their range amidst extreme rates of ocean warming (Ridgway 2007) and a dense human population. However, species have physiological limits, for example, otariids in temperate regions are sensitive to high temperatures (Gentry 1973; Ladds et al. 2017), and thermal energetic costs are often higher for pups and juveniles (Liwanag 2010). Species are also limited by habitat needs, in this case particular terrestrial features at haul-out and breeding sites (Ryan et al. 1997; Stevens and Boness 2003), and several of their haul-out sites at this margin of their range are currently not zoned as protected areas (Salton et al. 2021). Therefore, whilst males have reoccupied this part of the species’ range, these additional limitations could influence the successful reestablishment of a breeding population and future occupation by males.
Furthermore, ocean warming is altering prey distribution and abundance and thereby the habitat uses of marine predators (Schumann et al. 2013; Amador‐Capitanachi et al. 2020; Evans et al. 2020; Niella et al. 2020, 2021; d’Entremont et al. 2021; Florko et al. 2021). There have been recent losses of habitat and habitat-forming species at this margin of the seals’ range (Wernberg et al. 2011). Thus, whilst these predators demonstrate capability to exploit a dynamic environment and a high level of adaptiveness to change, a rapidly warming environment presents several risks that could limit population growth and expansion at this margin of their range. These risks would compromise the success of current conservation efforts that have seen these species reoccupy parts of their historic range. To mitigate such compromises, we encourage actions that support species to adapt to climate change (Hobday et al. 2016; Roberts et al. 2017; Miller et al. 2018; Wilson et al. 2020). In particular, both a larger and targeted network of protected areas on land and at sea (Salton et al. 2021), and informed, dynamic management of both these predators and their changing prey base (Nelms et al 2021).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Amador-Capitanachi MJ, Moreno-Sánchez XG, Ventura-Domínguez PD, Juárez-Ruiz A, González-Rodríguez E, Gálvez C, Norris T, Elorriaga-Verplancken FR (2020) Ecological implications of unprecedented warm water anomalies on interannual prey preferences and foraging areas of Guadalupe fur seals. Mar Mamm Sci 36:1254–1270. https://doi.org/10.1111/mms.12718
Araújo MS, Bolnick DI, Machado G, Giaretta AA, Dos Reis SF (2007) Using δ 13 C stable isotopes to quantify individual-level diet variation. Oecologia 152:643–654. https://doi.org/10.1007/s00442-007-0687-1
Araújo MS, Guimaraes PR Jr, Svanbäck R, Pinheiro A, Guimarães P, Reis SFD, Bolnick DI (2008) Network analysis reveals contrasting effects of intraspecific competition on individual vs. population diets. Ecology 89:1981–1993. https://doi.org/10.1890/07-0630.1
Araújo MS, Bolnick DI, Layman CA (2011) The ecological causes of individual specialisation. Ecol Lett 14:948–958. https://doi.org/10.1111/j.1461-0248.2011.01662.x
Arnould JP, Boyd I, Warneke R (2003) Historical dynamics of the Australian fur seal population: evidence of regulation by man? Can J Zool 81:1428–1436. https://doi.org/10.1139/z03-134
Atwood TC, Weeks J, Harmon P (2003) Spatial home-range overlap and temporal interaction in eastern coyotes: the influence of pair types and fragmentation. Can J Zool 81:1589–1597. https://doi.org/10.1139/z03-144
Aurioles-Gamboa D, Rodríguez-Pérez MY, Sánchez-Velasco L, Lavín MF (2013) Habitat, trophic level, and residence of marine mammals in the Gulf of California assessed by stable isotope analysis. Mar Ecol Prog Ser 488:275–290. https://doi.org/10.3354/meps10369
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Baylis A, Orben R, Arnould J, Peters K, Knox T, Costa D, Staniland I (2015) Diving deeper into individual foraging specializations of a large marine predator, the southern sea lion. Oecologia 179:1053–1065. https://doi.org/10.1007/s00442-015-3421-4
Bearhop S, Adams CE, Waldron S, Fuller RA, MacLeod H (2004) Determining trophic niche width: a novel approach using stable isotope analysis. J Anim Ecol 73:1007–1012. https://doi.org/10.1111/j.0021-8790.2004.00861.x
Bearhop S, Phillips RA, McGill R, Cherel Y, Dawson DA, Croxall JP (2006) Stable isotopes indicate sex-specific and long-term individual foraging specialisation in diving seabirds. Mar Ecol Prog Ser 311:157–164. https://doi.org/10.3354/meps311157
Bivand R, Lewin-Koh N (2021) maptools: tools for handling spatial objects. R package version 1.1-2. https://CRAN.R-project.org/package=maptools
Bivand R, Rundel C (2021) rgeos: interface to geometry engine—open source (‘GEOS’). R package version 0.5-8. https://CRAN.R-project.org/package=rgeos
Bolnick DI (2001) Intraspecific competition favours niche width expansion in Drosophila melanogaster. Nature 410:463–466. https://doi.org/10.1038/35068555
Bolnick DI, Yang LH, Fordyce JA, Davis JM, Svanbäck R (2002) Measuring individual-level resource specialization. Ecology 83:2936–2941. https://doi.org/10.1890/0012-9658(2002)083[2936:MILRS]2.0.CO;2
Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML (2003) The ecology of individuals: incidence and implications of individual specialization. Am Nat 161:1–28. https://doi.org/10.1086/343878
Bolnick DI, Svanbäck R, Araújo MS, Persson L (2007) Comparative support for the niche variation hypothesis that more generalized populations also are more heterogeneous. Proc Natl Acad Sci 104:10075–10079. https://doi.org/10.1073/pnas.0703743104
Bonner WN (1989) The natural history of seals. Kent, Christopher Helm (Publishers) Ltd, Bromley
Bonnet-Lebrun A-S, Phillips R, Manica A, Rodrigues AS (2018) Quantifying individual specialization using tracking data: a case study on two species of albatrosses. Mar Biol 165:1–15. https://doi.org/10.1007/s00227-018-3408-x
Brent SB (1978) Individual specialization, collective adaptation and rate of environmental change. Hum Dev 21:21–33. https://doi.org/10.1159/000271573
Burleigh A, Lynch T, Rogers T (2008) Status of the Steamers Head (NSW) Australian and New Zealand fur seal haul-out site and influence of environmental factors and stochastic disturbance on seal behaviour. In: Lunney D, Munn A, Meikle W (eds) Too close for comfort: contentious issues in human-wildlife encounters. Royal Zoological Society of New South Whales, pp 246–254
Carneiro AP, Bonnet-Lebrun A-S, Manica A, Staniland IJ, Phillips RA (2017) Methods for detecting and quantifying individual specialisation in movement and foraging strategies of marine predators. Mar Ecol Prog Ser 578:151–166. https://doi.org/10.3354/meps12215
Case TJ (1990) Invasion resistance arises in strongly interacting species-rich model competition communities. Proc Natl Acad Sci 87:9610–9614. https://doi.org/10.1073/pnas.87.24.9610
Case TJ, Taper ML (2000) Interspecific competition, environmental gradients, gene flow, and the coevolution of species’ borders. Am Nat 155:583–605. https://doi.org/10.1086/303351
Case TJ, Holt RD, McPeek MA, Keitt TH (2005) The community context of species’ borders: ecological and evolutionary perspectives. Oikos 108:28–46. https://doi.org/10.1111/j.0030-1299.2005.13148.x
Chapron G, Kaczensky P, Linnell JD, Von Arx M, Huber D, Andrén H, López-Bao JV, Adamec M, Álvares F, Anders O (2014) Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 346:1517–1519. https://doi.org/10.1126/science.1257553
Cherel Y, Kernaléguen L, Richard P, Guinet C (2009) Whisker isotopic signature depicts migration patterns and multi-year intra-and inter-individual foraging strategies in fur seals. Biol Let 5:830–832. https://doi.org/10.1098/rsbl.2009.0552
Chiaradia A, Costalunga A, Kerry K (2003) The diet of Little Penguins (Eudyptula minor) at Phillip Island, Victoria, in the absence of a major prey–Pilchard (Sardinops sagax). Emu 103:43–48. https://doi.org/10.1071/MU02020
Chilvers BL, Wilkinson IS (2009) Diverse foraging strategies in lactating New Zealand sea lions. Mar Ecol Prog Ser 378:299–308. https://doi.org/10.3354/meps07846
Connell JH (1983) On the prevalence and relative importance of interspecific competition: evidence from field experiments. Am Nat 122:661–696. https://doi.org/10.1086/284165
Cote J, Clobert J, Brodin T, Fogarty S, Sih A (2010) Personality-dependent dispersal: characterization, ontogeny and consequences for spatially structured populations. Philos Trans R Soc B Biol Sci 365:4065–4076. https://doi.org/10.1098/rstb.2010.0176
Crawley M, Wilson G (1976) The natural history and behaviour of the New Zealand fur seal (Arctocephalus forsteri). Biological Society, Victoria University of Wellington
Crespo EA (2021) Exploitation and recovery of the South American sea lion in the Southwestern Atlantic. In: Ethology and behavioral ecology of otariids and the odobenid. Springer, pp 521–537. https://doi.org/10.1007/978-3-030-59184-7_24
Davenport SR, Bax NJ (2002) A trophic study of a marine ecosystem off southeastern Australia using stable isotopes of carbon and nitrogen. Can J Fish Aquat Sci 59:514–530. https://doi.org/10.1139/f02-031
Dehnhard N, Achurch H, Clarke J, Michel LN, Southwell C, Sumner MD, Eens M, Emmerson L (2020) High inter-and intraspecific niche overlap among three sympatrically breeding, closely related seabird species: Generalist foraging as an adaptation to a highly variable environment? J Anim Ecol 89:104–119. https://doi.org/10.1111/1365-2656.13078
d’Entremont KJ, Guzzwell LM, Wilhelm SI, Friesen VL, Davoren GK, Walsh CJ, Montevecchi WA (2021) Northern Gannets (Morus bassanus) breeding at their southern limit struggle with prey shortages as a result of warming waters. ICES J Mar Sci. https://doi.org/10.1093/icesjms/fsab240
Duong T (2021) ks: kernel smoothing. In (Version 1.13.2) [R package]. https://CRAN.R-project.org/package=ks
Edelaar P, Bolnick DI (2019) Appreciating the multiple processes increasing individual or population fitness. Trends Ecol Evol 34:435–446. https://doi.org/10.1016/j.tree.2019.02.001
Eerkens JW, Sullivan K, Greenwald AM (2016) Stable isotope analysis of serial samples of third molars as insight into inter-and intra-individual variation in ancient diet. J Archaeol Sci Rep 5:656–663. https://doi.org/10.1016/j.jasrep.2015.11.003
Elorriaga-Verplancken F, Aurioles-Gamboa D, Newsome SD, Martínez-Díaz SF (2013) δ 15 N and δ 13 C values in dental collagen as a proxy for age-and sex-related variation in foraging strategies of California sea lions. Mar Biol 160:641–652. https://doi.org/10.1007/s00227-012-2119-y
Elorriaga-Verplancken FR, Acevedo-Whitehouse K, Norris T, Gutiérrez-Gutiérrez A, Amador-Capitanachi MJ, Juárez-Ruiz A, Sandoval-Sierra J, Gálvez C, Moreno-Sánchez XG (2021) Guadalupe fur seals and california sea lions: two sympatric otariids from the california current ecosystem. In: Ethology and behavioral ecology of otariids and the odobenid. Springer, pp 621–634. https://doi.org/10.1007/978-3-030-59184-7_28
Engebretsen KN, Beckmann JP, Lackey CW, Andreasen A, Schroeder C, Jackson P, Young JK (2021) Recolonizing carnivores: is cougar predation behaviorally mediated by bears? Ecol Evol 11:5331–5343. https://doi.org/10.1002/ece3.7424
Evans R, Hindell M, Kato A, Phillips LR, Ropert-Coudert Y, Wotherspoon S, Lea M-A (2020) Habitat utilization of a mesopredator linked to lower sea-surface temperatures & prey abundance in a region of rapid warming. Deep Sea Res Part II 175:104634. https://doi.org/10.1016/j.dsr2.2019.104634
Florko KR, Tai TC, Cheung WW, Ferguson SH, Sumaila UR, Yurkowski DJ, Auger-Méthé M (2021) Predicting how climate change threatens the prey base of Arctic marine predators. Ecol Lett 24:2563–2575. https://doi.org/10.1111/ele.13866
Franchini M, Corazzin M, Bovolenta S, Filacorda S (2021) The return of large carnivores and extensive farming systems: a review of stakeholders’ perception at an EU Level. Animals 11:1735. https://doi.org/10.3390/ani11061735
Franco-Trecu V (2014) 03). Individual trophic specialisation and niche segregation explain the contrasting population trends of two sympatric otariids. Mar Biol 161:609–618. https://doi.org/10.1007/s00227-013-2363-9
Franco-Trecu V, Aurioles-Gamboa D, Arim M, Lima M (2012) Prepartum and postpartum trophic segregation between sympatrically breeding female Arctocephalus australis and Otaria flavescens. J Mammal 93:514–521. https://doi.org/10.1644/11-MAMM-A-174.1
Freed LA, Cann RL (2014) Diffuse competition can be reversed: a case history with birds in Hawaii. Ecosphere 5:1–40. https://doi.org/10.1890/ES14-00289.1
Frey S, Fisher JT, Burton AC, Volpe JP (2017) Investigating animal activity patterns and temporal niche partitioning using camera-trap data: Challenges and opportunities. Remote Sens Ecol Conserv 3:123–132. https://doi.org/10.1002/rse2.60
Gentry RL (1973) Thermoregulatory behavior of eared seals. Behaviour 46:73–93. https://doi.org/10.1163/156853973X00175
Gerber LR, Hilborn R (2001) Catastrophic events and recovery from low densities in populations of otariids: implications for risk of extinction. Mammal Rev 31:131–150. https://doi.org/10.1046/j.1365-2907.2001.00081.x
Giménez J, Cañadas A, Ramírez F, Afán I, García-Tiscar S, Fernández-Maldonado C, Castillo JJ, de Stephanis R (2018) Living apart together: Niche partitioning among Alboran Sea cetaceans. Ecol Ind 95:32–40. https://doi.org/10.1016/j.ecolind.2018.07.020
Gompper ME (2002) Top Carnivores in the suburbs? Ecological and conservation issues raised by colonization of North-eastern North America by coyotes. Bioscience 52:185–190. https://doi.org/10.1641/0006-3568(2002)052[0185:TCITSE]2.0.CO;2
Gompper ME, Belant JL, Kays R (2015) Carnivore coexistence: America’s recovery. Science 347:382–383. https://doi.org/10.1126/science.347.6220.382-b
Gray JS (1997) Marine biodiversity: patterns, threats and conservation needs. Biodivers Conserv 6:153–175. https://doi.org/10.1023/A:1018335901847
Guo Q, Taper M, Schoenberger M, Brandle J (2005) Spatial-temporal population dynamics across species range: from centre to margin. Oikos 108:47–57. https://doi.org/10.1111/j.0030-1299.2005.13149.x
Hardin G (1960) The competitive exclusion principle. Science 131:1292–1297. https://doi.org/10.1126/science.131.3409.1292
Hardy N, Berry T, Kelaher BP, Goldsworthy SD, Bunce M, Coleman MA, Gillanders BM, Connell SD, Blewitt M, Figueira W (2017) Assessing the trophic ecology of top predators across a recolonisation frontier using DNA metabarcoding of diets. Mar Ecol Prog Ser 573:237–254. https://doi.org/10.3354/meps12165
Hersteinsson P, Macdonald DW (1992) Interspecific competition and the geographical distribution of red and arctic foxes Vulpes vulpes and Alopex lagopus. Oikos:505–515. https://doi.org/10.2307/3545168
Hobday AJ, Cochrane K, Downey-Breedt N, Howard J, Aswani S, Byfield V, Duggan G, Duna E, Dutra LX, Frusher SD (2016) Planning adaptation to climate change in fast-warming marine regions with seafood-dependent coastal communities. Rev Fish Biol Fisheries 26:249–264. https://doi.org/10.1007/s11160-016-9419-0
Hoskins AJ, Schumann N, Costa DP, Arnould JP (2017) Foraging niche separation in sympatric temperate-latitude fur seal species. Mar Ecol Prog Ser 566:229–241. https://doi.org/10.3354/meps12024
Irvine A, Bryden M, Corkeron P, Warneke R (1997) A census of fur seals at Montague Island, New South Wales. In: Hindell M, Kemper C (eds) Marine mammal research in the southern hemisphere: status, ecology and medicine. Surrey Beatty & Sons, pp 56–62
Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. J Anim Ecol 80:595–602. https://doi.org/10.1111/j.1365-2656.2011.01806.x
Jeglinski JW, Goetz KT, Werner C, Costa DP, Trillmich F (2013) Same size–same niche? Foraging niche separation between sympatric juvenile Galapagos sea lions and adult Galapagos fur seals. J Anim Ecol 82:694–706. https://doi.org/10.1111/1365-2656.12019
Jonsen I, Patterson T (2020) foieGras: fit continuous-time state-space and latent variable models for filtering Argos satellite (and other) telemetry data and estimating movement behaviour. 2019. R package version 0.4. 0
Jonsen I, Basson M, Bestley S, Bravington M, Patterson T, Pedersen MW, Thomson R, Thygesen UH, Wotherspoon S (2013) State-space models for bio-loggers: a methodological road map. Deep Sea Res Part II 88:34–46. https://doi.org/10.1016/j.dsr2.2012.07.008
Jonsen ID, Patterson TA, Costa DP, Doherty PD, Godley BJ, Grecian WJ, Guinet C, Hoenner X, Kienle SS, Robinson PW (2020) A continuous-time state-space model for rapid quality control of argos locations from animal-borne tags. Mov Ecol 8:1–13. https://doi.org/10.1186/s40462-020-00217-7
Kelly JF (2000) Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can J Zool 78:1–27. https://doi.org/10.1139/z99-165
Kernaléguen L, Cazelles B, Arnould JP, Richard P, Guinet C, Cherel Y (2012) Long-term species, sexual and individual variations in foraging strategies of fur seals revealed by stable isotopes in whiskers. PLoS ONE 7:e32916. https://doi.org/10.1371/journal.pone.0032916
Kernaléguen L, Arnould JP, Guinet C, Cherel Y (2015a) Determinants of individual foraging specialization in large marine vertebrates, the Antarctic and subantarctic fur seals. J Anim Ecol 84:1081–1091. https://doi.org/10.1111/1365-2656.12347
Kernaléguen L, Cherel Y, Knox TC, Baylis AM, Arnould JP (2015b) Sexual niche segregation and gender-specific individual specialisation in a highly dimorphic marine mammal. PLoS ONE 10:e0133018. https://doi.org/10.1371/journal.pone.0133018
Kernaléguen L, Dorville N, Ierodiaconou D, Hoskins AJ, Baylis AM, Hindell MA, Semmens J, Abernathy K, Marshall GJ, Cherel Y (2016) From video recordings to whisker stable isotopes: a critical evaluation of timescale in assessing individual foraging specialisation in Australian fur seals. Oecologia 180:657–670. https://doi.org/10.1007/s00442-015-3407-2
Kilgo JC, Ray HS, Ruth C, Miller KV (2010) Can coyotes affect deer populations in southeastern North America? J Wildl Manag 74:929–933. https://doi.org/10.2193/2009-263
Kirkwood R, Lynch M, Gales N, Dann P, Sumner M (2006) At-sea movements and habitat use of adult male Australian fur seals (Arctocephalus pusillus doriferus). Can J Zool 84:1781–1788. https://doi.org/10.1139/z06-164
Kirkwood R, Pemberton D, Gales R, Hoskins AJ, Mitchell T, Shaughnessy PD, Arnould JP (2010) Continued population recovery by Australian fur seals. Mar Freshw Res 61:695–701. https://doi.org/10.1071/MF09213
Kirkman SP, Yemane D, Oosthuizen W, Meÿer M, Kotze P, Skrypzeck H, Vaz Velho F, Underhill L (2013) Spatio-temporal shifts of the dynamic Cape fur seal population in southern Africa, based on aerial censuses (1972–2009). Mar Mamm Sci 29:497–524. https://doi.org/10.1111/j.1748-7692.2012.00584.x
Kleynhans EJ, Jolles AE, Bos MR, Olff H (2011) Resource partitioning along multiple niche dimensions in differently sized African savanna grazers. Oikos 120:591–600. https://doi.org/10.1111/j.1600-0706.2010.18712.x
Kliska K, McIntosh RR, Jonsen I, Hume F, Dann P, Kirkwood R, Harcourt R (2022) Environmental correlates of temporal variation in the prey species of Australian fur seals inferred from scat analysis. Royal Society Open Science 9(10):211723
Knox TC, Baylis AM, Arnould JP (2017) Habitat use and diving behaviour of male Australian fur seals. Mar Ecol Prog Ser 566:243–256. https://doi.org/10.3354/meps12027
Knox TC, Baylis AM, Arnould JP (2018) Foraging site fidelity in male Australian fur seals. Mar Biol 165:108. https://doi.org/10.1007/s00227-018-3368-1
Krebs C (2001) Ecology: the experimental analysis of distribution and abundance. Wesley Longman, San Francisco, CA, p 695
Kuhn CE, Baker JD, Towell RG, Ream RR (2014) Evidence of localized resource depletion following a natural colonization event by a large marine predator. J Anim Ecol 83:1169–1177. https://doi.org/10.1111/1365-2656.12202
Ladds MA, Slip D, Harcourt R (2017) Intrinsic and extrinsic influences on standard metabolic rates of three species of Australian otariid. Conserv Physiol 21:cow074
Lewis S, Sherratt T, Hamer K, Wanless S (2001) Evidence of intra-specific competition for food in a pelagic seabird. Nature 412:816–819. https://doi.org/10.1038/35090566
Liwanag HE (2010) Energetic costs and thermoregulation in northern fur seal (Callorhinus ursinus) pups: the importance of behavioral strategies for thermal balance in furred marine mammals. Physiol Biochem Zool 83:898–910. https://doi.org/10.1086/656426
Luiselli L (2006) Resource partitioning and interspecific competition in snakes: the search for general geographical and guild patterns. Oikos 114:193–211. https://doi.org/10.1111/j.2006.0030-1299.14064.x
Lyons E, DeLong R, Gulland F, Melin S, Tolliver S, Spraker T (2000) Comparative biology of Uncinaria spp. in the California sea lion (Zalophus californianus) and the northern fur seal (Callorhinus ursinus) in California. J Parasitol 86:1348–1352. https://doi.org/10.1645/0022-3395(2000)086[1348:CBOUSI]2.0.CO;2
MacArthur RH (1984) Geographical ecology: patterns in the distribution of species. Princeton University Press
MacArthur RH, Pianka ER (1966) On optimal use of a patchy environment. Am Nat 100:603–609. https://doi.org/10.1086/282454
MacArthur R, Levins R (1967) The limiting similarity, convergence, and divergence of coexisting species. Am Nat 101:377–385. https://doi.org/10.1086/282505
Majluf P, Trillmich F (1981) Distribution and abundance of sea lions (Otaria byronia) and fur seals (Arctocephalus australis) in Peru. Zeitschrift für Säugetierkunde 46:384–393. https://pub.uni-bielefeld.de/record/1781841
Martinez Cano I, Taboada FG, Naves J, Fernández-Gil A, Wiegand T (2016) Decline and recovery of a large carnivore: environmental change and long-term trends in an endangered brown bear population. Proc R Soc B Biol Sci 283:20161832. https://doi.org/10.1098/rspb.2016.1832
Matich P, Heithaus MR (2014) Multi‐tissue stable isotope analysis and acoustic telemetry reveal seasonal variability in the trophic interactions of juvenile bull sharks in a coastal estuary. J Anim Ecol 83:199–213. https://www.jstor.org/stable/24035058
McCutchan JH Jr, Lewis WM Jr, Kendall C, McGrath CC (2003) Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102:378–390. https://doi.org/10.1034/j.1600-0706.2003.12098.x
McIntosh RR, Kirkman SP, Thalman S, Southerland DR, Mitchell AT, Arnould JPY, Salton M, Slip D, Dann P, Kirkwood R (2018) Understanding meta-population trends of the Australian fur seal, with insights for adaptive monitoring. PLoS ONE 13:e0200253. https://doi.org/10.1371/journal.pone.0200253
Michener RH, Kaufman L (2007) Stable isotope ratios as tracers in marine food webs: an update. Stable Isot Ecol Environ Sci 2:238–282
Miller DD, Ota Y, Sumaila UR, Cisneros-Montemayor AM, Cheung WW (2018) Adaptation strategies to climate change in marine systems. Glob Change Biol 24:e1–e14. https://doi.org/10.1111/gcb.13829
Moleón M, Sánchez-Zapata JA, Real J, García-Charton JA, Gil-Sánchez JM, Palma L, Bautista J, Bayle P (2009) Large-scale spatio-temporal shifts in the diet of a predator mediated by an emerging infectious disease of its main prey. J Biogeogr 36:1502–1515. https://doi.org/10.1111/j.1365-2699.2009.02078.x
Navarro J, Votier SC, Aguzzi J, Chiesa JJ, Forero MG, Phillips RA (2013) Ecological segregation in space, time and trophic niche of sympatric planktivorous petrels. PLoS ONE 8:e62897. https://doi.org/10.1371/journal.pone.0062897
Nelms SE, Alfaro-Shigueto J, Arnould JP, Avila IC, Nash SB, Campbell E, Carter MI, Collins T, Currey RJ, Domit C, Franco-Trecu V (2021) Marine mammal conservation: over the horizon. Endanger Species Res 44:291–325
Newsome SD, Tinker MT, Monson DH, Oftedal OT, Ralls K, Staedler MM, Fogel ML, Estes JA (2009) Using stable isotopes to investigate individual diet specialization in California sea otters (Enhydra lutris nereis). Ecology 90:961–974. https://doi.org/10.1890/07-1812.1
Newsome SD, Clementz MT, Koch PL (2010) Using stable isotope biogeochemistry to study marine mammal ecology. Mar Mamm Sci 26:509–572. https://doi.org/10.1111/j.1748-7692.2009.00354.x
Newsome SD, Tinker MT, Gill VA, Hoyt ZN, Doroff A, Nichol L, Bodkin JL (2015) The interaction of intraspecific competition and habitat on individual diet specialization: a near range-wide examination of sea otters. Oecologia 178:45–59. https://doi.org/10.1007/s00442-015-3223-8
Niella Y, Smoothey AF, Peddemors V, Harcourt R (2020) Predicting changes in distribution of a large coastal shark in the face of the strengthening East Australian Current. Mar Ecol Prog Ser 642:163–177. https://doi.org/10.3354/meps13322
Niella Y, Butcher P, Holmes B, Barnett A, Harcourt R (2021) Forecasting intraspecific changes in distribution of a wide-ranging marine predator under climate change. Oecologia:1–14. https://doi.org/10.1007/s00442-021-05075-7
Novak M, Tinker MT (2015) Timescales alter the inferred strength and temporal consistency of intraspecific diet specialization. Oecologia 178:61–74. https://doi.org/10.1007/s00442-014-3213-2
Pablo-Rodríguez N, Aurioles-Gamboa D, Montero-Muñoz JL (2016) Niche overlap and habitat use at distinct temporal scales among the California sea lions (Zalophus californianus) and Guadalupe fur seals (Arctocephalus philippii townsendi). Mar Mamm Sci 32:466–489. https://doi.org/10.1111/mms.12274
Pacala S, Roughgarden J (1982) 07/30). Resource partitioning and interspecific competition in two two-species insular anolis lizard communities. Science 217:444–446. https://doi.org/10.1126/science.217.4558.444
Páez-Rosas D, Aurioles-Gamboa D, Alava JJ, Palacios DM (2012) Stable isotopes indicate differing foraging strategies in two sympatric otariids of the Galapagos Islands. J Exp Mar Biol Ecol 424:44–52. https://doi.org/10.1016/j.jembe.2012.05.001
Page B, McKenzie J, Goldsworthy SD (2005a) Dietary resource partitioning among sympatric New Zealand and Australian fur seals. Mar Ecol Prog Ser 293:283–302. https://doi.org/10.3354/meps293283
Page B, McKenzie J, Goldsworthy SD (2005b) Inter-sexual differences in New Zealand fur seal diving behaviour. Mar Ecol Prog Ser 304:249–264. https://doi.org/10.3354/meps304249
Page B, McKenzie J, Sumner MD, Coyne M, Goldsworthy SD (2006) Spatial separation of foraging habitats among New Zealand fur seals. Mar Ecol Prog Ser 323:263–279. https://doi.org/10.3354/meps323263
Peres-Neto PR, Legendre P, Dray S, Borcard D (2006) Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87:2614–2625. https://doi.org/10.1890/0012-9658(2006)87[2614:VPOSDM]2.0.CO;2
Petraitis PS (1979) Likelihood measures of niche breadth and overlap. Ecology 60:703–710. https://doi.org/10.2307/1936607
Pianka ER (1974) Niche overlap and diffuse competition. Proc Natl Acad Sci 71:2141–2145. https://doi.org/10.1073/pnas.71.5.2141
Pielou E (1972) Niche width and niche overlap: a method for measuring them. Ecology 53:687–692. https://doi.org/10.2307/1934784
Pigot AL, Tobias JA (2013) Species interactions constrain geographic range expansion over evolutionary time. Ecol Lett 16:330–338. https://doi.org/10.1111/ele.12043
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2021) nlme: Linear and nonlinear mixed effects models. R package version 3.1-153. https://CRAN.R-project.org/package=nlme
Prati S, Henriksen EH, Smalås A, Knudsen R, Klemetsen A, Sánchez-Hernández J, Amundsen P-A (2021) The effect of inter-and intraspecific competition on individual and population niche widths: a four-decade study on two interacting salmonids. Oikos 130:1679–1691. https://doi.org/10.1111/oik.08375
R Core Team (2020) R: a language and environment for statistical computing. In: R Foundation for Statistical Computing. http://www.R-project.org
Ratcliffe N, Adlard S, Stowasser G, McGill R (2018) Dietary divergence is associated with increased intra-specific competition in a marine predator. Sci Rep 8:1–10. https://doi.org/10.1038/s41598-018-25318-7
Ray GC (1991) Coastal-zone biodiversity patterns. Bioscience 41:490–498. https://doi.org/10.2307/1311807
Reddy CS, Yosef R, Calvi G, Fornasari L (2019) Inter-specific competition influences apex predator–prey populations. Wildl Res 46:628–638. https://doi.org/10.1071/WR19011
Ridgway KR (2007) Long‐term trend and decadal variability of the southward penetration of the East Australian Current. Geophys Res Lett 34. https://doi.org/10.1029/2007GL030393
Riverón S, Raoult V, Baylis AMM, Jones KA, Slip DJ, Harcourt RG (2021) Pelagic and benthic ecosystems drive differences in population and individual specializations in marine predators. Oecologia 196:891–904. https://doi.org/10.1007/s00442-021-04974-z
Roberts CM, O’Leary BC, McCauley DJ, Cury PM, Duarte CM, Lubchenco J, Pauly D, Sáenz-Arroyo A, Sumaila UR, Wilson RW (2017) Marine reserves can mitigate and promote adaptation to climate change. Proc Natl Acad Sci 114:6167–6175. https://doi.org/10.1073/pnas.1701262114
Robinson SA (2002) The foraging ecology of two sympatric fur seal species, Arctocephalus gazella and Arctocephalus tropicalis, at Macquarie Island during the austral summer. Mar Freshw Res 53:1071. https://doi.org/10.1071/MF01218
Rodríguez-Malagón MA, Speakman CN, Sutton GJ, Angel LP, Arnould JP (2021) Temporal and spatial isotopic variability of marine prey species in south-eastern Australia: Potential implications for predator diet studies. PLoS ONE 16:e0259961. https://doi.org/10.1371/journal.pone.0259961
Roughgarden J (1972) Evolution of niche width. Am Nat 106:683–718. https://doi.org/10.1086/282807
Ryan CJ, Hickling G, Wilson K-J (1997) Breeding habitat preferences of the New Zealand fur seal (Arctocephalus forsteri) on Banks Peninsula. Wildl Res 24:225–235. https://doi.org/10.1071/WR95068
Salton M, Kirkwood R, Slip D, Harcourt R (2019) Mechanisms for sex-based segregation in foraging behaviour by a polygynous marine carnivore. Mar Ecol Prog Ser 624:213–226. https://doi.org/10.3354/meps13036
Salton M, Carr M, Tarjan LM, Clarke J, Kirkwood R, Slip D, Harcourt R (2021) Protected area use by two sympatric marine predators repopulating their historical range. Endanger Species Res. https://doi.org/10.3354/esr01129
Schoener TW (1983) Field experiments on interspecific competition. Am Nat 122:240–285. https://doi.org/10.1086/284133
Schumann N, Gales NJ, Harcourt RG, Arnould JP (2013) Impacts of climate change on Australian marine mammals. Aust J Zool 61(2):146–159
Schwarz JF, Mews S, DeRango EJ, Langrock R, Piedrahita P, Páez-Rosas D, Krüger O (2021) Individuality counts: a new comprehensive approach to foraging strategies of a tropical marine predator. Oecologia 195:313–325. https://doi.org/10.1007/s00442-021-04850-w
Sequeira AM, Rodríguez J, Eguíluz VM, Harcourt R, Hindell M, Sims DW, Duarte CM, Costa DP, Fernández-Gracia J, Ferreira LC (2018) Convergence of marine megafauna movement patterns in coastal and open oceans. Proc Natl Acad Sci 115:3072–3077. https://doi.org/10.1073/pnas.1716137115
Shaughnessy PD, Goldsworthy SD (2015) Long-nosed fur seal: a new vernacular name for the fur seal, Arctocephalus forsteri, in Australia. Mar Mamm Sci 31:830–832. https://doi.org/10.1111/mms.12203
Shaughnessy PD, Briggs SV, Constable R (2001) Observations on seals at Montague Island, New South Wales. Aust Mammal 23:1–7. https://doi.org/10.1071/AM01001
Shaughnessy P, Goldsworthy S, Mackay A (2015) The long-nosed fur seal (Arctocephalus forsteri) in South Australia in 2013–14: abundance, status and trends. Aust J Zool 63:101–110. https://doi.org/10.1071/ZO14103
Stevens MA, Boness DJ (2003) Influences of habitat features and human disturbance on use of breeding sites by a declining population of southern fur seals (Arctocephalus australis). J Zool 260:145–152. https://doi.org/10.1017/S0952836903003583
Svanbäck R, Persson L (2004) Individual diet specialization, niche width and population dynamics: implications for trophic polymorphisms. J Anim Ecol 73:973–982. https://www.jstor.org/stable/3505532
Svanbäck R, Bolnick DI (2005) Intraspecific competition affects the strength of individual specialization: an optimal diet theory method. Evol Ecol Res 7:993–1012
Svanbäck R, Bolnick DI (2007) Intraspecific competition drives increased resource use diversity within a natural population. Proc R Soc B Biol Sci 274:839–844. https://doi.org/10.1098/rspb.2006.0198
Swanson HK, Lysy M, Power M, Stasko AD, Johnson JD, Reist JD (2015) A new probabilistic method for quantifying n-dimensional ecological niches and niche overlap. Ecology 96:318–324. https://doi.org/10.1890/14-0235.1
Syväranta J, Lensu A, Marjomäki TJ, Oksanen S, Jones RI (2013) An empirical evaluation of the utility of convex hull and standard ellipse areas for assessing population niche widths from stable isotope data. PLoS ONE 8:e56094. https://doi.org/10.1371/journal.pone.0056094
Thornton DH, Sunquist ME, Main MB (2004) Ecological separation within newly sympatric populations of coyotes and bobcats in south-central Florida. J Mammal 85:973–982. https://doi.org/10.1644/BEH-020
Tinker MT, Bentall G, Estes JA (2008) Food limitation leads to behavioral diversification and dietary specialization in sea otters. Proc Natl Acad Sci 105:560–565. https://doi.org/10.1073/pnas.0709263105
Tinker MT, Guimaraes PR Jr, Novak M, Marquitti FMD, Bodkin JL, Staedler M, Bentall G, Estes JA (2012) Structure and mechanism of diet specialisation: testing models of individual variation in resource use with sea otters. Ecol Lett 15:475–483. https://doi.org/10.1111/j.1461-0248.2012.01760.x
Tuomainen U, Candolin U (2011) Behavioural responses to human-induced environmental change. Biol Rev 86:640–657. https://doi.org/10.1111/j.1469-185X.2010.00164.x
Vales DG, Cardona L, García NA, Zenteno L, Crespo EA (2015) Ontogenetic dietary changes in male South American fur seals Arctocephalus australis in Patagonia. Mar Ecol Prog Ser 525:245–260. https://doi.org/10.3354/meps11214
Van Valen L (1965) Morphological variation and width of ecological niche. Am Nat 99:377–390. https://doi.org/10.1086/282379
Villegas-Amtmann S, Jeglinski JW, Costa DP, Robinson PW, Trillmich F (2013) Individual foraging strategies reveal niche overlap between endangered Galapagos pinnipeds. PLoS ONE 8:e70748. https://doi.org/10.1371/journal.pone.0070748
Wabakken P, Sand H, Liberg O, Bjärvall A (2001) The recovery, distribution, and population dynamics of wolves on the Scandinavian peninsula, 1978–1998. Can J Zool 79:710–725. https://doi.org/10.1139/z01-029
Warneke RM (1975) Dispersal and mortality of juvenile fur seals Arctocephalus pusillus doriferus in Bass Strait, southeastern Australia. Rapports Et Proces-Verbaux Des Reunions Conseil International Pour L’exploration De La Mer 169:296–302
Warneke R, Shaughnessy P (1985) Arctocephalus pusillus, the South African and Australian fur seal: taxonomy, evolution, biogeography, and life history. Studies of sea mammals in south latitudes, Adelaide
Wege M, Etienne MP, Chris Oosthuizen W, Reisinger RR, Bester MN, de Bruyn PN (2016) Trend changes in sympatric Subantarctic and Antarctic fur seal pup populations at Marion Island, Southern Ocean. Mar Mamm Sci 32:960–982. https://doi.org/10.1111/mms.12306
Wernberg T, Russell BD, Moore PJ, Ling SD, Smale DA, Campbell A, Coleman MA, Steinberg PD, Kendrick GA, Connell SD (2011) Impacts of climate change in a global hotspot for temperate marine biodiversity and ocean warming. J Exp Mar Biol Ecol 400:7–16. https://doi.org/10.1016/j.jembe.2011.02.021
Wickens P, York AE (1997) Comparative population dynamics of fur seals. Mar Mamm Sci 13:241–292. https://doi.org/10.1111/j.1748-7692.1997.tb00631.x
Wilson KL, Tittensor DP, Worm B, Lotze HK (2020) Incorporating climate change adaptation into marine protected area planning. Glob Change Biol 26:3251–3267. https://doi.org/10.1111/gcb.15094
Wolf JB, Harrod C, Brunner S, Salazar S, Trillmich F, Tautz D (2008) Tracing early stages of species differentiation: ecological, morphological and genetic divergence of Galápagos sea lion populations. BMC Evol Biol 8:1–14. https://doi.org/10.1186/1471-2148-8-150
Zaccarelli N, Mancinelli G, Bolnick DI (2013) RInSp: an R package for the analysis of individual specialisation in resource use. Methods Ecol Evol 4:1018–1023. https://doi.org/10.1111/2041-210X.12079
Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
We are grateful to the Australian Navy, New South Wales National Parks and Wildlife Service, Jervis Bay Marine Park and the Beecroft Ranger Station staff for logistical support and assistance with fieldwork. We thank Gemma Carrol, Matt Carr, Justin Clarke, Roger Kirkwood and David Slip for their invaluable field expertise and support.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. The research was financially supported by the Australian Marine Mammal Centre, and through the Australian Research Council Linkage grants LP110200603 & LP160100162. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
VR, IJ and RH conceived and designed the study. MS and RH sourced the resources and permits, and collected the data with support of field teams. MS, VR and IJ processed the data. MS analysed the data with feedback from VR, IJ and RH. MS wrote and prepared the manuscript, and VR, IJ and RH provided critical revisions of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics approval
All the procedures were conducted under Office of Environment and Heritage Animal Ethics Committee Approval (100322/03) and Macquarie University Ethics Committee Approval 2011/054. Research Permits (SL 100111 and SL 100746) and all relevant institutional and national guidelines for the care and use of animals were followed.
Consent to participate
Not applicable.
Consent for publication
All the authors read and gave approval for publication to the final version of the manuscript.
Additional information
Communicated by Helene Marsh.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salton, M., Raoult, V., Jonsen, I. et al. Niche partitioning and individual specialisation in resources and space use of sympatric fur seals at their range margin. Oecologia (2024). https://doi.org/10.1007/s00442-024-05537-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00442-024-05537-8