Abstract
Purpose
Glioblastoma (GB) is the most common primary malignant brain tumor with the highest incidence occurring in older adults with a median age at diagnosis of 64 years old. While treatment often improves survival it brings toxicities and adverse events (AE). Here we identify sex differences in treatment patterns and AE in individuals ≥ 66 years at diagnosis with GB.
Methods
Using the SEER-Medicare dataset sex differences in adverse events were assessed using multivariable logistic regression performed to calculate the male/female odds ratio (M/F OR) and 95% confidence intervals [95% CI] of experiencing an AE adjusted for demographic variables and Elixhauser comorbidity score.
Results
Males with GB were more likely to receive standard of care (SOC; Surgery with concurrent radio-chemotherapy) [20%] compared to females [17%], whereas females were more likely to receive no treatment [26%] compared to males [21%].
Females with GB receiving SOC were more likely to develop gastrointestinal disorders (M/F OR = 0.76; 95% CI,0.64–0.91, p = 0.002) or blood and lymphatic system disorders (M/F OR = 0.79; 95% CI,0.66–0.95, p = 0.012). Males with GB receiving SOC were more likely to develop cardiac disorders (M/F OR = 1.21; 95% CI,1.02–1.44, p = 0.029) and renal disorders (M/F OR = 1.65; 95% CI,1.37–2.01, p < 0.001).
Conclusions
Sex differences for individuals, 66 years and older, diagnosed with GB exist in treatment received and adverse events developed across different treatment modalities.
Similar content being viewed by others
Introduction
Glioblastoma (GB) is the most common type of glioma accounting for approximately 60% of all gliomas and 51% of all malignant brain tumors [1]. In the United States (US), the incidence rate for GB is 5 per 100,000 persons, with a 5-year relative survival of less than 7% [1]. In adults, the risk of developing a GB increases with age, with the median age at diagnosis of a GB being 64 years old [2]. Males have been shown to have increased GB risk and worse GB survival rates when compared to females [1, 3].
Despite aggressive multi-modal therapies, GB remains uniformly lethal. Progression-free survival is dictated by response to extent of surgical resection and radiation and chemotherapy. Current standard of care (SOC) for GB was established nearly two decades ago (i.e. the Stupp protocol) and confers only a modest increase in overall survival [4,5,6]. When first established, the SOC protocol for GB was limited to individuals under 70 year-old [4] and there is still a limited number of clinical trials involving elderly individuals [7]. The lack of rigorous information on clinical outcomes in the aging population often leads to treatment strategies based on physician and/or individual preference, taking into consideration prognostic survival benefits as well as potential toxicities and risk of adverse events (AE) [8,9,10,11]. AEs can range from mild disorders to life-threatening conditions, including increased risk of neurological deficiency after surgical intervention, fibrosis after radiation treatment, and psychiatric disorders and cardiovascular complications following chemotherapy [12,13,14,15,16]. Multimodal treatment approaches increase the risk of tissue toxicities compared to a single treatment approach [17].
Recent work has suggested the presence of a sex-bias in the development of AE in individuals receiving cancer treatment [18,19,20]. However, the impact of various treatment modalities on the development of AE in individuals with GB has not been closely examined. We aim to fill this gap in knowledge by utilizing the population-based data available from the Surveillance, Epidemiology and End Results (SEER) Program-Medicare dataset to investigate sex differences in AEs based on treatment modalities in individuals with GB.
Methods
Study design and setting
Individuals diagnosed with GB were obtained from the SEER-Medicare dataset, a population based data registry collecting demographic, clinical, and cause of death information from individuals with cancer covering about 28% of the US population [21]. Medicare is a US federally funded health insurance program primarily involving individuals that are 65 and older. Medicare claims are insurance billing data for treatment or diagnostic procedures. Medicare claims data are linked with the SEER dataset, through an agreement with the National Cancer Institute (NCI) and the Center for Medicare Services (CMS), providing additional information on older adults with cancer. This study was conducted at the NCI and was determined to be human subjects exempt by the National Institute of Health (NIH) Office of Human Subjects Research.
Claims data
The following claims files from SEER-Medicare were utilized in this study: Durable Medical Equipment (DME), Carrier Claims (NCH), Outpatient Claims, Medicare Provider Analysis and Review (MedPAR), and Medicare Part D Data. DME and NCH files contained data on physician-performed procedures and visits while the Outpatient Claims file contained outpatient procedures. The MedPAR file contained data on hospitalization and the Medicare Part D data contained information on prescription drugs. AE were collected from DME, NCH, Outpatient, and MedPAR data files.
Inclusion/exclusion criteria
A total of 12,165 individuals diagnosed between 2004 and 2017 were selected for this study. Individuals were restricted to those aged 66 years and above to ensure a full year of claims data prior to the diagnosis for evaluating their comorbidities. Individuals with GB were selected using International Classification of Disease for Oncology, Third Edition (ICD-O-3) histopathology codes (9440, 9441, 9442/ 3, 9445) consistent with the reporting of Central Brain Tumor Registry of the United States (CBTRUS) [22]. Individuals were excluded if they were either enrolled in a Health Maintenance Organization (HMO) or if they lacked continuous Medicare part A and part B enrollment for 1 year prior to the date of diagnosis through the end of adverse event observation time (6–12 months after diagnosis) or date of death/last follow up, whichever occurred first.
Data collected
First-line cancer treatment information was collected from claims data using the following categories: surgery, radiation, and Temozolomide. Temozolomide is the most common GB chemotherapy agent and a key component of the current SOC for GB. Treatment modalities were identified in the claims data using ICD9/10 procedure codes, Healthcare Common Procedure Coding System (HCPCS) codes, and Current Procedural Terminology (CPT) codes (Supplemental Table 1). The SEER registry contains reliable information regarding individual treatment by both surgery and radiation [23, 24]. Thus, the SEER database was utilized to identify surgery and radiation treatment patterns for individuals lacking treatment data from the claims dataset. This approach maximized sample size for this rare tumor type. Individuals with any treatment modality in either Medicare claims data or surgery and radiation data in the SEER dataset within 6 months following diagnosis were classified as having received treatment. Individuals whose first-line therapy started more than 6 months after diagnosis were classified as receiving no treatment. To assess the impact of various treatment modalities, individuals with GB were analyzed by specific treatment modality as follows: SOC (surgery, radiation, and Temozolomide), surgery alone, Temozolomide alone, radiation alone, and any other pairwise combination of surgery, Temozolomide, and radiation.
Demographic data assessed included sex (male, female), ethnicity (Hispanic, non-Hispanic), age at diagnosis, and race (White, Black, “Other”). The race category “Other”, consisted of Native American, Alaskan Indian, Asian and Pacific Islander, combined due to the insufficient sample size to assess these groups separately. The Elixhauser comorbidity score was calculated with R package ‘Comorbidity 1.0.0’, using claims data and including all comorbidities 1 year prior to the date of GB diagnosis. Comorbidity scores were grouped into the following categories: 0–3, 4–6, 7–9, and > 9 [25].
Adverse events (AE)
AEs categories were identified using the standardized classification in the common terminology criteria of adverse event (CTCAE) designed to identify AEs in clinical trials. The CTCAE uses the Medical Dictionary for Regulatory Activities (MedDRA) code system to identify the AEs (Supplemental Table 2). MedDRA AE codes were converted to Systematized Nomenclature of Medicine (SNOMED) codes using MedDRA mapping tool. The resulting SNOMED codes were then converted to ICD10 codes. Further, we analyzed individual AEs that occurred between 0 and 6 months after the start of any treatment or, for individuals that received no treatment, after the diagnosis. AEs that occurred between the date of diagnosis and the start of treatment were excluded from the study. AE groups that were either not relevant to individuals over the age of 66, specific to reproductive organs, specific to congenital disorders, or had a small, limited number of individuals were excluded in the analysis. These included congenital, familial, and genetic disorders; immune system disorders; hepatobiliary disorders; injury, poisoning, and procedural complications; reproductive system and breast disorders; investigations; and pregnancy, puerperium and perinatal conditions.
AE categories analyzed include blood and lymphatic system disorders; cardiac disorders; ear and labyrinth disorders; endocrine disorders; eye disorders; gastrointestinal disorders; general disorders and administration site conditions (conditions of a general kind that result from or treatment of a disease); infections and infestations; metabolism and nutrition disorders; musculoskeletal and connective tissue disorders; neoplasms benign, malignant and unspecified (including cysts and polyps); nervous system disorders; psychiatric; renal and urinary disorders; respiratory, thoracic and mediastinal disorders; skin and subcutaneous tissue disorders; and vascular disorders. All AEs were treated as binary outcomes (yes/no).
Statistical analysis
All statistical analyses were performed using R 4.1 software. Descriptive statistics, stratified by sex, were used to assess differences in the sex distribution of demographic and clinical factors. Groups of individuals with each treatment modality combination were analyzed separately. Counts are suppressed when fewer than 11 cases were reported within a cell, or where the inclusion of the count would allow for back-calculation of suppressed values. The suppressed cases are included in the total counts for a given category.
Continuous data were assessed using t-test and categorical data were assessed with either Fisher’s exact test or Pearson’s Chi-squared test where appropriate. Multivariable logistic regression, adjusted for demographic variables and Elixhauser comorbidity score, was performed to calculate the male/female odds ratio (M/F OR) and 95% confidence intervals [95% CI] of experiencing an AE. Separate multivariable logistic regression models were performed for each AE category, among each treatment modality. All M/F ORs are reported as the odds of males experiencing an adverse event compared to females. Pearson correlation was used to assess the relationship between individual AEs to elucidate if the presence of certain events were driving the chance of experiencing other events. P values less than 0.05 were considered statistically significant.
Results
Study population and individual characteristics
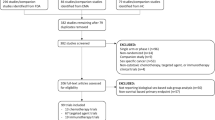
The SEER-Medicare dataset contained 244,133 individuals diagnosed with brain tumors. A total of 231,968 records were excluded for one or more reasons: (1) a histopathology other than GB, (2) brain tumor diagnosis before 2004, (3) a diagnosis of non-first sequence brain tumor, (4) age of diagnosis < 66 years, (5) and a record of HMO or non-continuous part A and part B enrollment (Fig. 1). After exclusion there were 12,165 individuals diagnosed with GB, consisting of 6,304 males (52%) and 5,861 females (48%).
Individuals diagnosed with GB were predominately White (92%, male to female ratio (m/f) 93%/92%) and non-Hispanic (93%). There was a significant sex difference in race (p < 0.001), but not in ethnicity (p = 0.3). The average age of an individual diagnosed with GB, in this Medicare population, was 75 years old. There was a significant sex difference in age of diagnosis (p < 0.001, m/f 75/76 years old) and Elixhauser score (p = 0.013) with more males having lower comorbidity score at diagnosis compared to females (0–3 m/f 48%/46%; 4–6 m/f 26%/25%; 7–9 m/f 16%/16%; >9 m/f 11%/13%). The average age of individuals with GB receiving SOC was 73 (m/f 73/73, p = 0.3) years old whereas the average age in those receiving no treatment was 79 (m/f 78/80, p < 0.001) years old. Only 2,218 (18%) individuals received SOC (surgery with concurrent radio-chemotherapy), while most individuals (59%) received other treatment regiments (surgery only, Temozolomide only, radiation only, and any other combination of surgery, Temozolomide, and radiation), and 2,830 (23%) received no cancer related treatment (Table 1). There was a significant sex difference in treatment modality received by individuals with GB: males were more likely to receive SOC compared to females (n = 1,238 [20%] males and 980 [17%] females, p < 0.001). A significant sex difference was observed in individuals receiving no treatment (1,307 males [21%], 1,523 females [26%], p < 0.001).
Adverse events (AE)
Results for individual AEs that occurred between 0 and 6 months after the start of treatment or, for individuals that received no treatment, after the diagnosis date (Fig. 2) are shown. Regardless of treatment modality, both males and females individually experienced on average 7 unique types of AEs. Nervous system disorders were the most common (78%) followed by vascular disorders (66%) (Table 2). All pairs of AEs had a correlation below 0.5 with the highest occurring between nervous system disorders and general and administration site conditions (r2 = 0.43) (Supplementary Fig. 1).
Renal and urinary disorders were more likely in males in most of the treatment modalities apart from Temozolomide only treatment, which had no significant sex difference. Females were more likely to develop endocrine disorders in most treatment modalities except for radiation only which did not show any significant sex difference. No significant sex difference in any of the treatment modality observed for the: eye disorders; neoplasms benign, malignant and unspecified including polyps; nervous system disorders, and vascular disorders.
Adverse events– no treatment
In patients receiving no treatment, males were more likely to develop cardiac disorders when receiving no treatment (M/F OR = 1.50; 95% CI,1.28–1.76, p = < 0.001) and were more likely to develop metabolism and nutrition disorders (M/F OR = 1.18; 95% CI,1.01–1.37, p = 0.033) (Fig. 3A). In addition, respiratory, thoracic and mediastinal disorders were more likely in males receiving no treatment (M/F OR = 1.30; 95% CI,1.11–1.52, p < 0.001) (Fig. 3A). In contrast, females were more likely to develop musculoskeletal and connective tissue disorders (M/F OR = 0.68; 95% CI,0.58–0.79, p < 0.001), infections and infestations (M/F OR = 0.68; 95% CI,0.58–0.79, p < 0.001), and psychiatric disorders (M/F OR = 0.75; 95% CI,0.64–0.88, p < 0.001) when receiving no treatment (Fig. 3A).
Adverse events- standard of care
In patients that received SOC, the combination of surgery, followed by concurrent radio-chemotherapy with Temozolomide, females had more types of AE when compared to males (Fig. 3B). Males were more likely to develop cardiac disorders (M/F OR = 1.21; 95% CI,1.02–1.44, p = 0.029) and renal disorders (M/F OR = 1.65; 95% CI,1.37–2.01, p < 0.001) when receiving SOC. Conversely, females were more likely to develop gastrointestinal disorders (M/F OR = 0.76; 95% CI,0.64–0.91, p = 0.002) (Fig. 3B) or blood and lymphatic system disorders (M/F OR = 0.79; 95% CI,0.66–0.95, p = 0.012) (Fig. 3B). Additionally, females were more likely to develop general and administration site conditions (M/F OR = 0.79; 95% CI,0.65–0.96, p = 0.02), infections and infestations (M/F OR = 0.72; 95% CI, 0.61–0.86, p < 0.001), and psychiatric disorders (M/F OR = 0.78; 95% CI,0.65–0.92, p = 0.004) when receiving SOC (Fig. 3B).
Adverse events– other modalities
Most GB patients received other treatment modalities, besides no treatment or SOC, including surgery and radiation, surgery and Temozolomide, radiation and Temozolomide, surgery alone, radiation alone, or Temozolomide only. Sex-differences in AE were also observed in these treatment modalities.
Males were more likely to develop cardiac disorders when receiving the combination of surgery and radiation (Fig. 3C) (M/F OR = 1.41; 95% CI,1.18–1.69, p < 0.001) and surgery alone (M/F OR = 1.31; 95% CI,1.11–1.55, p = 0.001) (Fig. 3D). Males were also more likely to develop ear and labyrinth disorders when receiving surgery only (M/F OR = 1.61; 95% CI,1.18–2.22, p = 0.003) (Fig. 3D) and respiratory, thoracic and mediastinal disorders when receiving the combination of surgery and radiation (M/F OR = 1.36; 95% CI,1.15–1.62, p < 0.001) (Fig. 3D), surgery and Temozolomide (M/F OR = 1.52; 95% CI,1.14–2.03, p = 0.005) (Fig. 3F) or surgery only (M/F OR = 1.49; 95% CI,1.27–1.76, p < 0.001) (Fig. 3D).
Females were more likely to develop gastrointestinal disorders (M/F OR = 0.82; 95% CI,0.69–0.97, p = 0.019) or infections and infestations (M/F OR = 0.7; 95% CI,0.59–0.83, p < 0.001) when receiving the combination of surgery and radiation (Fig. 3C). In contrast, males were more likely to develop gastrointestinal disorders when having a combination of surgery and Temozolomide (M/F OR = 1.49; 95% CI,1.12-2.00, p = 0.007) (Fig. 3F).
In addition, females were more likely to develop blood and lymphatic system disorders (M/F OR = 0.59; 95% CI,0.38–0.9 p = 0.016) and skin and subcutaneous tissue disorders (M/F OR = 0.58; 95% CI,0.34–0.98, p = 0.042) with a combination of radiation and Temozolomide (Fig. 3E). Musculoskeletal and connective tissue disorders were more likely in females receiving the combination of surgery and radiation (M/F OR = 0.81; 95% CI,0.68–0.96, p = 0.016) (Fig. 3C) or surgery only (M/F OR = 0.78; 95% CI,0.66–0.92, p = 0.003) (Fig. 3D). In those receiving radiation treatment only, females were more likely to develop infections and infestations (M/F OR = 0.57; 95% CI,0.41–0.79, p < 0.001) (Fig. 3G).
Discussion
Using SEER-Medicare data, this study provides evidence of sex differences in treatment patterns and subsequent AE following a GB diagnosis for adults ages 66 and older in the US. While previous studies have identified sex differences in cancer treatment-induced AEs [26,27,28], the analyses were limited to single treatment modalities. Here we assessed sex differences in AEs associated with both multimodal and single modality treatment for GB.
Most individuals with GB received either single or bimodal therapy (Table 1). Significantly more males with GB received SOC. In contrast, more females did not receive any type of treatment. This observation has been previously reported in other studies [29, 30]. A difference between those studies and the one here is that we focus on elderly individuals diagnosed with GB rather than patients across the age continuum. This study found that individuals with GB who did not receive treatment were significantly older than those receiving SOC. Further, females with GB who did not receive treatment were significantly older than males receiving no treatment and were more likely to have a higher number of comorbidities (Table 1). These results may suggest that there may be an age bias in the use of SOC to treat GB and that the presence of multiple comorbidities drives treatment decisions.
Elderly individuals often have comorbidities and due to perceived fragility may not be treated in the same way as younger individuals [31, 32]. These combined factors may result in both caregiver and patients being discouraged to pursue aggressive treatments [33]. We were interested to assess whether the differences in age and number of comorbidities in this dataset contributed to the apparent disparity in treatment between males and females with GB who did not receive treatment (Table 1). Further analysis of AE stratified by age group (66–75,75–85, >=85), demonstrated no significant differences between age groups (data not shown).
The Stupp protocol was established as the SOC for GB almost two decades ago [34, 35]. The studies performed establishing GB SOC were performed primarily in GB patients under 70 years of age [4]. Additional studies demonstrated GB SOC benefits in adults 60 years and older [36, 37]. Laperriere and colleagues expanded on these studies suggesting that the same SOC is recommended for individuals 65 and older with a ECOG performance status of 0–2, and suggesting that those older than 65 with higher ECOG scores should consider other treatment approaches [38]. The age and comorbidity scores of the Medicare population may contribute to the low level of individuals receiving SOC in this study.
When observing AEs post GB diagnosis among individuals who did not receive treatment, there are different sex biases observed compared to those who received SOC. While the odds of blood and lymphatic system disorders, gastrointestinal disorders, general and administrative site conditions were higher in females among those who received SOC, there was no statistically significant difference observed by sex among those who did not receive treatment. When looked at together, these findings may suggest that females are more likely to experience a higher variety of AEs after SOC. While these data demonstrate the presence of the sex-differences in AE. It is important to remain cognizant of the fact that these sex differences may be due to, or influenced by, biological, environmental exposure, and behavioral mechanisms.
Previous studies have shown that females receiving Temozolomide have increased risk of experiencing hematological toxicity [39]. In this study, females had significantly higher odds of experiencing blood and lymphatic system disorders when receiving the combination of radiation and Temozolomide. No significant sex differences were observed in other treatment modalities involving the use of Temozolomide. This result may suggest that in the absence of surgery, radiation treatment exacerbates Temozolomide’s potential for blood and lymphatic disorders in females. Deeper analysis into mechanism of such interactions warranted, though is beyond the scope of the current study.
A recent study utilizing mice models suggests that there are glioma-related gut microbiome alterations [40]. Additional studies have demonstrated that Temozolomide treatment further impacts the gut microbiome and that this effect may mitigate the changes induced by brain tumors, possibly through the mitigation of the tumor effects [41]. Based on these preliminary, biological studies, we may expect to see an impact of Temozolomide on gastrointestinal disorder AEs. It is important to note that these studies, did not account for sex as a biological variable. In this study, there were no sex differences in patients who did not receive treatment suggesting that the tumor itself does not promote the sex difference in gastrointestinal disorders observed here.
Further, we found that females had significantly higher odds of experiencing gastrointestinal disorders when receiving the combination of surgery and radiation. However, when given a combination of surgery and Temozolomide, the odds of GI AEs were significantly higher in males. These results may suggest that there may be a sex bias in the development of GI AE. Additional studies adjusting for sex as a biological variable in biological studies, will be required to provide insights on the impact of treatments on the gut microbiome and gastrointestinal related AE.
General and administration site conditions include symptomatic outcomes such as pain or fatigue. Females have been reported to suffer more from chronic pain when compared to men [42]. In addition, studies have demonstrated that female glioma patients suffer from fatigue to a greater extent than males [43]. In our study, there was no sex difference observed in patients who did not receive treatment, suggesting that the presence of the tumor alone did not contribute to a sex based difference in these AE. However, females were more likely to experience general and administration site conditions only after receiving SOC, with no other treatment modality showing a significant sex bias. The general and administration site conditions category consists of categories other than pain and fatigue, so these two AE likely do not contribute entirely to the results observed here. Currently, there is lack of studies focused on general and administration site conditions as a whole category and further analysis is required to dissect the contributing factors for these observations.
Receiving both chemotherapy and radiation therapy has been correlated to hearing problems [44, 45]. Further, surgical procedures in the brain can result in fluid buildup and swelling which may impact ear and labyrinth disorders [46, 47]. Thus, it may not be surprising to find these types of AEs in patients with brain tumors. There were no significant sex differences observed in those individuals receiving no treatment, perhaps suggesting that any ear and labyrinth disorders caused by the tumor itself does not have a sex bias. Interestingly, ear and labyrinth disorders were significantly more likely to occur in males who received surgery only. This may suggest that adverse events to surgery, may have a sex-bias and further studies will be necessary to fully evaluate this potential. While occupational exposures, through employment and lifestyle, may impact sex differences in hearing loss in males, to a higher extent than females, studies have suggest that this difference between males and females becomes nominal at older ages [48]. Studies have also implicated sex-hormone signaling pathways in hearing loss [49, 50]. Thus, it may be also interesting to hypothesize that these mechanisms are involved in the sex-differences in AE to treatment response that we see here, though the exact mechanisms remain to be elucidated.
There are several important limitations to this study. First, this study was limited to claims data associated with Medicare insurance, and it was not possible to identify procedures covered by private insurance, which may create bias. Second, this study assessed only Temozolomide, as it is the most recommended chemotherapy treatment for GB, although there might be an impact from other chemotherapy treatments as well. Third, it was not possible to ensure that surgery and radiation information extracted from the SEER was exactly within 6 months after the diagnosis, as SEER only provides year and month information of the first cancer therapy started without further time information on subsequent therapies. Next, the nature of Medicare beneficiaries prevents the collection of data prior to Medicare enrollment, which potentially limits the available amount of comorbidity data beyond one year prior to diagnosis. Finally, as GB can occur at any age, these results are not generalizable across all ages and are instead informative for older US adults only.
Conclusions
In conclusion, our study found that sex differences exist in GB treatment receipt. Males with GB were more likely to receive SOC. Across different treatment modalities sex differences exist in the odds of experiencing an AE for individuals 66 and older diagnosed with GB. Females were more likely to develop more types of AEs after SOC compared to males. While sex differences exist in occupational/environmental exposures, social behavior, seeking medical care, and types of comorbidities, and are important aspects to analyze, this work is not intended to begin to elucidate how these pre-treatment differences may directly impact treatment response. Nonetheless, regardless of the underlying mechanism, this work demonstrates that there are sex differences in AE in response to treatment. Thus, health care providers should consider how sex biases may impact treatment as well as the potential of developing an AE when discussing and developing treatment plans for individuals 66 or older with GB.
Data availability
The datasets used to conduct this study are available upon approval of a research protocol from the National Cancer Institute SEER-Medicare Program. Instructions for obtaining these data are available at https://healthcaredelivery.cancer.gov/seermedicare/obtain/.
References
Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C, Barnholtz-Sloan JS (2023) CBTRUS Statistical Report: primary brain and other Central Nervous System tumors diagnosed in the United States in 2016–2020. Neurooncology 25:iv1–iv99. https://doi.org/10.1093/neuonc/noad149
Tan AC, Ashley DM, López GY, Malinzak M, Friedman HS, Khasraw M (2020) Management of glioblastoma: state of the art and future directions. Cancer J Clin 70:299–312. https://doi.org/10.3322/caac.21613
Oronsky B, Reid TR, Oronsky A, Sandhu N, Knox SJ (2020) A review of newly diagnosed Glioblastoma. Front Oncol 10:574012. https://doi.org/10.3389/fonc.2020.574012
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med 352:987–996. https://doi.org/10.1056/NEJMoa043330
Fernandes C, Costa A, Osório L, Lago RC, Linhares P, Carvalho B, Caeiro C (2017) Current Standards of Care in Glioblastoma Therapy. In: De Vleeschouwer S (ed) Glioblastoma. Codon Publications Copyright: The Authors., Brisbane (AU)
Jordan JT, Gerstner ER, Batchelor TT, Cahill DP, Plotkin SR (2016) Glioblastoma care in the elderly. Cancer 122:189–197. https://doi.org/10.1002/cncr.29742
Young JS, Chmura SJ, Wainwright DA, Yamini B, Peters KB, Lukas RV (2017) Management of glioblastoma in elderly patients. J Neurol Sci 380:250–255. https://doi.org/10.1016/j.jns.2017.07.048
Liu Y, Wasilewski A, Mohile NA (2020) Disparities in patient enrollment on glioblastoma clinical trials. CNS Oncol 9:Cns59. https://doi.org/10.2217/cns-2020-0008
Murthy VH, Krumholz HM, Gross CP (2004) Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA 291:2720–2726. https://doi.org/10.1001/jama.291.22.2720
Pellerino A, Bruno F, Internò V, Rudà R, Soffietti R (2020) Current clinical management of elderly patients with glioma. Expert Rev Anticancer Ther 20:1037–1048. https://doi.org/10.1080/14737140.2020.1828867
Minniti G, Niyazi M, Alongi F, Navarria P, Belka C (2021) Current status and recent advances in reirradiation of glioblastoma. Radiat Oncol 16:36. https://doi.org/10.1186/s13014-021-01767-9
Lipitz-Snyderman A, Pfister D, Classen D, Atoria CL, Killen A, Epstein AS, Anderson C, Fortier E, Weingart SN (2017) Preventable and mitigable adverse events in cancer care: measuring risk and harm across the continuum. Cancer 123:4728–4736. https://doi.org/10.1002/cncr.30916
Jairam V, Lee V, Park HS, Thomas CR Jr, Melnick ER, Gross CP, Presley CJ, Adelson KB, Yu JB (2019) Treatment-related complications of systemic therapy and Radiotherapy. JAMA Oncol 5:1028–1035. https://doi.org/10.1001/jamaoncol.2019.0086
Yang M, Moon C (2013) Neurotoxicity of cancer chemotherapy. Neural Regen Res 8:1606–1614. https://doi.org/10.3969/j.issn.1673-5374.2013.17.009
Wong JM, Panchmatia JR, Ziewacz JE, Bader AM, Dunn IF, Laws ER, Gawande AA (2012) Patterns in neurosurgical adverse events: intracranial neoplasm surgery. Neurosurg Focus 33:E16. https://doi.org/10.3171/2012.7.Focus12183
Moloney EC, Brunner M, Alexander AJ, Clark J (2015) Quantifying fibrosis in head and neck cancer treatment: an overview. Head Neck 37:1225–1231. https://doi.org/10.1002/hed.23722
Morris ZS, Harari PM (2014) Interaction of radiation therapy with molecular targeted agents. J Clin Oncol 32:2886–2893. https://doi.org/10.1200/jco.2014.55.1366
Unger JM, Vaidya R, Albain KS, LeBlanc M, Minasian LM, Gotay CC, Henry NL, Fisch MJ, Lee SM, Blanke CD Hershman DL Sex Differences in Risk of Severe Adverse Events in Patients Receiving Immunotherapy, Targeted Therapy, or Chemotherapy in Cancer Clinical Trials. Journal of Clinical Oncology 0: JCO.21.02377 https://doi.org/10.1200/jco.21.02377
Özdemir BC, Csajka C, Dotto GP, Wagner AD (2018) Sex differences in efficacy and toxicity of systemic treatments: an undervalued issue in the era of Precision Oncology. J Clin Oncol 36:2680–2683. https://doi.org/10.1200/jco.2018.78.3290
Chmielewski-Stivers N, Petit B, Ollivier J, Monceau V, Tsoutsou P, Quintela Pousa A, Lin X, Limoli C, Vozenin M-C (2021) Sex-specific differences in toxicity following systemic Paclitaxel treatment and localized Cardiac Radiotherapy. Cancers 13:3973
Edward R, Berchick EH, Jessica C, Barnett (2018) Health Insurance Coverage in the United States: 2017. United States Census Bureau
Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C, Barnholtz-Sloan Jill S (2022) CBTRUS Statistical Report: primary brain and other Central Nervous System tumors diagnosed in the United States in 2015–2019. Neurooncology 24:v1–v95. https://doi.org/10.1093/neuonc/noac202
Cooper GS, Virnig B, Klabunde CN, Schussler N, Freeman J, Warren JL (2002) Use of SEER-Medicare data for measuring cancer surgery. Med Care 40:IV–43
Virnig BA, Warren JL, Cooper GS, Klabunde CN, Schussler N, Freeman J (2002) Studying radiation therapy using SEER-Medicare-linked data. Med Care 40:IV–49
Elixhauser A, Steiner C, Harris DR, Coffey RM (1998) Comorbidity measures for Use with Administrative Data. Med Care 36:8–27
Unger JM, Vaidya R, Albain KS, LeBlanc M, Minasian LM, Gotay CC, Henry NL, Fisch MJ, Lee SM, Blanke CD, Hershman DL (2022) Sex differences in risk of severe adverse events in patients receiving immunotherapy, targeted therapy, or Chemotherapy in Cancer clinical trials. J Clin Oncol 40:1474–1486. https://doi.org/10.1200/jco.21.02377
Wilcox NS, Rotz SJ, Mullen M, Song EJ, Ky Hamilton B, Moslehi J, Armenian SH, Wu JC, Rhee JW, Ky B (2022) Sex-Specific Cardiovascular risks of Cancer and its therapies. Circ Res 130:632–651. https://doi.org/10.1161/circresaha.121.319901
Hershman DL, McBride RB, Eisenberger A, Tsai WY, Grann VR, Jacobson JS (2008) Doxorubicin, cardiac risk factors, and cardiac toxicity in elderly patients with diffuse B-cell non-hodgkin’s lymphoma. J Clin Oncol 26:3159–3165. https://doi.org/10.1200/jco.2007.14.1242
Stabellini N, Krebs H, Patil N, Waite K, Barnholtz-Sloan JS (2021) Sex differences in Time to treat and outcomes for gliomas. Front Oncol 11:630597. https://doi.org/10.3389/fonc.2021.630597
Ostrom QT, Krebs HL, Patil N, Cioffi G, Barnholtz-Sloan JS (2021) Racial/ethnic disparities in treatment pattern and time to treatment for adults with glioblastoma in the US. J Neurooncol 152:603–615. https://doi.org/10.1007/s11060-021-03736-4
Gállego Pérez-Larraya J, Delattre JY (2014) Management of elderly patients with gliomas. Oncologist 19:1258–1267. https://doi.org/10.1634/theoncologist.2014-0170
Mason M, Laperriere N, Wick W, Reardon DA, Malmstrom A, Hovey E, Weller M, Perry JR (2016) Glioblastoma in the elderly: making sense of the evidence. Neurooncol Pract 3:77–86. https://doi.org/10.1093/nop/npv027
Braun K, Ahluwalia MS (2017) Treatment of Glioblastoma in older adults. Curr Oncol Rep 19:81. https://doi.org/10.1007/s11912-017-0644-z
Tavelin B, Malmström A (2022) Sex differences in glioblastoma-findings from the Swedish National Quality Registry for primary brain tumors between 1999–2018. J Clin Med 11. https://doi.org/10.3390/jcm11030486
Yang W, Warrington NM, Taylor SJ, Whitmire P, Carrasco E, Singleton KW, Wu N, Lathia JD, Berens ME, Kim AH, Barnholtz-Sloan JS, Swanson KR, Luo J, Rubin JB (2019) Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Sci Transl Med 11:eaao5253. https://doi.org/10.1126/scitranslmed.aao5253
Zhang C, Wang X, Hao S, Su Z, Zhang P, Li Y, Song G, Yu L, Wang J, Ji N, Xie J, Gao Z (2016) Analysis of treatment tolerance and Factors Associated with overall survival in Elderly patients with Glioblastoma. World Neurosurg 95:77–84. https://doi.org/10.1016/j.wneu.2016.07.079
Kushnir I, Tzuk-Shina T (2011) Efficacy of treatment for glioblastoma multiforme in elderly patients (65+): a retrospective analysis. Isr Med Assoc J 13:290–294
Laperriere N, Weller M, Stupp R, Perry JR, Brandes AA, Wick W, van den Bent MJ (2013) Optimal management of elderly patients with glioblastoma. Cancer Treat Rev 39:350–357. https://doi.org/10.1016/j.ctrv.2012.05.008
Bae SH, Park MJ, Lee MM, Kim TM, Lee SH, Cho SY, Kim YH, Kim YJ, Park CK, Kim CY (2014) Toxicity profile of temozolomide in the treatment of 300 malignant glioma patients in Korea. J Korean Med Sci 29:980–984. https://doi.org/10.3346/jkms.2014.29.7.980
Herbreteau A, Aubert P, Croyal M, Naveilhan P, Billon-Crossouard S, Neunlist M, Delneste Y, Couez D, Aymeric L (2022) Late-stage glioma is Associated with deleterious alteration of gut bacterial metabolites in mice. Metabolites 12. https://doi.org/10.3390/metabo12040290
Patrizz A, Dono A, Zorofchian S, Hines G, Takayasu T, Husein N, Otani Y, Arevalo O, Choi HA, Savarraj J, Tandon N, Ganesh BP, Kaur B, McCullough LD, Ballester LY, Esquenazi Y (2020) Glioma and temozolomide induced alterations in gut microbiome. Sci Rep 10:21002. https://doi.org/10.1038/s41598-020-77919-w
Sorge RE, Totsch SK (2017) Sex differences in Pain. J Neurosci Res 95:1271–1281. https://doi.org/10.1002/jnr.23841
Valko PO, Siddique A, Linsenmeier C, Zaugg K, Held U, Hofer S (2014) Prevalence and predictors of fatigue in glioblastoma: a prospective study. Neurooncology 17:274–281. https://doi.org/10.1093/neuonc/nou127
Schultz C, Goffi-Gomez MV, Pecora Liberman PH, Pellizzon AC, Carvalho AL (2010) Hearing loss and complaint in patients with head and neck cancer treated with radiotherapy. Arch Otolaryngol Head Neck Surg 136:1065–1069. https://doi.org/10.1001/archoto.2010.180
Marosi C (2012) Chap. 57 - complications of chemotherapy in neuro-oncology. In: Grisold W, Soffietti R (eds) Handbook of clinical neurology. Elsevier, pp 873–885
Jackson C, Westphal M, Quiñones-Hinojosa A (2016) Chap. 12 - complications of glioma surgery. In: Berger MS, Weller M (eds) Handbook of clinical neurology. Elsevier, pp 201–218
Satzer D, Guillaume DJ (2016) Hearing loss in hydrocephalus: a review, with focus on mechanisms. Neurosurg Rev 39:13–25. https://doi.org/10.1007/s10143-015-0650-2
Homans NC, Metselaar RM, Dingemanse JG, van der Schroeff MP, Brocaar MP, Wieringa MH, Baatenburg de Jong RJ, Hofman A, Goedegebure A (2017) Prevalence of age-related hearing loss, including sex differences, in older adults in a large cohort study. Laryngoscope 127:725–730. https://doi.org/10.1002/lary.26150
Shuster BZ, Depireux DA, Mong JA, Hertzano R (2019) Sex differences in hearing: probing the role of estrogen signaling. J Acoust Soc Am 145:3656–3663. https://doi.org/10.1121/1.5111870
Gillies GE, McArthur S (2010) Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev 62:155–198. https://doi.org/10.1124/pr.109.002071
Acknowledgements
This project utilizes data from the California Cancer registry, as such the collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Sect. 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 1NU58DP007156; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors.
Funding
The research performed by Jill S. Barnholtz-Sloan, Mantas Dmukauskas, Gino Cioffi and Kristin A. Waite was provided by the Division of Cancer Epidemiology and Genetics (DCEG) of the National Cancer Institute (NCI).
Author information
Authors and Affiliations
Contributions
MD: investigation, data curation, methodology, formal analysis and interpretation, visualization, writing– original draft and editing. GC: investigation, data curation methodology, formal analysis and interpretation, visualization, writing– original draft and editing. KAW: investigation, visualization, interpretation, funding acquisition, project administration, supervision, writing– original draft and editing. AES: methodology, interpretation, writing-original draft and editing. CN: formal analysis and interpretation, visualization, writing-original draft and editing. MP: formal analysis and interpretation, visualization, writing-original draft and editing. QTO: investigation, methodology, interpretation, visualization, writing– review and editing. JSBS: conceptualization, data curation, funding acquisition, investigation, methodology, project administration, resources, supervision, writing– review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare. Jill S. Barnholtz-Sloan is a full-time paid employee of the NIH/NCI. Mantas Dmukauskas is a full time fellow of the NIH/NCI. Gino Cioffi and Kristin A. Waite are full-time contractors of the NIH/NCI.
Ethics approval
This study was conducted at the NCI and was determined to be human subjects exempt by the National Institute of Health (NIH) Office of Human Subjects Research.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
: Supplemental Figure 1: Correlation Matrix Between Each AE Used in This Study. Correlation between each adverse event developed by individuals with glioblastoma is calculated and shown in a matrix format. More intense red color indicates higher correlation between the adverse events
Supplementary Material 2
: Supplementary Table 1. Codes Used to Identify Treatment Patterns. Table contains treatment codes that were used to identify 3 treatment categories: surgery, radiation and Temozolomide. Supplementary Table 2. CTCAE AE categories. Adverse events with corresponding medDRA codes that were used in this study
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dmukauskas, M., Cioffi, G., Waite, K.A. et al. Sex differences in adverse events in Medicare individuals ≥ 66 years of age post glioblastoma treatment. J Neurooncol (2024). https://doi.org/10.1007/s11060-024-04652-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11060-024-04652-z