Abstract
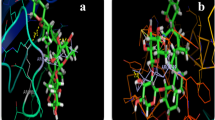
Enzyme inhibition is one of the leading drug development methods for the treatment of many diseases. Due to the possible side effects and low bioavailability of existing drugs, studies are continuing for the discovery of new drugs. In this study, in vitro elastase, acetylcholinesterase inhibition activities of newly synthesized N,S-substituted polyhalogenated nitrobuta-1,3-dienes derivatives (compounds 3, 4a, 4b, 4c, 4d, 4e, and 4f), as well as their antioxidant properties, were investigated. Results was showed that compounds 4a (IC50 = 22.10 ± 0.49 µM), 4b (IC50 = 53.98 ± 1.77 µM), and 4f (IC50 = 32.01 ± 1.33 µM) showed higher elastase inhibition effect than positive control, ursolic acid (IC50 = 479.11 ± 15.53 µM) and in silico adsorption, distribution, metabolism, excretion, and toxicity (ADMET) and molecular docking studies were carried out in line with the results. As a result of molecular docking studies with iGemdock, DockThor, and Autodock Vina, it was determined that there was a higher binding relevance for compounds 4a (− 84.41/− 8.439 kcal/mol), 4b (− 86.32/− 7.878 kcal/mol), and 4f (− 86.32/− 8.530 kcal/mol) than ursolic acid (− 77.67/− 7.024 kcal/mol) for iGemdock and DockThor. It has been shown by DPPH, ABTS, and reducing power experiments that all compounds also show antioxidant properties. In conclusion, both in vitro and in silico molecular docking studies of compounds 4a, 4b, and 4f show that these three compounds are potent inhibitors of elastase.







Similar content being viewed by others
References
L. Bianchi, G. Giorgi, M. Maccagno, G. Petrillo, C. Scapolla, C. Tavani, Tetrahedron 72, 7050 (2016)
S. Karimi, S. Ma, Y. Liu, K. Ramig, E.M. Greer, K. Kwon, W.F. Berkowitz, G. Subramaniam, Tetrahedron Lett. 58, 2223 (2017)
L. Bianchi, M. Maccagno, M. Pani, G. Petrillo, C. Scapolla, C. Tavani, Tetrahedron 71, 7421 (2015)
V.A. Zapol’skii, J.C. Namyslo, M. Gjikaj, D.E. Kaufmann, Beilstein J. Org. Chem. 10, 1638 (2014)
M. Bürgi, V.A. Zapol’skii, B. Hinkelmann, M. Köster, D.E. Kaufmann, F. Sasse, H. Hauser, M. Etcheverrigaray, R. Kratje, M. Bollati-Fogolín, M. Oggero, J. Biotechnol. 233, 6 (2016)
C. Tavani, L. Bianchi, A. De Palma, G.I. Passeri, G. Punzi, C.L. Pierri, A. Lovece, M.M. Cavalluzzi, C. Franchini, G. Lentini, G. Petrillo, Bioorg. Med. Chem. Lett. 27, 3980 (2017)
G. Bolger, S. Roy, V.A. Zapol’skii, D.E. Kaufmann, M. Schnürch, M.D. Mihovilovic, R.K. Nandy, W. Tegge, J. Med. Microbiol. 65, 678 (2016)
V.A. Zapol’skii, R. Fischer, J.C. Namyslo, D.E. Kaufmann, Bioorg. Med. Chem. 17, 4206 (2019)
V.A. Zapol’skii, J.C. Namyslo, G. Sergeev, M. Brönstrup, M. Gjikaj, D.E. Kaufmann, Eur. J. Org. Chem. 2015, 7763 (2015)
G. Sergeev, S. Roy, M. Jarek, V. Zapolskii, D.E. Kaufmann, R.K. Nandy, W. Tegge, BMC Microbiol. 14, 49 (2014)
R. Fischer, P. Jeschke, A. Lubos-Erdelen, P. Lösel, U. Reckmann, D. E. Kaufmann, V. A. Zapol’skii, A. G. Bayer, US Patent, US 7,332,512 B2 (2008)
S. Mahavir, D. E. Kaufmann, V. A. Zapol’skii, W. Oehlmann, European Patent Application EP 2 829 536 A1 20150128 (2015)
Y.-H. Won, M.-S. Park, Arch. Pharm. Res. 33, 189 (2010)
H. Nian, B. Delage, J.T. Pinto, R.H. Dashwood, Carcinogenesis 29, 1816 (2008)
K.H. Kyung, Curr. Opin. Biotechnol. 23, 142 (2012)
S. Melino, R. Sabelli, M. Paci, Amino Acids 41, 103 (2010)
N. Onul, O. Ertik, N. Mermer, R. Yanardag, J. Chem. 2018, 1 (2018)
B.H.S. Cho, S. Xu, Comp. Biochem. Physiol. C Toxicol. Pharmacol. 126, 195 (2000)
F. Antonicelli, G. Bellon, L. Debelle, W. Hornebeck, Curr. Top. Dev. Biol. 79, 99 (2007)
M.P. Giovannoni, I.A. Schepetkin, M.T. Quinn, N. Cantini, L. Crocetti, G. Guerrini, A. Iacovone, P. Paoli, P. Rossi, G. Bartolucci, M. Menicatti, C. Vergelli, J. Enzyme Inhib. Med. Chem. 330, 1108 (2018)
E. Deryugina, A. Carré, V. Ardi, T. Muramatsu, J. Schmidt, C. Pham, J.P. Quigley, iScience 23, 101799 (2020)
B. Donarska, K.Z. Łączkowski, Future Med. Chem. 12, 1809 (2020)
A. Nunes, J. Marto, L.M. Gonçalves, S. Simões, R. Félix, A. Ascenso, F. Lopes, H.M. Ribeiro, Pharmaceutics 12, 358 (2020)
J.A. Voynow, S. Zheng, A.B. Kummarapurugu, Front. Pharmacol. 11, 1011 (2020)
H. Akıncıoğlu, İ Gülçin, Mini-Rev. Med. Chem. 20, 703 (2020)
G. Marucci, M. Buccioni, D.D. Ben, C. Lambertucci, R. Volpini, F. Amenta, Neuropharmacology 190, 108352 (2021)
V.E. Semenov, I.V. Zueva, M.A. Mukhamedyarov, S.V. Lushchekina, E.O. Petukhova, L.M. Gubaidullina, E.S. Krylova, L.F. Saifina, O.A. Lenina, K.A. Petrov, Molecules 25, 4191 (2020)
N. Maddu, in: Antioxidants’, Ed. E. Shalaby, IntechOpen, (2019)
A.K. Mitra, J. Chem. Rev. 2, 243 (2020)
D. Sayın, D.T. Çakır, N. Gençer, O. Arslan, J. Enzyme Inhib. Med. Chem. 27, 595 (2020)
Y.A. Oldekop, R.V. Kaberdin, V.I. Potkin, Zh. Prikl. Khim. 22, 1389 (1986)
C. Ibis, N. Onul, Phosphorus Sulfur Silicon Relat. Elem. 81, 2411 (2006)
J.-Y. Moon, E.-Y. Yim, G. Song, N.H. Lee, C.-G. Hyun, EurAsian J. Biosci. 4, 41 (2010)
K. Ingkaninan, P. Temkitthawon, K. Chuenchom, T. Yuyaem, W. Thongnoi, J. Ethnopharmacol. 89, 261 (2023)
W. Brand-Williams, M.E. Cuvelier, C. Berset, LWT—Food Sci. Tech. 28, 25 (1995)
M.B. Arnao, A. Cano, M. Acosta, Food Chem. 73, 239 (2001)
M. Oyaizu, Jpn. J. Nutr. Diet 44, 307 (1986)
J. Jiménez, S. Doerr, G. Martínez-Rosell, A.S. Rose, G. De Fabritiis, Bioinformatics 33, 3036 (2017)
S. Kim, P.A. Thiessen, E.E. Bolton, J. Chen, G. Fu, A. Gindulyte, L. Han, J. He, S. He, B.A. Shoemaker, J. Wang, B. Yu, J. Zhang, S.H. Bryant, Nucleic Acids Res. 44, D1202 (2015)
A.K. Rappe, C.J. Casewit, K.S. Colwell, W.A. Goddard III., W.M. Skiff, J. Am. Chem. Soc. 114, 10024 (1992)
K.-C. Hsu, Y.-F. Chen, S.-R. Lin, J.-M. Yang, BMC Bioinform. 12, S33 (2011)
C.S. de Magalhães, D.M. Almeida, H.J.C. Barbosa, L.E. Dardenne, Inf. Sci. 289, 206 (2014)
K.B. Santos, I.A. Guedes, A.L.M. Karl, L.E. Dardenne, J. Chem. Inform. Model. 60, 667 (2020)
I.A. Guedes, A.M.S. Barreto, D. Marinho, E. Krempser, M.A. Kuenemann, O. Sperandio, L.E. Dardenne, M.A. Miteva, Sci. Rep. 11, 3198 (2021)
O. Trott, A.J. Olson, J. Comput. Chem. 31, 455 (2009)
J. Eberhardt, D. Santos-Martins, A.F. Tillack, S. Forli, J. Chem. Inf. Model. 61, 3891 (2021)
D.E.V. Pires, T.L. Blundell, D.B. Ascher, J. Med. Chem. 58, 4066 (2015)
M. Jamroz, A. Kolinski, S. Kmiecik, Nucleic Acids Res. 41, W42 (2013)
S. Kmiecik, D. Gront, M. Kolinski, L. Wieteska, A.E. Dawid, A. Kolinski, Chem. Rev. 116, 7898 (2016)
S. Ahmad, M. Saleem, N. Riaz, Y.S. Lee, R. Diri, A. Noor, D. Almasri, A. Bagalagel, M.F. Elsebai, Front. Pharmacol. 11, 688 (2020)
E. Polo, L. Prent-Peñaloza, Y.A.R. Núñez, L. Valdés-Salas, J. Trilleras, J. Ramos, J.A. Henao, A. Galdámez, A. Morales-Bayuelo, M. Gutiérrez, J. Mol. Struct. 1224, 129307 (2021)
K. Jakimiuk, J. Gesek, A.G. Atanasov, M. Tomczyk, J. Enzyme Inhib. Med. Chem. 36, 1016 (2021)
N.F. Brás, R. Gonçalves, N. Mateus, P.A. Fernandes, M.J. Ramos, V. de Freitas, J. Agric. Food Chem. 58, 10668 (2010)
O. Ertik, F. Danışman Kalındemirtaş, B. Kaya, R. Yanardag, S. ErdemKuruca, O. Şahin, B. Ülküseven, Polyhedron 202, 115192 (2021)
N. Hosseini Nasab, H. Raza, Y.S. Eom, M. Hassan, A. Kloczkowski, S.J. Kim, Bioorg. Med. Chem. 86, 117292 (2023)
N. Onul, O. Ertik, N. Mermer, R. Yanardag, J. Biochem. Mol. Toxicol. 32, e22021 (2017)
G. Pizzino, N. Irrera, M. Cucinotta, G. Pallio, F. Mannino, V. Arcoraci, F. Squadrito, D. Altavilla, A. Bitto, Oxid. Med. Cell. Longev. 2017, 1 (2017)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors also declare that they have no confict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bayrak, B.B., Ertik, O., Onul, N. et al. Synthesis novel N,S-substituted nitrobutadiene derivatives: some metabolic enzyme inhibition properties and antioxidant activities and in silico ADMET and molecular docking studies. J IRAN CHEM SOC 21, 1299–1315 (2024). https://doi.org/10.1007/s13738-024-02999-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-024-02999-8