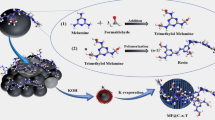
Abstract
Propylene is one of the most important olefins in the industry, and conventional separation of propylene/propane relies on low-temperature distillation, which consumes huge amounts of energy. The adsorption separation technique requires low energy consumption and has developed into an important method for the separation of propylene/propane. In this work, chitosan is used as the carbon source, and Cu(II) is immobilized in the molecule through the coordination of –OH and –NH2 in chitosan with Cu(II). By freeze-drying and high-temperature calcination, Cu(II) in chitosan was reduced to Cu(I), resulting in a porous carbon material containing Cu(I). The adsorption properties of the materials were tested for propylene and propane at 298 K, 1 bar. The Langmuir–Freundlich model was used to fit the adsorption curves and the IAST theory was combined to calculate the selectivity of propylene/propane. The adsorption capacity of carbon materials for propane/propylene under different preparation conditions showed that the best adsorption separation performance for propylene/propane was obtained when the freezing temperature difference was 15 ℃ − (− 18 ℃) and the concentrations of Cu(II) were 0.025 mol/L and 0.0125 mol/L. The breakthrough experiment using a propylene/propane mixture (VC3H6/VC3H8 = 50/50) revealed that the Chitosan-Cu (0.025 mol/L) material exhibited a lower adsorption capacity for propane, as compared to its higher adsorption capacity for propylene. Therefore, the adsorption selectivity of propylene/propane reached an impressive 3.877, revealing the potential of carbon materials in the separation of propylene/propane.
Graphical abstract










Similar content being viewed by others
References
Abbasi S, Khosravi-Nikou MR, Shariati A (2023) Selective separation of propane from the propylene-propane mixture using pure silica zeolites: a molecular dynamic simulation. Chem Eng Process 184:109294. https://doi.org/10.1016/j.cep.2023.109294
Andrade M, Parnell AJ, Bernardo G, Mendes A (2021) Propane selective carbon adsorbents from phenolic resin precursor. Micropor Mesopor Mater 320:111071. https://doi.org/10.1016/j.micromeso.2021.111071
Bae YS, Lee CY, Kim KC, Farha OK, Nickias P, Hupp JT, Nguyen ST, Snurr RQ (2012) High propene/propane selectivity in isostructural metal-organic frameworks with high densities of open metal sites. Angew Chem Int Ed 51:1857–1860. https://doi.org/10.1002/anie.201107534
Basaldella EI, Tara JC, Armenta GA, Iglesias MEP (2007) Cu/SBA -15 as adsorbent for propane/propylene separation. J Porous Mater 14:273–278. https://doi.org/10.1007/s10934-006-9062-6
Cadiau A, Adil K, Bhatt PM, Belmabkhout Y, Eddaoudi M (2016) A metal-organic framework-based splitter for separating propylene from propane. Science 353:137–140. https://doi.org/10.1126/science.aaf6323
Chang ZD, Lin RB, Ye YX, Duan CY, Chen BL (2019) Construction of a thiourea-based metal-organic framework with open Ag+ sites for the separation of propene/propane mixtures. J Mater Chem A 7:25567–25572. https://doi.org/10.1039/C9TA08614E
Choi C, Nam JP, Nah JW (2016) Application of chitosan and chitosan derivatives as biomaterials. J Ind Eng Chem 33:1–10. https://doi.org/10.1016/j.jiec.2015.10.028
da Silva FA, Rodrigues AE (1999) Adsorption equilibria and kinetics for propylene and propane over 13X and 4A zeolite pellets. Ind Eng Chem Res 38:2051–2057. https://doi.org/10.1021/ie980640z
da Silva FA, Rodrigues AE (2001a) Vacuum swing adsorption for propylene/propane separation with 4A zeolite. Ind Eng Chem Res 40:5758–5774. https://doi.org/10.1021/ie0008732
da Silva FA, Rodrigues AE (2001b) Propylene/propane separation by vacuum swing adsorption using 13X zeolite. AIChE J 47:341–357. https://doi.org/10.1002/aic.690470212
da Silva FA, Silva JA, Rodrigues AE (1999) A general package for the simulation of cyclic adsorption processes. Adsorption 5:229–244. https://doi.org/10.1023/A:1008974908427
Deville S, Saiz E, Nalla RK, Tomsia AP (2006) Freezing as a path to build complex composites. Science 311:515–518. https://doi.org/10.1126/science.1120937
Ding Q, Zhang S (2022a) Recent advances in the development of metal-organic frameworks for propylene and propane separation. Energy Fuels 36:7337–7361. https://doi.org/10.1021/acs.energyfuels.2c01427
Ding Q, Zhang S (2022b) Recent advances in the development of metal-organic frameworks for propylene and propane separation. Energy Fuels 36:7337–7736. https://doi.org/10.1021/acs.energyfuels.2c01427
Du SJ, Huang JW, Anjum AW, Xiao J, Li Z (2021) A novel mechanism of controlling ultramicropore size in carbons at sub-angstrom level for molecular sieving of propylene/propane mixtures. J Mater Chem a 9:23873–23881. https://doi.org/10.1039/D1TA07261G
Eldridge RB (1993) Olefin/paraffin separation technology: a review. Ind Eng Chem Res 32:2208–2212. https://doi.org/10.1021/ie00022a002
Ferreira AFP, Santos JC, Plaza MG, Lamia N, Loureiro JM, Rodrigues AE (2011) Suitability of Cu-BTC extrudates for propane-propylene separation by adsorption processes. Chem Eng J 167:1–12. https://doi.org/10.1016/j.cej.2010.07.041
Gao F, Wang YQ, Wang X, Wang SH (2016) Selective CO adsorbent CuCl/AC prepared using CuCl2 as a precursor by a facile method. RSC Adv 6:34439–34446. https://doi.org/10.1039/c6ra03116a
Gao JK, Cai YL, Qian XF, Liu PX, Wu H, Zhou W, Liu DX, Li LB, Lin RB, Chen BL (2021) A microporous hydrogen-bonded organic framework for the efficient capture and purification of propylene. Angew Chem Int Ed 60:20400–20406. https://doi.org/10.1002/anie.202106665
Geier SJ, Mason JA, Bloch ED, Queen WL, Hudson MR, Brown CM, Long JR (2013) Selective adsorption of ethylene over ethane and propylene over propane in the metal-organic frameworks M2(dobdc) (M=Mg, Mn, Fe Co, Ni, Zn). Chem Sci 4:2054–2061. https://doi.org/10.1039/C3SC00032J
Jarvelin H, Fair JR (1993) Adsorptive separation of propylene-propane mixtures. Ind Eng Chem Res 32:2201–2207. https://doi.org/10.1021/ie00022a001
Jiang HF, Chen Y, Song SQ, Guo ZY, Zhang ZQ, Zheng CY, He GW, Wang HJ, Wu H, Huang T, RenYX LX, Zhang JF, Yin Y, Jiang Y, Guiver MD (2022) Confined facilitated transport within covalent organic frameworks for propylene/propane membrane separation. Chem Eng J 439:135657. https://doi.org/10.1016/j.cej.2022.135657
Jorg M, Lamia N, Rodrigues AE (2009) Molecular simulation of propane/propylene separation on the metal-organic framework Cu-BTC. Colloids Surf A 357:104–108. https://doi.org/10.1016/j.colsurfa.2009.08.025
Ko CH, Han SS, Park JH, Cho SH, Kim JN (2006) Silver nitrate impregnated pellet-type adsorbents for propylene/propane separation. Ind Eng Chem Res 45:9129–9135. https://doi.org/10.1021/ie051119p
Lee CY, Bae YS, Jeong NC, Farha OK, Sarjeant AA, Stern CL, Nickias P, Snurr RQ, Hupp JT, Nguyen ST (2011) Kinetic separation of propene and propane in metal-organic frameworks: controlling diffusion rates in plate-shaped crystals via tuning of pore apertures and crystallite aspect ratios. J Am Chem Soc 133:5228–5231. https://doi.org/10.1021/ja200553m
Liu PX, Chen KM, Chen Y, Wang XQ, Yang JF, Li LB, Li JP (2022) Linker micro-regulation of a hofmann-based metal-organic framework for efficient propylene/propane separation. Inorg Chem Front 9:1082–1090. https://doi.org/10.1039/d1qi01562a
Neagu M, Cursaru DL (2013) Thermal energy approach of a conventional propylene/propane splitter. Rev Chim 64:880–885. https://doi.org/10.37358/Rev.Chim.1949
Padin J, Yang RT (2000) New sorbents for olefin/paraffin separations by adsorption via π-complexation: synthesis and effects of substrates. Chem Eng Sci 55:2607–2616. https://doi.org/10.1016/S0009-2509(99)00537-0
Patrulea V, Negrulescu A, Mincea MM, Pitulice LD, Spiridon OB, Ostafe V (2013) Optimization of the removal of copper (II) ions from aqueous solution on chitosan and cross-linked chitosan beads. BioResources 8:1147–1165. https://doi.org/10.15376/biores.8.1.1147-1165
Shahmirzadi MAA, Kargari A, Matsuura T (2022) Separation of propylene/propane using IL/Silver ion facilitated transport: Insights from computational molecular approach. J Mol Liq 360:119480. https://doi.org/10.1016/j.molliq.2022.119480
Sholl DS, Lively RP (2016) Seven chemical separations to change the world. Nature 532:435–437. https://doi.org/10.1038/533316a
Takahashi A, Yang FH, Yang RT (2004) Aromatics/aliphatics separation by adsorption: new sorbents for selective aromatics adsorption by π -complexation. Ind Eng Chem Res 39:3856–3867. https://doi.org/10.1021/ie000376l
Tong YS, Xing JC, Lou CY, Yuan DH, Huang W, Chen ZA, Liu ZM, Xu YP (2021) Efficient separation of propylene and propane on SAPO-17 molecular sieve. Can J Chem 99:570–575. https://doi.org/10.1139/cjc-2020-0489
van Miltenburg A, Gascon J, Zhu WD, Kapteijn F, Moulijn JA (2008) Propylene/propane mixture adsorption on faujasite sorbents. Adsorption 14:309–321. https://doi.org/10.1007/s10450-007-9101-x
Wang Y, Huang NY, Zhang XW, He H, Huang RK, Ye ZM, Li Y, Zhou DD, Liao PQ, Chen XM, Zhang JP (2019) Selective aerobic oxidation of a metal-organic framework boosts thermodynamic and kinetic propylene/propane selectivity. Angew Chem Int Ed 58:7692–7696. https://doi.org/10.1002/anie.201902209
Wang XB, Zhang PX, Zhang ZQ, Yang LF, Ding Q, Cui XL, Wang J, Xing HB (2020) Efficient separation of propene and propane using anion-pillared metal-organic frameworks. Ind Eng Chem Res 59:3531–3537. https://doi.org/10.1021/acs.iecr.9b06294
Wu ZB, Han SS, Cho SH, Kim JN, Chue K, Chue KT, Yang RT (1997) Modification of resin-type adsorbents for ethane/ethylene separation. Ind Eng Chem Res 36:2749–2756. https://doi.org/10.1021/ie970185r
Xiong Y, Woodward RT, Danaci D, Evans A, Tian T, Azzan H, Ardakani M, Petit C (2021) Understanding trade-offs in adsorption capacity, selectivity and kinetics for propylene/propane separation using composites of activated carbon and hypercrosslinked polymer. Chem Eng J 426:131628. https://doi.org/10.1016/j.cej.2021.131628
Xiong Y, Tian T, L’Hermitte A, Mendez ASJ, Danaci D, Platero-Prats AE, Petit C (2022) Using silver exchange to achieve high uptake and selectivity for propylene/propane separation in zeolite Y. Chem Eng J 446:137104. https://doi.org/10.1016/j.cej.2022.137104
Yoon BH, Lee EJ, Kim HE, Koh YH (2007) Highly aligned porous silicon carbide ceramics by freezing polycarbosilane/camphene solution. J Am Ceram Soc 90:1753–1759. https://doi.org/10.1111/j.1551-2916.2007.01703.x
Yoon JW, Kim AR, Kim MJ, Yoon TU, Kim JH, Bae YS (2019) Low-temperature Cu(I) loading on a mesoporous metal-organic framework for adsorptive separation of C3H6/C3H8 mixtures. Micropor Mesopor Mat 279:271–277. https://doi.org/10.1016/j.micromeso.2018.12.041
Yu ZY, Li Y, Feng ZP, Zhang ZH, Li P, Chen Y, Chen SS, Li PW, Yang ZM (2019) Cu+-containing physically crosslinked chitosan hydrogels with shape memory. Express Polym Lett 13:785–793. https://doi.org/10.3144/expresspolymlett.2019.67
Yuan YF, Wang YS, Zhang XL, Li WC, Hao GP, Han L, Lu AH (2021) Wiggling mesopores kinetically amplify the adsorptive separation of propylene/propane. Angew Chem Int Ed 60:19063–19067. https://doi.org/10.1002/anie.202106523
Zhang ZQ, Ding Q, Cui XL, Jiang XM, Xing HB (2020) Fine-tuning and selective-binding within an anion-functionalized ultramicroporous metal-organic framework for efficient olefin/paraffin separation. ACS Appl Mater Interfaces 12:40229–40235. https://doi.org/10.1021/acsami.0c07800
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22125802). The authors gratefully acknowledge these grants.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jin, D., Tu, Y., Zhang, Z. et al. Preparation of chitosan-copper material and its application on propylene-propane adsorption separation. Chem. Pap. (2024). https://doi.org/10.1007/s11696-024-03422-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11696-024-03422-5