Abstract
The environmental contamination caused by antibiotics is increasingly conspicuous due to their widespread manufacture and misuse. Plasma has been employed in recent years for the remediation of antibiotic pollution in the environment. In this work, a falling-film dielectric barrier discharge was used to degrade the antibiotic tetracycline (TC) in water. The reactor combined the gas-liquid discharge and active gas bubbling to improve the TC degradation performance. The discharge characteristics, chemical species' concentration, and degradation rates at different parameters were systematically studied. Under the optimized conditions (working gas was pure oxygen, liquid flow rate was 100 mL/min, gas flow rate was 1 L/min, voltage was 20 kV, single treatment), TC was removed beyond 70% in a single flow treatment with an energy efficiency of 145 mg/(kW·h). The reactor design facilitated gas and liquid flow in the plasma area to produce more ozone in bubbles after a single flow under pure oxygen conditions, affording fast TC degradation. Furthermore, long-term stationary experiment indicated that long-lived active species can sustain the degradation of TC. Compared with other plasma treatment systems, this work offers a fast and efficient degradation method, showing significant potential in practical industrial applications.
Export citation and abstract BibTeX RIS
1. Introduction
Antibiotics are extensively used in human and animal disease control, agricultural production, animal husbandry, and aquaculture. However, the environmental contamination caused by antibiotics is increasingly conspicuous due to their widespread manufacture and misuse. Specifically, antibiotics can endanger other higher organisms through food chain transmission, destroy ecosystem balance, and induce the expression of antibiotic-resistance genes [1, 2]. Conventional wastewater treatment processes mainly include physical [3, 4], chemical [5], biological [6], and photodegradation [7]. Physical methods generally serve as a collection role without further degradation. The use of chemical reagents in the chemical method might result in secondary contamination. Additionally, the specific impact of antibiotics on microorganisms hinders the application of biological methods.
The advanced oxidation process (AOPs) has the potential to generate highly oxidative species in situ, it is investigated as an effective method for the degradation of antibiotics [8]. Among these methods, non-thermal plasma (NTP) technology, powered by electrical energy and coupled with renewable energy sources, has gained attention as a promising AOP due to its simplicity, high efficiency, and absence of secondary contamination during liquid remediation [9]. Plasma can produce chemically diverse active species (e.g., ·OH, ·HO2, ·O, O3, and H2O2), which can further store or react with liquids without secondary contamination [10]. In addition, high-energy electrons, shock waves, UV radiation, and electrohydraulic cavitation might have an additional effect on antibiotic removal [11].
Dielectric barrier discharge (DBD) is a popular method for generating low-temperature plasma by high-voltage discharges [12]. It is convenient, stable, extensible [13], and can be applied in high conductivity solutions and complex water quality [14, 15], being widely used in wastewater treatment [16]. For example, Yu et al [17] used a liquid film DBD to degrade N, N-diethyl-m-toluidine in water (DEET) and found that the degradation efficiency and rate constant reached 76.8% and 0.0526 min, respectively, after a 27 min treatment. Hijosa-Valsero et al [18] compared a flat plate reactor and a coaxial thin film flow device for the treatment of cyanide solution. Their results showed that the degradation rate of the plate reactor was higher than that of the coaxial liquid film reactor, but the energy efficiency of the latter was superior. Additionally, the degradation efficiency of both reactors was higher than that of the Fenton-like method and the TiO2 photocatalytic method. Kozakova et al [19] employed three plasma systems to decompose two organic textile dyes. The highest discoloration efficiency of over 90% was reached in the water-falling film DBD when maximal energy of 270 and 450 kJ/L was applied into Reactive Yellow 125 and Direct Red 79 dye solutions, respectively. In another work, Magureanu et al [8] found that the thin liquid film configuration of the reactor could provide a large surface-to-volume ratio due to the small penetration depth of the reactive species. In summary, the liquid film configuration of DBD shows excellent antibiotic removal in water and might develop into an appropriate alternative in wastewater treatment.
In practical industrial applications, it is essential for water treatment devices to operate at a high treatment rate. In existing studies, many systems operate in a fixed volume of wastewater and need a long time to obtain a higher degradation rate. While cyclic treatment can completely degrade the antibiotics and achieve a high degradation, it also requires a long treatment time and hardly meets the fast treatment. Therefore, this study aimed to induce a coaxial falling-film DBD reactor to further improve the treatment efficiency and speed. By increasing the gap between the media, more gases and liquids could enter the reactor, increasing the treatment rate. During treatment, the untreated wastewater flowed through the reactor and was treated just in a single flow, and the reactor combined the gas-liquid discharge and active gas bubbles to improve the degradation performance. This approach ensured that the generated active species were fully and effectively utilized, enabling a relatively high degradation rate of antibiotics in water. Figure 1(a) illustrates the research framework delineated in this article.
Fig. 1 (a) Efficiently degradation of the antibiotic (TC) is by strong oxidants, such as H2O2, O3, and ·OH, produced in situ by the dielectric barrier discharge plasma in single flow treatment. (b) The diagram of the treatment system.
Download figure:
Standard image2. Experimental section
2.1. Experimental setup
Figure 1(b) shows the diagram of the treatment system, which consists of an AC power supply (CTP-2000 K, 30 kV, 10 kHz, Suman), a falling-film DBD reactor, a gas and liquid inlet system, as well as the electrical and chemical measurement system. The reactor contains a coaxial DBD cylinder, consisting of two quartz tubes and electrodes with a length of 30 cm. The ground electrode is composed of the metal powder filled in the inner quartz glass tube with an outer diameter of 0.8 cm. Metal powder can be completely filled inside the quartz tube to eliminate the gap between the electrode and the quartz tube, thus mitigating the potential effects of this gap on discharge. The high-voltage electrode is a copper mesh (18 cm in length) attached to the outer quartz tube with a diameter of 2.5 cm. The thickness of the quartz dielectric was approximately 1 mm. The double-layer media design not only serves as a dielectric but also separates the electrode from the liquid and gas to prevent the electrode from being contaminated and lost. A metal flange fixes the reactor, and there are inlet and outlet holes on its top and side, respectively. The antibiotic wastewater is pumped into the reactor through a peristaltic pump (UIP, 0‒660 mL/min, Kamoer). The working gases are pure Ar and O2, and the gas flow rates are controlled by two glass rotor flowmeters (LZB-3WB, 0‒1 L/min, Shuang Huan), respectively. The liquid flows from the upper part of the reactor and down along the outside of the inner quartz glass tube, forming a water film. At the same time, the gas will enter the reactor through the inlet hole and fill the reactor, thus the gas-liquid phase discharge occurs in the reactor. In each experiment, a 500 mL solution is processed. Under the optimal conditions set for this experiment (liquid flow rate is 100 mL/min), the treatment time of these solutions requires 5 min.
UV-Vis spectrophotometer (UV3500s, Onlab) is employed for the detection of concentrations of TC, O3, and H2O2 in the liquid phase. EEMs (F-7000, HITACHI) is utilized for the detection of ·OH. The emission spectrometer (USB2000+, Ocean) is used to detect the emission spectra during gas discharge processes. The voltage and current are monitored by a high-voltage probe (PVM‐1, divider ratio is 2000:1, NorthStar) and a current probe (6595, divider ratio is 0.5 V/A; Pearson), respectively, and recorded by an oscilloscope (DPO2024B, Tektronix). Figure 2(a) shows a typical Lissajous figure. The value of the charge required to build the Lissajous figure is obtained by a capacitance (CM = 3 nF) in series with the reactor. The discharge power of the reactor is
Fig. 2 (a) Typical Lissajous figure (voltage = 20 kV), (b) tetracycline absorbance in solution before and after degradation, absorbance of (c) O3 and (d) H2O2 in solution with different ratios of O2 and Ar (The experimental parameters: liquid flow rate is 100 mL/min, gas flow rate is 1 L/min, voltage is 20 kV, single treatment).
Download figure:
Standard image

where P is the discharge power, f is the voltage frequency, U is the voltage applied to the reactor, UM is the voltage applied to the capacitor, I is the current flowing through the capacitor, and Q is the amount of charge that passes through the capacitor.
2.2. Treatment process and chemical characterization
In the experiment, TC solution with an original concentration of 5 mg/L was treated. By adjusting the gas-mixing ratio, discharge voltage, gas and liquid flow rate, the TC concentrations were quantitatively measured using the UV-Vis at the wavelength of 357 nm. The TC degradation rate at different parameters was calculated as follows:

where C0 and C1 refer to the original concentration and concentration after treatment, respectively.
The concentration of H2O2 was determined photometrically by titanium sulfate [20]. The concentration of O3 was quantified using the potassium indigo trisulfate in the phosphoric acid environment [20]. In addition, hydroxyl groups were determined using terephthalic acid in three-dimensional fluorescence spectroscopy [21].
The energy yield of the treatment system for the TC degradation was defined as follows:

where L and P refer to the liquid flow rate and discharge power, respectively.
Figure 2(b) shows the absorbance changes before and after the degradation of tetracycline. It can be seen that after degradation, the absorbance of the solution at 357 nm significantly decreases, which proved that tetracycline degradation occurs. Figures 2(c) and (d) show the change of absorbance of ozone and hydrogen peroxide detection reagents with different mixing ratios of oxygen and argon, respectively, which also proved the existence of ozone and hydrogen peroxide in the solution. It was of significance to mention that all the aforementioned substances were synthesized and detected within a medium of deionized water (pH = 7.1), devoid of any interference from tetracycline.
3. Results and discussion
3.1. Physicochemical properties of the falling-film DBD plasma
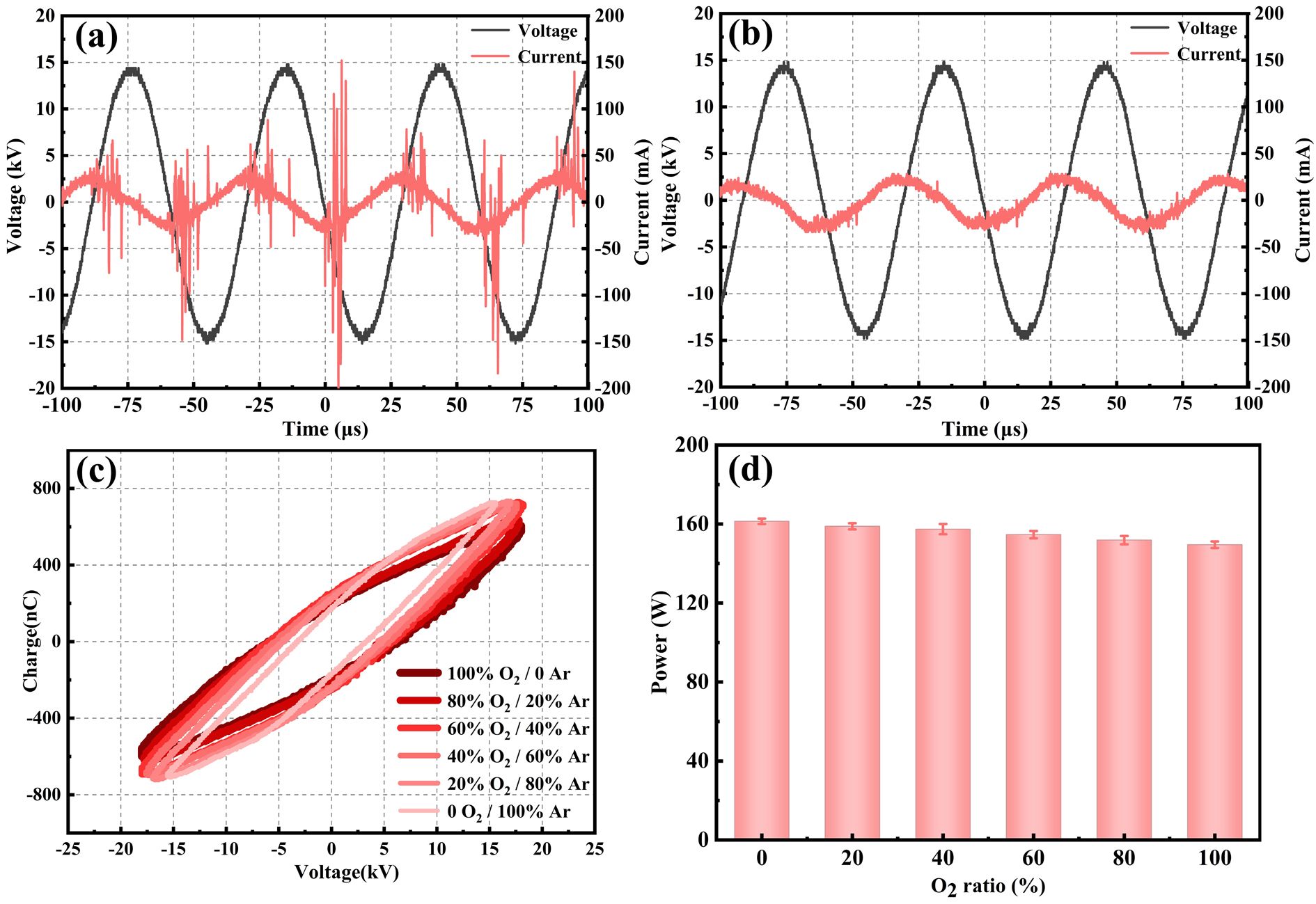
The electrical characteristics of the reactor were first exhibited, including the voltage, current, and power. Figures 3(a) and (b) show the current and voltage waveforms under oxygen and argon, respectively. It can be observed that the phase of the discharge current is approximately 90° earlier than the applied voltage because the DBD reactor works as a capacitive load. In the current waveform, the discharge pulses represented the micro-discharge channels in the reactor, corresponding to a filamentary discharge mode [22]. Once the discharge occurs, the micro discharge channels appear until the applied voltage reaches the peak. When the voltage was negative, the discharge pulse amplitude increased, which indicated that the discharge was concentrated in the negative half. At the applied voltage of 15 kV, the amplitude of current pulses under argon gas conditions was smaller, and the pulses were more uniformly dispersed. It is suggested that filamentary discharge was not the primary discharge mode occurring in the reactor with argon gas because argon is a single atomic molecule and easily breaks down. The voltage-current waveform of argon gas discharge revealed the coexistence of filamentary discharge and arc discharge. In water treatment, it is generally believed that the filamentary discharge is more favorable to producing active species [23].
Fig. 3 Voltage and current waveforms under the conditions of (a) oxygen, (b) argon, (c) Lissajous at different Ar/O2 ratios, (d) discharge power at different Ar/O2 ratios.
Download figure:
Standard imageFigure 3(c) shows the Lissajous figures at different Ar/O2 ratios. The slope of each side of the parallelogram changed with the gas mixture ratios, which indicated that the change of gas atmosphere affects the capacitance value of the reactor and its resonant frequency. The addition of Ar increased the effective dielectric capacitance and enhanced the charge transfer process [24]. Figure 3(d) illustrates the discharge power under various gas compositions. The discharge power of the reactor can be obtained about 150 W according to equation (
Figure 4 shows the emission spectra in the oxygen and argon atmosphere, which record the particles produced during the discharge process. The presence of water film resulted in the quenching of ·OH, thus the ·OH peak at 309 nm was not obvious in the figure. In contrast, the peak at 309 nm in the argon atmosphere was stronger than that in the oxygen atmosphere, indicating that more ·OH was produced in the argon atmosphere. In the emission spectrum of pure argon, high-intensity Ar atomic spectral lines were detected in the range of 690‒850 nm, and the excited oxygen atomic spectral lines at 777 nm were weaker than those in pure oxygen atmosphere. ·OH radical and excited oxygen atom are both active substances with strong oxidation, which might promote the degradation of tetracycline under the condition of pure oxygen.
Fig. 4 Emission spectra under (a) oxygen and (b) argon atmospheres.
Download figure:
Standard image3.2. Reactive species for antibiotic degradation
To investigate the effect of different active species on tetracycline degradation, three representatively active species including ozone, hydrogen peroxide, and hydroxyl radicals were tested because we believed that antibiotics could be removed by the active species dissolved in water. Notably, deionized water was used as a substitute for antibiotic effluent to accurately determine the concentration of active species at different working gases because other substances might consume the active species.
Figures 5(a)–(c) show the trends of the active species at different O2/Ar ratios. The concentration of O3 dissolved in water increased with the oxygen ratio, and the highest concentration of O3 was obtained under pure oxygen conditions, reaching about 8 mM (figure 5(a)). O3 was produced by cycloaddition, electrophilic and nucleophilic reactions with organic substances because of its special dipole structure and electrophilic and nucleophilic properties [25]. The production of O3 occurred mainly in the process of micro-discharge in the DBD. During a single micro discharge, electrons were accelerated in the electric field, and the accelerated high-energy electrons collide with oxygen molecules to form oxygen atoms, and the three-body collision reaction will form O3. The following reactions can describe this process [26].
Fig. 5 Change trend of (a) O3, (b) H2O2, (c) ·OH with oxygen ratio, (d) degradation rate and energy yield with different oxygen ratios (liquid flow rate = 100 mL/min, gas flow rate = 1 L/min, voltage = 20 kV, single treatment).
Download figure:
Standard image


where M referred to O or O2
According to the Penning effect, the presence of sub-stable argon will promote the ionization of oxygen, which will produce more ozone. However, the results were different from the expected because the power supply injected enough energy into the reactor at a discharge voltage of 20 kV to produce a plasma with enough high-energy electrons to ionize the oxygen fully. The positive effect of argon addition on oxygen ionization is not obvious.
The trend of ·OH and H2O2 concentration in water was opposite to the ozone concentration trend. More ·OH and H2O2 were produced under pure Ar conditions where the concentration of H2O2 and ·OH reached about 191 mM and 29 μM, respectively. Argon molecules were more prone to excitation, and the presence of argon led to an increased population of high-energy electrons, thereby promoting the generation of OH radicals. During the discharge process, the production of ·OH was divided into two phases: the accelerated high-energy electrons hit the water molecules to produce ·OH (reaction (




The generation of H2O2 was also inseparable from the high-energy electrons produced during the discharge process. The high-energy electrons collide with oxygen molecules to produce oxygen atoms, which then react with water molecules to produce H2O2, while the two ·OHs produced during the discharge process merge to produce H2O2. The following reactions can describe this process [28, 29].





Figure 5(d) shows the TC degradation rate and energy efficiency as a function of oxygen ratio. It can be seen that the degradation rate of TC increased with the oxygen ratio. Specifically, the degradation rate was only 13% under pure Ar conditions. When the gas was pure oxygen, the degradation rate reached about 70%, which was significantly enhanced. Lu et al [30] also arrived at similar findings, namely that better degradation rates were achieved under pure oxygen conditions. Meanwhile, the energy efficiency increased with the oxygen ratio, reaching 0.145 g/(kW·h) at pure oxygen, which meant that the addition of argon increased the reactor's energy consumption at the same voltage. When oxygen was used as the working gas, the active substance produced in the reactor was mainly ozone. In contrast, the active substance produced was mainly short-lived radicals in pure argon-feeding gas. The gas passing through the reactor was also passed into the water in this experiment. The oxygen will produce ozone after passing through the discharge area of the reactor. The ozone can continue deleting the antibiotics in the water after dissolving in the water. In contrast, argon gas was passed into the water through the discharge area. The free radicals have easily disappeared, thus they cannot continue to degrade the antibiotics in the water. Therefore, the degradation rate of the antibiotics in the water will be higher when oxygen was used as the working gas under the treatment mode designed in this experiment.
To test the hypothesis, the solutions treated with oxygen and argon were allowed to settle for 2.5 h each. The results of the experiment are presented in table 1, which shows that after oxygen treatment, there was a continued increase in antibiotic degradation rate even after 2.5 h of standing. Specifically, the antibiotic degradation rate of the treated solution increased from 70.3% to 83.49% following a resting period of 2.5 h, indicating an approximate 10% increase. The degradation rate has actually increased by approximately 10% within the span of an hour. In contrast, under argon conditions, there was only a slight rise from 13.2% to 16.4%, indicating no significant increase in the degradation rate. This phenomenon provides evidence for the hypothesis that long-lived active substances dissolved in water continue to degrade antibiotics under oxygen conditions.
Table 1. Change of degradation rate of treated solution after standing for 2.5 h.
| Time (h) | O2 | Ar |
| 0 | 70.3% | 13.2% |
| 0.5 | 79.3% | 15.4% |
| 1 | 82.2% | 15.8% |
| 1.5 | 83.2% | 16.1% |
| 2 | 83.4% | 16.2% |
| 2.5 | 83.5% | 16.4% |
It was observed that as the oxygen proportion increased, the degradation rate aligned with the trend of ozone concentration, while it contrasted with the trends in H2O2 and ·OH concentrations. This suggested that under the experimental conditions, ozone played a primary role in degradation. Subsequent experiments further indicate that ozone, as a long-lived active species, can continuously degrade TC in water.
3.3. Parameter optimization
The effect of gas flow rate, liquid flow rate, and discharge voltage on TC degradation was investigated to obtain the optimal parameters under the pure oxygen atmosphere, as shown in figures 6(a)–(c), respectively. It should be mentioned that when selecting the optimal conditions, the degradation rate was used as the priority index, and the energy yield was used as the index for auxiliary judgment.
Fig. 6 Effect of (a) liquid flow rate (gas flow rate = 1 L/min, voltage = 20 kV), (b) gas flow rate (liquid flow rate = 100 mL/min, voltage = 20 kV), (c) peak voltage on the degradation rate (gas flow rate = 1 L/min, liquid flow rate = 100 mL/min), (d) degradation rate and energy yield after cyclic degradation.
Download figure:
Standard imageAn obvious decrease in TC degradation was observed as the liquid flow rate increased (figure 6(a)). Specifically, the degradation rate was 80.1% at 50 mL/min and decreased to 70.9% at 100 mL/min. The degradation rate finally dropped to 53.87% when the liquid flow rate was 250 mL/min, approximately decreased by 30%. At the same input voltage, the liquid flow rate did not have a large effect on the discharge power, so the energy yield and degradation rate changed in the same trend. The energy yield was 0.156 g/(kW·h) at 50 mL/min and decreased to 0.142 g/(kW·h) at 100 mL/min. The flow rate of the liquid directly affected the thickness of the liquid film, which determined the average penetration depth of the active substance in the deep water [31]. When the liquid film is thinner, the diffusion of the active species generated in the gas and the gas-liquid surface is easier to the whole liquid film. The active species could react more fully with the antibiotic. In addition, when the liquid flow rate was low, the liquid was more likely to adhere to the medium surface and stay longer in the discharge region due to the effect of liquid surface tension. The combined effect of these two factors determined a full degradation of TC in a lower liquid flow rate. It is worth noting that in equation (
Figure 6(b) shows the effect of the gas flow rate on the TC degradation rate. The TC degradation rate dramatically increased first and then decreased slowly with the gas flow rate. The highest degradation rate of 70.49% was obtained at a gas flow rate of 1 L/min. The effect of gas flow rate on discharge power is also small, so the changing trend of energy yield and degradation rate was the same, and the maximum value was 0.145 g/(kW·h) at 1 L/min. First, less reactive species were produced at a lower gas flow rate, giving rise to insufficient reaction of plasma and TC. As the gas flow rate kept increasing, more gas entered the reactor simultaneously, providing abundant raw material for active. The increase in gas flow rate also facilitated the transfer of active species (mainly O3) from the gas phase to the liquid phase [32]. Therefore, the degradation rate of antibiotics in water increased continuously with the increase of the gas flow rate. However, a decreased TC degradation rate was observed when the gas flow rate exceeded 1 L/min. It was due to the fact that the generated active species existed in the gas phase did not fully diffuse into the liquid phase and was blown away by the large airflow, reducing the residence time of the active species in the reactor [33]. As a result, 1 L/min was chosen as this reactor's optimal gas flow rate.
Figure 6(c) shows the effect of discharge voltage on TC degradation rate. The TC degradation rate climbed linearly with the discharge voltage, reached the highest degradation rate of 70.37% at 20 kV, and then slightly decreased. The discharge voltage directly affected the discharge power and the frequency and number of energetic electrons produced [34]. Therefore, the input voltage has a more significant effect on the energy yield, which reaches a maximum of 0.289 g/(kW·h) and then decreases continuously. The positive effect of filamentary discharge in the counter reactor on the production of active species increased with the discharge voltage [35]. However, when the voltage is extensive, the filamentary discharge gradually changes to spark discharge and arc discharge, which are not conducive to producing active species, so, the TC degradation decreases [36]. The thermal effects caused by elevated voltage levels impair the generation of ozone, consequently leading to a diminished rate of degradation [37]. Ansari et al [38] also mentioned that increasing the applied voltage may not improve system performance significantly. In this context, 20 kV was chosen as this reactor's optimal discharge voltage.
The above experiments investigated the effects of gas flow rate, liquid flow rate, and discharge voltage on antibiotic degradation and obtained the optimal working parameters of this reactor with a liquid flow rate of 100 mL/min, gas flow rate of 1 L/min, and voltage of 20 kV. The energy yield was 0.145 g/(kW·h) under optimal condition.
In order to verify the superiority of single-treatment degradation, multiple cycles of degradation were also investigated (figure 6(d)). A fixed volume of antibiotic solution was collected after plasma treatment and used for the next treatment. The experimental results are shown in the figure. After several treatments, the degradation rate can reach more than 95%, but the cost of energy consumption and time was considered to be unreasonable. Each additional processing doubled the energy and time consumption. This also illustrated the scope of application of this study, which may be more suitable for the pretreatment of low-concentration wastewater, where there are often more stringent requirements for treatment speed. This result also provided a positive impact for subsequent research: by using a multistage plasma reactor in series, it is possible to further improve the antibiotic degradation rate while maintaining the processing speed.
3.4. Result comparsion
Table 2 shows the experimental conditions, degradation rate and energy consumption of similar experiments and compares them with the work in this paper. It can be found that this work has no obvious advantage in energy consumption but has some advantages in processing speed. From the table, a rule can be found that when the initial concentration of antibiotics is higher, better energy yield can be achieved. This may be due to the limitation of the reaction rate and the inability of lower concentration antibiotics to completely consume the active species. Higher initial concentration also leads to a better degradation speed. However, antibiotic concentrations in water are typically in the microgram and nanogram per litre levels [39]. Compared with other experiments with low initial concentration, this work achieved better treatment speed. The conditions of this experiment were suitable for the treatment of low concentration wastewater. The maximum degradation efficiency achieved in this experiment is 70.37%, although complete degradation is not attainable. However, under conditions of pre-treatment or coarse treatment, this degradation rate is considered acceptable. Cyclic processing is generally carried out in similar experiments. However, it not only increases the processing time, but also requires an additional liquid circulation system and storage device in practical application. Therefore, the single-processing mode proposed in this paper is more suitable for practical application. To meet the requirements for higher degradation rates, a possible approach is to employ a series of reactors, which can simultaneously enhance the degradation efficiency while maintaining a high processing rate within a single treatment. Therefore, this experiment can fulfil various demands by utilizing a series of reactors in a cascading manner. At the same time, it can be seen from the comparison in the table that pulse power supply has a higher energy yield. However, considering the life span and manufacturing cost of pulse power and AC power supply, whether the cost advantage brought by the lower energy consumption of pulse power can make up for its manufacturing and use costs in actual use needs further consideration.
Table 2. Comparison of antibiotic degradation in different DBD reactors.
| Discharge type | Electrical parameters | Experimental conditions | Removal/Speed (mg/min) | Energy yield (g/(kW·h)) | Reference |
| Surface discharge plasma | AC, 19 kV | Solution: C0 = 50 mg/L, 500 mLGas: air, 2.5 L/min | 93.3%/1.16 | ~ | [40] |
| Corona | AC, 36 W | Solution: C0 = 50 mg/L, 250 mLGas: air, 1 L/min | 61.9%/0.32 | 0.54 | [41] |
| Gas phase DBD | AC, 7 kV, 6 kHz | Solution: C0 = 40 mg/L, 900 mLGas: air, 1 L/min | 82.6%/1.98 | 58.0 | [42] |
| Gliding arc | AC, 15 kV | Solution: C0 = 5 mg/L, 70 mLGas: O2, 13 L/min | 94.95%/0.011 | ~ | [43] |
| DBD (Mn/γ-Al2O3) | AC, 50 Hz, 1.3 W | Solution: 100 mLGas: air, 150 mL/min | 99.3%/ | 91.7 | [44] |
| Liquid falling-film DBD | AC, 20 kV, 15 kHz | Solution: C0 = 5 mg/L, 500 mLGas: O2, 1 L/min | 70.37%/0.35 | 0.145 | This work |
4. Conclusions
The degradation of TC antibiotic in water was rapidly achieved by a liquid-film type dielectric barrier discharge reactor driven by an AC power supply. The discharge characteristics, chemical species concentration, and degradation rates at different parameters were studied. The experimental results showed that oxygen discharge exhibited filamentary discharge characteristics, which promoted the degradation of antibiotics. Gas-liquid discharge and active gas bubbles improved the degradation rate of antibiotics under single flow treatment. Although more hydroxyl radicals were produced under pure argon conditions, they could not exist in the liquid phase for a long time and continuously degrade antibiotics in water due to their short lifetime. By contrast, more ozone was produced under pure oxygen conditions, which also had strong oxidizing properties and lasting treatment effects, obtaining the highest degradation rate. Therefore, under the experimental conditions, ozone was considered to play a primary role in the degradation of antibiotics. The best TC degradation rate in the reactor was 70.37% under the conditions of pure oxygen with a gas flow rate of 1 L/min, a liquid flow rate of 100 mL/min, and an applied voltage of 20 kV, with an energy yield of 0.145 g/(kW·h). This work provides a new and sustainable approach to realize the efficient degradation of antibiotics in water by a single treatment process.