Abstract
Objective
To assess amantadine use and associated factors in the patients with Parkinson’s disease (PD).
Background
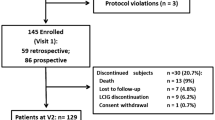
Immediate-release amantadine is approved for the treatment of PD and is largely used in clinical practice to treat “levodopa-induced dyskinesia (LIDs). Its use varies according to countries and PD stages. The prospective NS-Park cohort collects features of PD patients followed by 26 French PD Expert Centres.
Methods
Variables used for the analyses included demographics, motor and non-motor PD symptoms and motor complications [motor fluctuations (MFs), LIDs)], antiparkinsonian pharmacological classes and levodopa equivalent daily dose (LEDD). We evaluated: (i) prevalence of amantadine use and compared clinical features of amantadine users vs. non-users (cross-sectional analysis); (ii) factors associated with amantadine initiation (longitudinal analysis); (iii) amantadine effect on LIDs, MFs, apathy, impulse control disorders and freezing of gait (Fog) (longitudinal analysis).
Results
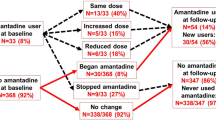
Amantadine use prevalence was 12.6% (1,585/12,542, median dose = 200 mg). Amantadine users were significantly younger, with longer and more severe PD symptoms, greater LEDD and more frequent use of device-aided/surgical treatment. Factors independently associated with amantadine initiation were younger age, longer PD duration, more frequent LIDs, MFs and FoG, higher LEDD and better cognitive function. 9 of the 658 patients on amantadine had stopped it at the following visit, after 12–18 months (1.3%). New users of amantadine presented a higher improvement in LIDs and MF compared to amantadine never users.
Conclusions
About 12% of PD patients within the French NS-Park cohort used amantadine, mostly those with younger age and more severe PD. Amantadine initiation was associated with a subsequent reduction in LIDs and MFs.

Similar content being viewed by others
Data availability
Anonymized data from this study will be available from the corresponding author upon reasonable request from any qualified researcher, following the EU General Data Protection Regulation. The study protocol and statistical analysis plan will be shared upon request.
References
Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, Daniels C, Deutschländer A, Dillmann U, Eisner W, Gruber D, Hamel W, Herzog J, Hilker R, Klebe S, Kloss M, Koy J, Krause M, Kupsch A, Lorenz D, Lorenzl S, Mehdorn HM, Moringlane JR, Oertel W, Pinsker MO, Reichmann H, Reuss A, Schneider GH, Schnitzler A, Steude U, Sturm V, Timmermann L, Tronnier V, Trottenberg T, Wojtecki L, Wolf E, Poewe W, Voges J (2006) A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med 355(9):896–908. https://doi.org/10.1056/NEJMoa060281
Deuschl G, Antonini A, Costa J, Śmiłowska K, Berg D, Corvol JC, Fabbrini G, Ferreira J, Foltynie T, Mir P, Schrag A, Seppi K, Taba P, Ruzicka E, Selikhova M, Henschke N, Villanueva G, Moro E (2022) European Academy of Neurology/Movement Disorder Society-European Section Guideline on the Treatment of Parkinson’s Disease: I. Invasive Therapies. Mov Disord 37(7):1360–1374. https://doi.org/10.1002/mds.29066
Dorsey ER, Sherer T, Okun MS, Bloem BR (2018) The emerging evidence of the Parkinson Pandemic. J Parkinsons Dis 8(s1):S3-s8. https://doi.org/10.3233/jpd-181474
Elmer LW, Juncos JL, Singer C, Truong DD, Criswell SR, Parashos S, Felt L, Johnson R, Patni R (2018) Pooled Analyses of Phase III Studies of ADS-5102 (Amantadine) Extended-Release Capsules for Dyskinesia in Parkinson’s Disease. CNS Drugs 32(4):387–398. https://doi.org/10.1007/s40263-018-0498-4
Fox SH, Katzenschlager R, Lim SY, Barton B, de Bie RMA, Seppi K, Coelho M, Sampaio C (2018) International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord 33(8):1248–1266. https://doi.org/10.1002/mds.27372
Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, Giladi N, Holloway RG, Moore CG, Wenning GK, Yahr MD, Seidl L (2004) Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord 19(9):1020–1028. https://doi.org/10.1002/mds.20213
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23(15):2129–2170. https://doi.org/10.1002/mds.22340
Hauser RA, Walsh RR, Pahwa R, Chernick D, Formella AE (2021) Amantadine ER (Gocovri(®)) significantly increases ON time without any dyskinesia: pooled analyses from pivotal trials in Parkinson’s disease. Front Neurol 12:645706. https://doi.org/10.3389/fneur.2021.645706
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55(3):181–184. https://doi.org/10.1136/jnnp.55.3.181
Kaasinen V, Luo S, Martinez-Martin P, Goetz CG, Stebbins GT (2023) Cross-cultural differences in patient perceptions of dyskinesia in Parkinson’s disease. Mov Disord 38(4):688–692. https://doi.org/10.1002/mds.29335
LeWitt PA (2015) Levodopa therapy for Parkinson’s disease: Pharmacokinetics and pharmacodynamics. Mov Disord 30(1):64–72. https://doi.org/10.1002/mds.26082
Lim SY, Tan AH, Ahmad-Annuar A, Klein C, Tan LCS, Rosales RL, Bhidayasiri R, Wu YR, Shang HF, Evans AH, Pal PK, Hattori N, Tan CT, Jeon B, Tan EK, Lang AE (2019) Parkinson’s disease in the Western Pacific Region. Lancet Neurol 18(9):865–879. https://doi.org/10.1016/s1474-4422(19)30195-4
Mariani LL, Doulazmi M, Chaigneau V, Brefel-Courbon C, Carrière N, Danaila T, Defebvre L, Defer G, Dellapina E, Doé de Maindreville A, Geny C, Maltête D, Meissner WG, Rascol O, Thobois S, Torny F, Tranchant C, Vidailhet M, Corvol JC, Degos B (2019) Descriptive analysis of the French NS-Park registry: towards a nation-wide Parkinson’s disease cohort? Parkinsonism Relat Disord 64:226–234. https://doi.org/10.1016/j.parkreldis.2019.04.012
Orayj K, Lane E (2019) Patterns and determinants of prescribing for Parkinson’s disease: a systematic literature review. Parkinson’s Dis. https://doi.org/10.1155/2019/9237181
Ory-Magne F, Corvol JC, Azulay JP, Bonnet AM, Brefel-Courbon C, Damier P, Dellapina E, Destée A, Durif F, Galitzky M, Lebouvier T, Meissner W, Thalamas C, Tison F, Salis A, Sommet A, Viallet F, Vidailhet M, Rascol O (2014) Withdrawing amantadine in dyskinetic patients with Parkinson disease: the AMANDYSK trial. Neurology 82(4):300–307. https://doi.org/10.1212/wnl.0000000000000050
Pringsheim T, Day GS, Smith DB, Rae-Grant A, Licking N, Armstrong MJ, de Bie RMA, Roze E, Miyasaki JM, Hauser RA, Espay AJ, Martello JP, Gurwell JA, Billinghurst L, Sullivan K, Fitts MS, Cothros N, Hall DA, Rafferty M, Hagerbrant L, Hastings T, O’Brien MD, Silsbee H, Gronseth G, Lang AE (2021) Dopaminergic therapy for motor symptoms in early parkinson disease practice guideline summary: a Report of the AAN Guideline Subcommittee. Neurology 97(20):942–957. https://doi.org/10.1212/wnl.0000000000012868
Rascol O, Negre-Pages L, Damier P, Delval A, Derkinderen P, Destée A, Fabbri M, Meissner WG, Rachdi A, Tison F, Perez-Lloret S (2020) Utilization patterns of amantadine in Parkinson’s disease patients enrolled in the French COPARK Study. Drugs Aging 37(3):215–223. https://doi.org/10.1007/s40266-019-00740-2
Rascol O, Fabbri M, Poewe W (2021) Amantadine in the treatment of Parkinson’s disease and other movement disorders. Lancet Neurol 20(12):1048–1056. https://doi.org/10.1016/s1474-4422(21)00249-0
Rascol O, Ory-Magne F, Azulay JP, Defebvre L, Houeto JL, Maltete D, Remy P, Foubert-Samier A, Sommet A, Thalamas C, Thobois S, Corvol JC, Ns-Park/Fcrin N (2023) Decreased occurrence of dyskinesia when combining amantadine to L-DOPA in early parkinson disease (PD): the PREMANDYSK Trial. Mov Disord 38
Rosa MM, Ferreira JJ, Coelho M, Freire R, Sampaio C (2010) Prescribing patterns of antiparkinsonian agents in Europe. Mov Disord 25(8):1053–1060. https://doi.org/10.1002/mds.23038
Thomas A, Bonanni L, Gambi F, Di Iorio A, Onofrj M (2010) Pathological gambling in Parkinson disease is reduced by amantadine. Ann Neurol 68(3):400–404. https://doi.org/10.1002/ana.22029
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25(15):2649–2653. https://doi.org/10.1002/mds.23429
Verhagen Metman L, Del Dotto P, van den Munckhof P, Fang J, Mouradian MM, Chase TN (1998) Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson’s disease. Neurology 50(5):1323–1326. https://doi.org/10.1212/wnl.50.5.1323
Warren Olanow C, Kieburtz K, Rascol O, Poewe W, Schapira AH, Emre M, Nissinen H, Leinonen M, Stocchi F (2013) Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson’s disease. Mov Disord 28(8):1064–1071. https://doi.org/10.1002/mds.25364
Weintraub D, Sohr M, Potenza MN, Siderowf AD, Stacy M, Voon V, Whetteckey J, Wunderlich GR, Lang AE (2010) Amantadine use associated with impulse control disorders in Parkinson disease in cross-sectional study. Ann Neurol 68(6):963–968. https://doi.org/10.1002/ana.22164
Zhang ZX, Chen H, Chen SD, Shao M, Sun SG, Qu QM, Zhang BR, Liu YM, Xu Q, Wan X, Li L, Wen HB, Chen X, Chen HB, Liu ZG, Wang J, Wang G (2014) Chinese culture permeation in the treatment of Parkinson disease: a cross-sectional study in four regions of China. BMC Res Notes 7:65. https://doi.org/10.1186/1756-0500-7-65
Acknowledgements
Authors thank the patients for their participation. The authors would also like to thank the coinvestigators of the NSPARK/F-CRIN network study group listed in the “online-only text”. Co-investigators: Amiens: Mickael AUBIGNAT; Besançon: Eloi MAGNIN; Bordeaux: Pr Pierre BURBAUD, Pr Dominique GUEHL, Dr Alexandra FOUBERT-SAMIER, Dr Brice LAURENS, Dr Thomas BORAUD, Dr Sylvain VERGNET, Dr David BENDETOWICZ; Caen: Thomas PALPACUER; Clermont-Ferrand: Bérengère DEBILLY, Philippe DEROST, Charlotte BEAL; Créteil—Henri Mondor: Hayet SALHI, Alice DORMEUIL, Aimée PETIT, Alban GRAVIER; Dijon: Gwendoline DUPONT, Lucie GARNIER; Grenoble : Valérie FRAIX, Anna CASTRIOTO, Sara MEONI; Lille: Nicolas CARRIERE; Lyon: Teodor DANAILA, Chloé LAURENCIN, Stéphane THOBOIS; Marseille: Jean-Philippe AZULAY, Frédérique FLUCHERE; Montpellier: Mahmoud CHARIF, Marie-Christine PICOT; Nancy: Lucie HOPES; Nantes: Anne-Gaelle CORBILLE, Tiphaine ROUAUD, Pascal DERKINDEREN; Nice: Cosmin ALECU, Charlotte HERAUD; Nîmes: Marie DE VERDAL; Paris—Pitié- Salpêtrière: Bertrand DEGOS, Graziella MANGONE, Sara SAMBIN, Aymeric LANORE, Thomas COURTIN, Louise-Laure MARIANI, David BENDETOWICZ, Fouad KHOURY, Poornima MENON, Florence CORMIER-DEQUAIRE, Emmanuel FLAMAND-ROZE, David GRABLI, Elodie HAINQUE, Marie VIDHAILLET, Aurélie MENERET, Cécile DELORME, Cendrine FOUCARD, Florian VON RAISON, Alexis ELBAZ, Andreas HARTMANN, Vincent LECLERCQ; Poitiers: Solène ANSQUER; Rennes: Frederique LEH, Marion LECLERCQ; Rouen: Guillaume COSTENTIN; Strasbourg: Ouhaid LAGHA BOUKBIZA; Toulouse: Fabienne ORY-MAGNE, Christine BREFEL COURBON, Clemence LEUNG, Hélène CATALA. Data collection contributors (Project Managers, Clinical Research Associate, Study Nurses, Secretary, Neuropsychologist): Amiens: Astrid CAUSEL; Besançon: Emilie Gaiffe; Bordeaux: Sandrine DUPOUY, Sandrine VILLARS, Wei-Ho LAI ; Caen: Rachida BARI, Damien CHEVANNE; Clermont-Ferrand: Elodie DURAND, Isabelle RIEU, Stephane BERNARD, Corinne GARSAULT; Créteil—Henri Mondor: Noel BOUDJEMA ; Dijon: Pascale GREBENT; Grenoble: Andrea KISTNER, Pierre PELISSIER; Lille: Valérie SANTRAINE; Limoges: Thomas GAUDIN, Pierre BOUTET; Lyon: Catherine CAIRE; Marseille: Manel NOUIRA; Montpellier : Claudia VERNA; Nancy: Amory JARDEL, Salomé PUISIEUX, Guillemette CLEMENT, Lili LE MONNIER; Nantes: Régis FRENAIS, Séverine LE DILY, Rachel CHAIGNEAU; Nice: Vanessa FERRIER, Elodie DAVID; Nîmes: Leslie FRA, Elsa FOUCARAN; Paris—Pitié- Salpêtrière: Carole DONGMO-KENFACK, Florence BEAUZOR, Mickael LE, Sonia MESSAR, Sophie LIOT; Poitiers: Emilie RABOIS; Reims: Margaux BONNAIRE-VERDIER; Rennes: Françoise KESTENS, Rozenn GOURHAN, Sandra LOPEZ-ALFARO, Jean-François HOUVENAGHEL, Mélanie ALEXANDRE, Christine BOURDONNAIS; Rouen: Linda VERNON, Ahmed BOUMEDIENE; Strasbourg: Céline Julie, Aurette Lobstein, Nadine Longato, Marie-Pierre Mitterle, Clélie Philips, Hugo Rummel ; Toulouse: Stéphanie BRAS, Estelle HARROCH, Claudia GILLET.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Isabelle Benatru, Giovani Castelnovo, Anne-Gaelle Corbille, Jean-Christophe Corvol, Anne Doe de Maindreville, Sophie Drapier, Alexandre Eusabio, Solène Frismand, Christian Gény, Caroline Giordana Reuther, Jean-Luc Houeto, David Maltête, Ana Marques, Wassilios Meissner, Stephane Prange Philippe Rémy, Vanessa Rousseau, Vincent Schneider, Melissa Tir and Ouhaid Lagha-Boukbiza report no disclosures. Margherita Fabbri received Honoraria to speak from AbbVie, ORKYN, and BIAL, consultancies from BIAL and LVL Medical; Grant from France Parkinson, HORIZON 2022 and MSA Coalition. Matthieu Bereau reports grants from France Parkinson Foundation, reimbursement of travel expenses to scientific meetings from Asten, Boston Scientific, Elivie and Medtronic, honoraria from AbbVie, Aguettan, Allergan, Asdia and Merz Pharma for lecturing outside the submitted work. Christine Brefel-Courbon has received research grant from Association France Parkinson, and fees for lectures and consultancies from Aguettant, Orkyn, NHC, Zambon and AbbVie. Luc Defebvre served on the Scientific Advisory Board for Abbvie and has received honoraria from pharmaceutical companies for consultancy and lectures including Abbvie, Novartis, Aguettant, Orkyn. E Moro has received honoraria from Medtronic for consulting services. She has also received research grant support from Abbott, France Parkinson, and Ipsen. Fabienne Ory-Magne has received honoraria for serving as an advisory board member from Abbvie, Medtronic, Orphalan, Aguettant and Orkyn, and for consultancy activities from Aguettant, Abbvie, Orphalan, Ellivie, Homeperf and Orkyn. Olivier Rascol has acted as a scientific advisor for drug companies developing antiparkinsonian medications (Abbott, Abbvie, Acorda, Adamas, BIAL, Biogen, Boehringer-Ingelheim, Cynapsus, GSK, Impax, Merck, Osmotica, Oxford-Biomedica, Lundbeck, Novartis, Prexton, Servier, Sunovion, TEVA, UCB, Zambon). Claire Thiriez reports no financial disclosure; congress travel grant from Orkyn, Abbvie, homeperf, ADELIA, ASDIA, Ipsen. Philippe Damier has received honoraria from Teva, Kyowa Kirin for consulting services and from Abbvie, Roche, Novartis, Teva for conferences.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fabbri, M., Rousseau, V., Corvol, JC. et al. Amantadine use in the French prospective NS-Park cohort. J Neural Transm (2024). https://doi.org/10.1007/s00702-024-02772-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00702-024-02772-4