Abstract
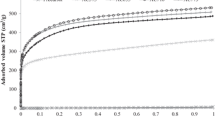
The carbonization temperature of carbon precursors before their activation is an important factor affecting the porous structure and properties of the resulting activated carbons. In this work сorrelation between the textural and adsorption properties of asphalt-based porous carbons and the carbonization temperature has been found. Additionally, the optimal carbonization temperature, and reasons why the carbonization temperature affects the main textural characteristics of the activated carbon were established. A series of porous carbons has been prepared from petroleum asphalt by a two-stage method, including carbonization of asphalt at different temperatures from 450 to 800 °C and KOH activation. To reveal the reasons of the correlation the carbonized samples were studied by TG-DTG, IR-Fourier, TEM methods. It is shown that the carbonization temperature effects on the structural defects, distance between the graphene layers, the reactivity and thermal stability of the carbonized asphalts. These specificities contribute to formation of porous structures of the activated carbons. The carbonization temperature 500–600 °С of the petroleum asphalt is found to be the optimal for further activation. The KOH activation of the petroleum asphalts carbonized at 500–600 °С provides microporous carbon with the high specific surface area (about 2000 m2g-1) and the CO2 uptake (3.3 mmolg-1). Additionally, the specific surface area of the activated carbons is shown can be predicted from the temperature of 50% (T50%) mass loss of the carbonized petroleum asphalt. The linear dependence of the T50% on BET surface area can be fitted by T50%=640–0.424SBET with determination coefficient R2 equal to 0.96.







Similar content being viewed by others
Data availability
Not Applicable.
References
Marsh, H., Reinoso, F.R.: Activated Carbon Elsevier (2006)
Yang, L., Feng, Y., Cao, M., Yao, J.: Two-step preparation of hierarchical porous carbon from KOH-activated wood sawdust for supercapacitor. Mater. Chem. Phys. 238, 121956–121973 (2019). https://doi.org/10.1016/j.matchemphys.2019.121956
Lia, C., Wanga, Y., Xiao, N., Li, H., Ji, Y., Guo, Z., Liu, C., Qiu, J.: Nitrogen-doped porous carbon from coal for high efficiency CO2 electrocatalytic reduction. Carbon. 151, 46–52 (2019). https://doi.org/10.1016/j.carbon.2019.05.042
Zhang, S., Shi, X., Wróbel, R., Chen, X., Mijowska, E.: Low-cost nitrogen-doped activated carbon prepared by polyethylenimine (PEI) with a convenient method for supercapacitor application. Electrochim. Acta. 294, 183–191 (2019). https://doi.org/10.1016/j.electacta.2018.10.111
Zbair, M., Ainassaari, K., Drif, A., Ojala, S., Bottlinger, M., Pirilä, M., Brahmi, R.: Toward new benchmark adsorbents: Preparation and characterization of activated carbon from argan nut shell for bisphenol A removal. ESPR. 25, 1869–1882 (2017). https://doi.org/10.1007/s11356-017-0634-6
Siddiqui, M.N., Ali, I., Asim, M., Chanbasha, B.: Quick removal of nickel metal ions in water using asphalt-based porous carbon. J. Mol. Liq. 308, 113078–113087 (2020). https://doi.org/10.1016/j.molliq.2020.113078
Jang, E., Choi, S.W., Lee, K.B.: Effect of carbonization temperature on the physical properties and CO2 adsorption behavior of petroleum coke-derived porous carbon. Fuel. 248, 85–92 (2019). https://doi.org/10.1016/j.fuel.2019.03.051
Liu, J., Liu, Y., Li, P., Wang, L., Zhang, H., Liu, H., Liu, J., Wang, Y., Tian, W., Wang, X., Li, Z., Wu, M.: Fe-N-doped porous carbon from petroleum asphalt for highly efficient oxygen reduction reaction. Carbon. 126, 1–8 (2018). https://doi.org/10.1016/j.carbon.2017.10.004
Strausz, O.P., Peng, P., Murgich, J.: About the colloidal nature of asphaltenes and the mw of covalent monomeric units. Energy Fuels. 1, 809–822 (2002). https://doi.org/10.1021/ef0002795
Groenzin, H., Mullins, O.C., Eser, S., Mathews, J., Yang, M.G., Jones, D.: Molecular size of asphaltene solubility fractions. Energy Fuels. 17, 498–503 (2003). https://doi.org/10.1021/ef010239g
Javed, H., Luong, D.X., Lee, C.-G., Zhang, D., Tour, J.M., Alvarez, P.J.J.: Efficient removal of bisphenol-A by ultra-high surface area porous activated carbon derived from asphalt. Carbon. 140, 441–448 (2018). https://doi.org/10.1016/j.carbon.2018.08.038
Liang, W., Zhang, Y., Wang, X., Wu, Y., Zhou, X.: Asphalt-derived high surface area activated porous carbons for the effective adsorption separation of ethane and ethylene. Chem. Eng. Sci. 162, 192–202 (2017). https://doi.org/10.1016/j.ces.2017.01.003
Heidarinejad, Z., Dehghani, M.H., Heidari, M., Javedan, G., Ali, I., Sillanpää, M.: Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 18, 393–415 (2020). https://doi.org/10.1007/s10311-019-00955-0
Yamashita, Y., Ouchi, K.: Influence of alkali on the carbonization process-I: Carbonization of 3,5-dimethylphenol-formaldehyde resin with NaOH. Carbon. 20, 41–45 (1982). https://doi.org/10.1016/0008-6223(82)90072-0
Katesa, J., Junpiromand, S., Tangsathitkulchai, C.: Effect of carbonization temperature on properties of char and activated carbon from coconut shell. Suranaree J. Sci. Technol. 20, 269–278 (2013). https://www.thaiscience.info/journals/Article/SJST/10966578.pdf
Sun, S., Yu, Q., Li, M., Zhao, H., Wang, Y., Ji, X.: Effect of carbonization temperature on characterization and water vapor adsorption of coffee-shell activated carbon. Adsorpt. Sci. Technol. 38, 9–10 (2020). https://doi.org/10.1177/0263617420950994
Zhai, D., Li, B., Du, H., Wang, G., Kang, F.: The effect of pre-carbonization of mesophase pitch-based activated carbons on their electrochemical performance for electric double-layer capacitors. J. Solid State Electrochem. 15, 787–794 (2011). https://doi.org/10.1007/s10008-010-1156-z
Gorbunova, O.V., Baklanova, O.N., Gulyaeva, T.I., Arbuzov, A.B., Trenikhin, M.V., Lavrenov, A.V.: Effect of thermal pretreatment on porous structure of asphalt-based carbon. J. Mater. Sci. 57, 7239–7249 (2022). https://doi.org/10.1007/s10853-022-07106-x
Jagiello, J., Olivier, J.P.: 2D-NLDFT adsorption models for carbon slit-shaped pores with surface energetical heterogeneity and geometrical corrugation. Carbon. 55, 70–80 (2013). https://doi.org/10.1016/j.carbon.2012.12.011
Zhu, W., Miser, D.E., Chan, W.G., Hajaligol, M.R.: HRTEM investigation of some commercially available furnace carbon blacks. Carbon. 42, 1841–1845 (2004). https://doi.org/10.1016/j.carbon.2004.01.077
Kruk, M., Jaroniec, M., Ryoo, R., Joo, S.H.: Characterization of ordered mesoporous carbons synthesized using mcm-48 silicas as templates. Phys. Chem. B. 104, 796–7968 (2000). https://doi.org/10.1021/jp000861u
Gorbunova, O.V., Baklanova, O.N., Gulyaeva, T.I.: Porous structure of PEG-mediated silica controlled by solution pH. Microporous Mesoporous Mater. 307, 110468–110472 (2020). https://doi.org/10.1016/j.micromeso.2020.110468
Thommes, M., Kaneko, K., Neimark, A.V., Olivier, J.P., Rodriguez-Reinoso, F., Rouquerol, J., Sing, K.S.W.: Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 87, 1051–1069 (2015). https://doi.org/10.1515/pac-2014-1117
Seaton, N.A., Walton, J.P.R.B., Quirke, N.: A new analysis method for the determination of the pore size distribution of porous carbons from nitrogen adsorption measurements. Carbon. 27, 13–22n (1989). https://doi.org/10.1016/0008-6223(89)90035-3
Abd, A.A., Naji, S.Z., Hashim, A.S., Othman, M.R.: Carbon dioxide removal through physical adsorption using carbonaceous and non-carbonaceous adsorbents: A review. J. Environ. Chem. Eng. 8, 104142–1014164 (2020). https://doi.org/10.1016/j.jece.2020.104142
Thermal Analysis Application No HB 451, Mettler Toledo TA Application Handbook Elastomers: Vol.2. https://www.mt.com/us/en/home/supportive_content/matchar_apps/MatChar_HB451, accessed 22.03.24
Kaneko, K., Ishii, C., Ruike, M., Kuwabara, H.: Origin of superhigh surface area and microcrystalline graphitic structures of activated carbons. Carbon. 30, 1075–1088 (1992). https://doi.org/10.1016/0008-6223(92)90139-N
Ding, H., Hesp, S.A.M.: Variable-temperature Fourier-transform infrared spectroscopy study of asphalt binders from the SHRP materials reference. Fuel. 298, 120819 (2021). https://doi.org/10.1016/j.fuel.2021.120819
Xing, C., Liu, L., Cui, Y., Ding, D.: Analysis of base bitumen chemical composition and aging behaviors via atomic force microscopy-based infrared spectroscopy. Fuel. 264, 116845 (2020). https://doi.org/10.1016/j.fuel.2019.116845
Hou, X., Lv, S., Chen, Z., Xiao, F.: Applications of Fourier transform infrared spectroscopy technologies on asphalt materials. Measurement. 121, 304–316 (2018). https://doi.org/10.1016/j.measurement.2018.03.001
Burg, P., Cagniant, D.: Characterization of Carbon Surface Chemistry. Chem. Phys. Carbon. 30, 129–175 (2008). https://doi.org/10.1201/9781420042993.ch3
Ehrburger, P., Addoun, A., Addoun, F., Ddonnet, J.B.: Carbonization of coals in the presence of alkaline hydroxides and carbonates: Formation of activated carbons. Fuel. 65, 1447–1449 (1986). https://doi.org/10.1016/0016-2361(86)90121-3
Chunlan, L., Shaoping, X., Yixiong, G., Shuqin, L., Changhou, L.: Effect of pre-carbonization of petroleum cokes on chemical activation process with KOH. Carbon. 43, 2295–2301 (2005). https://doi.org/10.1016/j.carbon.2005.04.009
Sadezky, A., Muckenhuber, H., Grothe, H., Niessner, R., Pöschl, U.: Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon. 43, 1731–1742 (2005). https://doi.org/10.1016/j.carbon.2005.02.018
Moseenkov, S.I., Kuznetsov, V.L., Zolotarev, N.A., Kolesov, B.A., Prosvirin, I.P., Ishchenko, A.V., Zavorin, A.V.: Investigation of Amorphous Carbon in Nanostructured Carbon materials (a comparative study by TEM, XPS, Raman Spectroscopy and XRD). Materials. 16, 1112 (2023). https://doi.org/10.3390/ma16031112
Chuang, C.-C., Liu, W.-L., Chen, W.-J., Huang, J.-H.: Temperature and substrate dependence of structure and growth mechanism of carbon nanofiber. Appl. Surf. Sci. 254, 4681–4687 (2008). https://doi.org/10.1016/j.apsusc.2008.01.097
Acknowledgements
The studies were carried out using facilities of the shared research center “National Center of Investigation of Catalysts” at Boreskov Institute of Catalysis and shared research center at the Omsk Scientific Center, Siberian Branch of the Russian Academy of Sciences.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation within the governmental order for Boreskov Institute of Catalysis (FWUR-2024-0039).
Author information
Authors and Affiliations
Contributions
O.V. Gorbunova took part in methodology, writing & editing the article, investigation. O.N. Baklanova contributed in writing and editing of the article. T.I. Gulyaeva, A.V. Vasilevich, A.B. Arbuzov, M.V. Trenikhin carried out measurements and investigation. A.V. Lavrenov took part in supervision.
Corresponding author
Ethics declarations
Ethical approval
Not Applicable.
Competing interests
There are no competing interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gorbunova, O.V., Baklanova, O.N., Gulyaeva, T.I. et al. An easy way to predict and direct the porous structure of activated carbons derived from petroleum asphalt. Adsorption (2024). https://doi.org/10.1007/s10450-024-00467-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10450-024-00467-6