Abstract
Introduction
Neoadjuvant systemic therapy (NAST) is vital in the management of HER2-positive (HER2+) breast cancer. Nevertheless, the indications for NAST in tumors <2 cm remain controversial.
Method
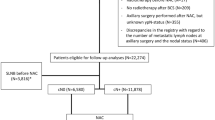
A total of 7961 patients were screened from the Surveillance, Epidemiology, and End Result database. Independent prognostic factors were identified using multivariate Cox analysis. Subgroup analyses and Kaplan–Meier analyses were used to simulate whether NAST would provide a survival benefit with different high-risk characteristics. Nomograms were constructed, and an internal validation cohort was employed.
Results
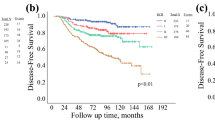
Of the 7961 included patients, 1137 (14.3%) underwent NAST. In the total population, NAST was associated with poorer overall survival (OS) and breast cancer-specific survival (BCSS) (OS: P = 0.00093; BCSS: P < 0.0001). Multivariate Cox analysis confirmed that NAST markedly affected the prognosis of enrolled patients. Besides, a direct association between T, N, age, subtype, and prognosis was observed. Subgroup analyses yielded in these three subgroups, T1c, hormone receptor-negative, and 61–69 years of age, NAST and AST had comparable OS, while NAST possessed worse BCSS. Notably, even in the N3, we still did not observe any additional benefit of NAST. The calculated C-index of 0.72 and 0.73 confirmed the predictability of the nomograms. The AUCs exhibit consistency in the training and validation cohorts.
Conclusion
Our findings suggest that NAST does not provide additional benefit to patients with T1 HER2+ breast cancer, even in the presence of lymph node metastasis, T1c, or hormone receptor negativity. This study facilitates the implementation of individualized management strategies.







Similar content being viewed by others
Data availability
The Surveillance, Epidemiology and End Results (SEER)-Medicare-linked data that support the findings of this study are available from the National Cancer Institute but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
References
Sirhan Z, Thyagarajan A, Sahu RP. The efficacy of tucatinib-based therapeutic approaches for HER2-positive breast cancer. Mil Med Res. 2022;9(1):39.
Dittrich A, Gautrey H, Browell D, Tyson-Capper A. The HER2 signaling network in breast cancer-like a spider in its web. J Mammary Gland Biol Neoplasia. 2014;19(3–4):253–70.
Di Fiore PP, Pierce JH, Kraus MH, Segatto O, King CR, Aaronson SA. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science. 1987;237(4811):178–82.
Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 1988;54(1):105–15.
Pernas S, Tolaney SM. Clinical trial data and emerging strategies: HER2-positive breast cancer. Breast Cancer Res Treat. 2022;193(2):281–91.
Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK, Winer EP. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;373(20):1989.
Hassett MJ, Li H, Burstein HJ, Punglia RS. Neoadjuvant treatment strategies for HER2-positive breast cancer: cost-effectiveness and quality of life outcomes. Breast Cancer Res Treat. 2020;181(1):43–51.
Tausch C, Däster K, Hayoz S, Matrai Z, Fitzal F, Henke G, et al. Trends in use of neoadjuvant systemic therapy in patients with clinically node-positive breast cancer in Europe: prospective TAXIS study (OPBC-03, SAKK 23/16, IBCSG 57–18, ABCSG-53, GBG 101). Breast Cancer Res Treat. 2023;201(2):215–25.
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72.
Asselain B, Barlow W, Bartlett J, Bergh J, Bergsten-Nordström E. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19(1):27–39.
Curigliano G, Burstein HJ, Gnant M, Loibl S, Cameron D, Regan MM, et al. Understanding breast cancer complexity to improve patient outcomes: the St Gallen international consensus conference for the primary therapy of individuals with early breast cancer 2023. Ann Oncol. 2023;34:970–86.
Curigliano G, Burstein HJ, Winer EP, Gnant M, Dubsky P, Loibl S, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen international expert consensus conference on the primary therapy of early breast cancer 2017. Ann Oncol. 2017;28:1700–12.
Zhang S, Liu Y, Liu X, Liu Y, Zhang J. Prognoses of patients with hormone receptor-positive and human epidermal growth factor receptor 2-negative breast cancer receiving neoadjuvant chemotherapy before surgery: a retrospective analysis. Cancers (Basel). 2023;15(4):1157.
Zhou ZR, Wang WW, Li Y, Jin KR, Wang XY, Wang ZW, et al. In-depth mining of clinical data: the construction of clinical prediction model with R. Ann Transl Med. 2019;7(23):796.
Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23(19):4265–74.
Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377–84.
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32.
Gianni L, Pienkowski T, Im YH, Tseng LM, Liu MC, Lluch A, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17(6):791–800.
Giordano SH, Elias AD, Gradishar WJ. NCCN guidelines updates: breast cancer. J Natl Compr Canc Netw. 2018;16(5s):605–10.
Chan A, Delaloge S, Holmes FA, Moy B, Iwata H, Harvey VJ, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17(3):367–77.
von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122–31.
Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372(2):134–41.
von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–28.
Donegan WL. Tumor-related prognostic factors for breast cancer. CA Cancer J Clin. 1997;47(1):28–51.
Fisher B. Some thoughts concerning the primary therapy of breast cancer. Recent Results Cancer Res. 1976;57:150–63.
Englander K, Chintapally N, Gallagher J, Elleson K, Sun W, Whiting J, et al. Factors Influencing Lymph Node Positivity in HER2/neu+ breast cancer patients. Curr Oncol. 2023;30(3):2825–33.
Mamtani A, Barrio AV, King TA, Van Zee KJ, Plitas G, Pilewskie M, et al. How often does neoadjuvant chemotherapy avoid axillary dissection in patients with histologically confirmed nodal metastases? Results of a prospective study. Ann Surg Oncol. 2016;23(11):3467–74.
Samiei S, Simons JM, Engelen SME, Beets-Tan RGH, Classe JM, Smidt ML. Axillary pathologic complete response after neoadjuvant systemic therapy by breast cancer subtype in patients with initially clinically node-positive disease: a systematic review and meta-analysis. JAMA Surg. 2021;156(6): e210891.
Russo J, Frederick J, Ownby HE, Fine G, Hussain M, Krickstein HI, et al. Predictors of recurrence and survival of patients with breast cancer. Am J Clin Pathol. 1987;88(2):123–31.
Smith JA 3rd, Gamez-Araujo JJ, Gallager HS, White EC, McBride CM. Carcinoma of the breast: analysis of total lymph node involvement versus level of metastasis. Cancer. 1977;39(2):527–32.
Gajdos C, Tartter PI, Bleiweiss IJ. Lymphatic invasion, tumor size, and age are independent predictors of axillary lymph node metastases in women with T1 breast cancers. Ann Surg. 1999;230(5):692–6.
Chadha M, Chabon AB, Friedmann P, Vikram B. Predictors of axillary lymph node metastases in patients with T1 breast cancer A multivariate analysis. Cancer. 1994;73(2):350–3.
Zeidman M, Schmidt H, Alberty-Oller JJ, Pisapati KV, Ahn S, Mazumdar M, et al. Trends in neoadjuvant chemotherapy versus surgery-first in stage I HER2-positive breast cancer patients in the national cancer data base (NCDB). Breast Cancer Res Treat. 2021;187(1):177–85.
Korde LA, Somerfield MR, Carey LA, Crews JR, Denduluri N, Hwang ES, et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol. 2021;39(13):1485–505.
Tolaney SM, Tarantino P, Graham N, Tayob N, Parè L, Villacampa G, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer: final 10-year analysis of the open-label, single-arm, phase 2 APT trial. Lancet Oncol. 2023;24(3):273–85.
Fehrenbacher L, Capra AM, Quesenberry CP Jr, Fulton R, Shiraz P, Habel LA. Distant invasive breast cancer recurrence risk in human epidermal growth factor receptor 2-positive T1a and T1b node-negative localized breast cancer diagnosed from 2000 to 2006: a cohort from an integrated health care delivery system. J Clin Oncol. 2014;32(20):2151–8.
Parsons BM, Uprety D, Smith AL, Borgert AJ, Dietrich LL. A US registry-based assessment of use and impact of chemotherapy in stage I HER2-positive breast cancer. J Natl Compr Canc Netw. 2018;16(11):1311–20.
Sobhani N, Roviello G, Corona SP, Scaltriti M, Ianza A, Bortul M, et al. The prognostic value of PI3K mutational status in breast cancer: a meta-analysis. J Cell Biochem. 2018;119(6):4287–92.
Miller TW, Hennessy BT, González-Angulo AM, Fox EM, Mills GB, Chen H, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120(7):2406–13.
Turner N, Dent RA, O’Shaughnessy J, Kim SB, Isakoff SJ, Barrios C, et al. Ipatasertib plus paclitaxel for PIK3CA/AKT1/PTEN-altered hormone receptor-positive HER2-negative advanced breast cancer: primary results from cohort B of the IPATunity130 randomized phase 3 trial. Breast Cancer Res Treat. 2022;191(3):565–76.
Arciero CA, Guo Y, Jiang R, Behera M, O’Regan R, Peng L, et al. ER(+)/HER2(+) breast cancer has different metastatic patterns and better survival than ER(−)/HER2(+) breast cancer. Clin Breast Cancer. 2019;19(4):236–45.
Mislang AR, Biganzoli L. Adjuvant systemic therapy in older breast cancer women: can we optimize the level of care? Cancers (Basel). 2015;7(3):1191–214.
Zheng S, Li L, Chen M, Yang B, Chen J, Liu G, et al. Benefits of neoadjuvant therapy compared with adjuvant chemotherapy for the survival of patients with HER2-positive breast cancer: a retrospective cohort study at FUSCC. Breast. 2022;63:177–86.
Zhang H, Barner JC, Moczygemba LR, Rascati KL, Park C, Kodali D. Comparing survival outcomes between neoadjuvant and adjuvant chemotherapy within breast cancer subtypes and stages among older women: a SEER-Medicare analysis. Breast Cancer. 2023;30(3):489–96.
Acknowledgements
We acknowledge the data support of the SEER program, as well as the Instrument Analysis Center of Xi’an Jiaotong University.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors had full access to the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, LD.C. and DD.L.; Methodology, XY.Z. and LY.D.; Investigation, XT.R., LD.C., and Q.H.; Formal Analysis, LD.C. and DD.L.; Resources, PN.L. and LY.D.; Writing—Original Draft, LD.C. and DD.L.; Writing—Review & Editing, H.W, XB.M. and HF.K.; Visualization, H.W.; Supervision, XB.M. and HF.K.; Funding Acquisition, XB.M. and HF.K.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Declaration of generative AI in scientific writing
The authors declare that this study was conducted without any AI or AI-assisted writing and did not use any AI or AI-assisted technology.
Consent for publication
The authors declare that this study does not contain data from any person. Therefore, the statement of consent for publication does not apply.
Ethical approval and informed consent
This study has been approved by the Ethics Committee of The Second Affiliated Hospital of Xi'an Jiaotong University. The access to and use of SEER data did not require informed patient consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chang, L., Liu, D., Zhao, X. et al. Can neoadjuvant systemic therapy provide additional benefits for T1 HER2+ breast cancer patients: a subgroup analysis based on different high-risk signatures. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03472-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12094-024-03472-x