Abstract
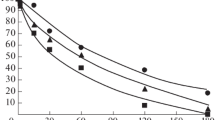
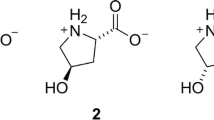
Objective: The resistance of carnosine, pyrrolylcarnosine (PC) and salicylcarnosine (SC) to the action of leucine aminopeptidase and carboxypeptidases B and Y was evaluated. Methods: Proteolysis of carnosine and its derivatives under the action of leucine aminopeptidase, carboxypeptidases Y and B, or under the action of enzyme systems of plasma membranes of rat brain cells or blood plasma. Results and Discussion: It was found that proteolysis of carnosine, PC and SC under the action of leucine aminopeptidase does not occur. Carboxypeptidases B and Y, as well as the enzyme systems of blood plasma and plasma membranes of rat brain cells, degraded peptides containing β-alanyl, N-pyrrolyl, N-salicylic fragments to varying degrees. In all cases, histidine was formed. The formation of pyrrole or salicylic acid did not occur. Conclusions: It was found that carnosine, PC and SC showed high resistance to the action of amino- and carboxypeptidases in in vitro experiments.


Similar content being viewed by others
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
Agyei, D., Ahmed, I., Akram, Z., Iqbal, H.M.N., and Danquah, M.K., Protein Pept. Lett., 2017, vol. 24, pp. 94–101. https://doi.org/10.2174/0929866523666161222150444
Boldyrev, A.A., Aldini, G., and Derave, W., Physiol. Rev., 2013, vol. 93, pp. 1803–1845. https://doi.org/10.1152/physrev.00039.2012
Hipkiss, A.R., In: Advances in Food and Nutrition Research, Taylor S.L., Ed., Burlington: Academic Press, 2009, vol. 57, pp. 87–154.
Bellia, F., Vecchio, G., and Rizzarelli, E., Molecules, 2014, vol. 19, pp. 2299–2329. https://doi.org/10.3390/molecules19022299
Tanashyan, M.M., Fedorova, T.N., Stvolinskij, S.L., Andreeva, L.A., Nagaev, I.Yu., Migulin, V.A., Shabalina, A.A., Trubitsyna, I.E., Lopachev, A.V., Kulikova, O.I., and Abaimov, D.A., Patent RU 2694061 C1, publ. 09.07.2019.
Fedorova, T.N., Stvolinskij, S.L., Migulin, V.A., Lopachev, A.V., Khutorova, A.V., Kulikova, O.I., Muzychuk, O.A., and Abaimov, D.A., Patent RU 2777391 С1, publ. 03.08.2022.
Baltazar, M.T., Dinis-Oliveira, R.J., Duarte, J.A., Bastos, M.L., and Carvalho, F., Curr. Med. Chem., 2011, vol. 18, pp. 3252–3264. https://doi.org/10.2174/092986711796391552
Bhardwaj, V., Gumber, D., Abbot, V., Dhimana, S., and Sharma, P., RSC Adv., 2015, vol. 5, pp. 15233– 15266. https://doi.org/10.1039/C4RA15710A
Pegova, A., Abe, H., and Boldyrev, A., Comp. Biochem. Physiol. B Biochem. Mol. Biol., 2000, vol. 127, pp. 443–446. https://doi.org/10.1016/S0305-0491(00)00279-0
Shevchenko, K.V., Nagaev, I.Yu., Andreeva, L.A., Shevchenko, V.P., and Myasoedov, N.F., Biochem. Moscow Suppl. Ser. B: Biomed. Chem., 2019, vol. 13, pp. 179–201. https://doi.org/10.1134/S1990750819030089
Ovchinnikov, Yu.A., Bioorganic Chemistry, M.: Prosveshchenie, 1987.
Funding
This work was done within the framework of the state assignment according to the plan of research development plan of the National Research Centre “Kurchatov Institute” and withing the framework of the topic no. 1021052806528-6-1.6.4;3.1.1;3.1.9;3.1.8;3.2.25 “Fundamental aspects of neuroplasticity within the framework of translational neurology model” of the Research Center of Neurology.
Author information
Authors and Affiliations
Contributions
All authors made equal contributions to the writing of the article.
Corresponding author
Ethics declarations
All applicable international, national and/or institutional guidelines for animal care were followed.
Experimental procedures for obtaining tissues from rats were approved by the local ethics committee of the Society for Experimental Animal Care (National Research Centre “Kurchatov Institute”): protocol no. 708n, August 23, 2010. No conflict of interest was declared by the authors.
Additional information
Publisher's Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations: PC, pyrrolylcarnosine; SC, salicylcarnosine; HCD, histidine-containing dipeptides; RBMF, rat brain microsomal fraction; PMSF, phenylmethylsylfonyl fluoride.
Rights and permissions
About this article
Cite this article
Shevchenko, K.V., Nagaev, I.Y., Kulikova, O.I. et al. Proteinase Resistance of Carnosine, Pyrrolylcarnosine and Salicylcarnosine. Russ J Bioorg Chem 50, 401–407 (2024). https://doi.org/10.1134/S1068162024020201
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162024020201