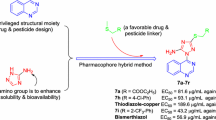
A series of twenty one novel compounds derived from indole-3-acetic acid, the structure of which includes 1,3,4-thiadiazole, thioether, and amide moieties were designed, synthesized, and evaluated for their in vitro antibacterial activity against three bacterial strains. The bioassay results showed that among the synthesized compounds, N-{5-[(2-fluorobenzyl)sulfanyl]-1,3,4-thiadiazol-2-yl}-3-(1H-indol-3-yl)-propanamide demonstrated the best inhibition rate against Pseudomonas syringae pv. actinidiae and N-{5-[(4-chlorobenzyl)sulfanyl]-1,3,4-thiadiazol-2-yl}-3-(1H-indol-3-yl)propanamide possessed the best inhibition rate against Xanthomonas oryzae pv. oryzae and Xanthomonas axonopodis pv. citri, in all cases superior to that of bactericides thiodiazole copper and bismerthiazol.

Similar content being viewed by others
References
Serizawa, S.; Ichikawa, T.; Takikawa, Y.; Tsuyumu, S.; Goto, M. Jpn. J. Phytopathol. 1989, 55, 427.
Colombi, E.; Straub, C.; Künzel, S.; Templeton, M. D.; McCann, H. C.; Rainey, P. B. Environ. Microbiol. 2017, 19, 819.
(a) Na, J.-I.; Kim, S.-Y.; Kim, J.-H.; Youn, S.-W.; Huh, C.-H.; Park, K.-C. Laser Surg. Med. 2011, 43, 200. (b) De, A.; Sarkar, S.; Majee, A. Chem. Heterocycl. Compd. 2021, 57, 410.
Cheng, Z.-Q.; Zhu, K.-K.; Zhang, J.; Song, J.-L.; Muehlmann, L. A.; Jiang, C.-S.; Liu, C.-L.; Zhang, H. Bioorg. Chem. 2019, 83, 277.
Palomba, M.; Pompei, S.; Roscini, L.; Bagnoli, L. ARKIVOC 2019, (ii), 163.
Vaca, J.; Salazar, F.; Ortiz, A.; Sansinenea, E. J. Antibiot. 2020, 73, 798.
Meng, T.; Hou, Y.; Shang, C.; Zhang, J.; Zhang, B. Arch. Pharm. 2021, 354, 2000266.
Mowery, P.; Filkorn, M. M.; Hurysz, B.; Kwansare, D. O.; Lafferty, M. M.; McFadden, M. A.; Neerukonda, N. D.; Patel, R. R.; Pierce, K.; Sockett, K. A.; Truax, N. J.; Webster, N. R.; Pelkey, E. T. Bioorg. Med. Chem. Lett. 2021, 41, 127991.
Wang, T.; Li, L.; Zhou, Y.; Lu, A.; Li, H.; Chen, J.; Duan, Z.; Wang, Q. J. Agric. Food Chem. 2021, 69, 10093.
Newaz, A. W.; Yong, K.; Lian, X.-Y.; Zhang, Z. Tetrahedron 2022, 104, 132598.
Yu, D.; Yang, C.; Liu, Y.; Lu, T.; Li, L.; Chen, G.; Liu, Z.; Li, Y. Sci. Rep. 2023, 13, 4877.
(a) Sandham, D. A.; Adcock, C.; Bala, K.; Barker, L.; Brown, Z.; Dubois, G.; Budd, D.; Cox, B.; Fairhurst, R. A.; Furegati, M.; Leblanc, C.; Manini, J.; Profit, R.; Reilly, J.; Stringer, R.; Schmidt, A.; Turner, K. L.; Watson, S. J.; Willis, J.; Williams, G.; Wilson, C. Bioorg. Med. Chem. Lett. 2009, 19, 4794. (b) Sandham, D. A.; Arnold, N.; Aschauer, H.; Bala, K.; Barker, L.; Brown, L.; Brown, Z.; Budd, D.; Cox, B.; Docx, C.; Dubois, G.; Duggan, N.; England, K.; Everatt, B.; Furegati, M.; Hall, E.; Kalthoff, F.; King, A.; Leblanc, C. J.; Manini, J.; Meingassner, J.; Profit, R.; Schmidt, A.; Simmons, J.; Sohal, B.; Stringer, R.; Thomas, M.; Turner, K. L.; Walker, C.; Watson, S. J.; Westwick, J.; Willis, J.; Williams, G.; Wilson, C. Bioorg. Med. Chem. 2013, 21, 6582. (c) Khan Jadoon, M. S.; Pelletier, J.; Sévigny, J.; Iqbal, J. RSC Adv. 2023, 13, 29496.
Zhang, Z.; Long, Y.; Yin, X.; Wang, W.; Li, W.; Chen, T.; Chen, J.; Chen, X.; Wang, B.; Ma, J. J. Agric. Food Chem. 2023, 71, 13566.
(a) Jain, A. K.; Sharma, S.; Vaidya, A.; Ravichandran, V.; Agrawal, R. K. Chem. Biol. Drug Des. 2013, 81, 557. (b) Li, Y.; Geng, J.; Liu, Y.; Yu, S.; Zhao, G. ChemMedChem 2013, 8, 27. (c) Hu, Y.; Li, C.-Y.; Wang, X.-M.; Yang, Y.-H.; Zhu, H.-L. Chem. Rev. 2014, 114, 5572. (d) Han, X.; Yu, L. Y.; Hu, S. Y.; Liu, H. X. Curr. Top. Med. Chem. 2021, 21, 2546. (e) Uliankin, E. B.; Kostyuchenko, A. S.; Fisyuk, A. S. Chem. Heterocycl. Compd. 2023, 59, 88.
Lv, M.; Liu, G.; Jia, M.; Xu, H. Bioorg. Chem. 2018, 81, 88.
Gan, X.; Hu, D.; Chen, Z.; Wang, Y.; Song, B. Bioorg. Med. Chem. Lett. 2017, 27, 4298.
Li, Q.; An, R.; Xu, Y.; Zhou, M.; Li, Y.; Guo, C.; Wang, R. Bioorg. Med. Chem. Lett. 2020, 30, 127114.
Wu, Z.; Shi, J.; Chen, J.; Hu, D.; Song, B. J. Agric. Food Chem. 2021, 69, 8660.
Tang, C.; Chen, X.; Yang, S.; Guo, W.; Yang, X.; Li, P.; Wang, X. Phosphorus, Sulfur Silicon Relat. Elem. 2023, 198, 627.
Men, Y.; Li, Z.; Wang, H.; Liu, Y.; Liu, X.; Chen, B. Phosphorus, Sulfur Silicon Relat. Elem. 2023, 198, 591.
Bastas, K. K.; Karakaya A. Plant Dis. 2012, 96, 452.
Jiang, N.; Yan, J.; Liang, Y.; Shi, Y.; He, Z.; Wu, Y.; Zeng, Q.; Liu, X.; Peng, J. Rice 2020, 13, 3.
Das, A. K. J. Appl. Hortic. 2003, 5, 52.
Tang, C.; Wang, W.; Luo, G.; Song, C.; Bao, Z.; Li, P.; Hao, G.; Chi, Y. R.; Jin, Z. Angew. Chem., Int. Ed. 2022, 61, e202206961.
(a) Kong, X.-W.; Zhang, Y.; Wang, T.; Lai, Y.-S.; Peng, S.-X. Chem. Biodivers. 2008, 5, 1743. (b) Ruan, X.; Zhang, C.; Jiang, S.; Guo, T.; Xia, R.; Chen, Y.; Tang, X.; Xue, W. Molecules 2018, 23, 3132. (c) Ahadi, H.; Shokrzadeh, M.; Hosseini-khah, Z; Ghassemi barghi, N.; Ghasemian, M.; Emadi, E.; Zargari, M.; Razzaghi-Asl, N.; Emami, S. Bioorg. Chem. 2020, 105, 104383.
The study was performed with the financial support by the Science and Technology Foundation of Guizhou Province (Qiankehe ZK[2024]655, ZK[2023]441), the Qiandongnan Science and Technology Plan Project (Qiandongnan kejichu [2021]17) and the Qiandongnan Science and Technology Plan Project (Qiandongnan kehe J [2022]40).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2024, 60(1/2), 92–98
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, C., Shao, J., Si, C. et al. Discovery of indole-3-acetic acid derivatives containing 1,3,4-thiadiazole thioether and amide moieties as novel antibacterial agents. Chem Heterocycl Comp 60, 92–98 (2024). https://doi.org/10.1007/s10593-024-03298-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-024-03298-z