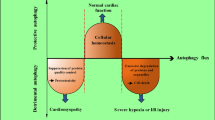
Abstract
Cardiomyocytes undergo a variety of cell death events during myocardial ischemia‒reperfusion injury (MIRI). Understanding the causes of cardiomyocyte mortality is critical for the prevention and treatment of MIRI. Among the various types of cell death, autosis is a recently identified type of autophagic cell death with distinct morphological and chemical characteristics. Autosis can be attenuated by autophagy inhibitors but not reversed by apoptosis or necrosis inhibitors. In recent years, it has been shown that during the late phase of reperfusion, autosis is activated, which exacerbates myocardial injury. This article describes the characteristics of autosis, autophagic cell death, and the relationship between autophagic cell death and autosis; reviews the mechanism of autosis in MIRI; and discusses its clinical significance.



Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Wang Y, Hou M, Duan S et al (2022) Macrophage-targeting gene silencing orchestrates myocardial microenvironment remodeling toward the anti-inflammatory treatment of ischemia-reperfusion (IR) injury. Bioact Mater 17:320–333. https://doi.org/10.1016/j.bioactmat.2022.01.026
Chen M, Li X, Yang H et al (2020) Hype or hope: Vagus nerve stimulation against acute myocardial ischemia-reperfusion injury. Trends Cardiovasc Med 30:481–488. https://doi.org/10.1016/j.tcm.2019.10.011
Carreira RS, Facundo HTF, Kowaltowski AJ (2005) Mitochondrial K+ transport and cardiac protection during ischemia/reperfusion. Braz J Med Biol Res 38:345–352. https://doi.org/10.1590/S0100-879X2005000300004
Eltzschig HK, Eckle T (2011) Ischemia and reperfusion—from mechanism to translation. Nat Med. https://doi.org/10.1038/nm.2507
Wang R, Wang M, He S et al (2020) Targeting calcium homeostasis in myocardial ischemia/reperfusion injury: an overview of regulatory mechanisms and therapeutic reagents. Front Pharmacol 11:872. https://doi.org/10.3389/fphar.2020.00872
Kormos A, Nagy N, Acsai K et al (2014) Efficacy of selective NCX inhibition by ORM-10103 during simulated ischemia/reperfusion. Eur J Pharmacol 740:539–551. https://doi.org/10.1016/j.ejphar.2014.06.033
Lefer DJ, Nichols CG, Coetzee WA (2009) Sulfonylurea receptor 1 subunits of ATP-sensitive potassium channels and myocardial ischemia/reperfusion injury. Trends Cardiovasc Med 19:61–67. https://doi.org/10.1016/j.tcm.2009.04.008
Mishra PK, Adameova A, Hill JA et al (2019) Guidelines for evaluating myocardial cell death. Am J Physiol Heart Circ Physiol 317:H891–H922. https://doi.org/10.1152/ajpheart.00259.2019
Condorelli G, Roncarati R, Ross J et al (2001) Heart-targeted overexpression of caspase3 in mice increases infarct size and depresses cardiac function. Proc Natl Acad Sci U S A 98:9977–9982. https://doi.org/10.1073/pnas.161120198
Jeremias I, Kupatt C, Martin-Villalba A et al (2000) Involvement of CD95/Apo1/Fas in cell death after myocardial ischemia. Circulation 102:915–920. https://doi.org/10.1161/01.cir.102.8.915
Chen Z, Chua CC, Ho YS et al (2001) Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. Am J Physiol Heart Circ Physiol 280:H2313-2320. https://doi.org/10.1152/ajpheart.2001.280.5.H2313
Zhang X, Ren Z, Xu W, Jiang Z (2022) Necroptosis in atherosclerosis. Clin Chim Acta 534:22–28. https://doi.org/10.1016/j.cca.2022.07.004
Grootjans S, Vanden Berghe T, Vandenabeele P (2017) Initiation and execution mechanisms of necroptosis: an overview. Cell Death Differ 24:1184–1195. https://doi.org/10.1038/cdd.2017.65
Del Re DP, Amgalan D, Linkermann A et al (2019) Fundamental mechanisms of regulated cell death and implications for heart disease. Physiol Rev 99:1765–1817. https://doi.org/10.1152/physrev.00022.2018
Griffiths EJ, Halestrap AP (1995) Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J 307(Pt 1):93–98. https://doi.org/10.1042/bj3070093
Honda HM, Korge P, Weiss JN (2005) Mitochondria and ischemia/reperfusion injury. Ann N Y Acad Sci 1047:248–258. https://doi.org/10.1196/annals.1341.022
Liu Y, Li L, Wang Z et al (2023) Myocardial ischemia-reperfusion injury Molecular mechanisms and prevention. Microvascular Res 149:104565. https://doi.org/10.1016/j.mvr.2023.104565
Sparvero LJ, Tian H, Amoscato AA et al (2021) Direct mapping of phospholipid ferroptotic death signals in cells and tissues by gas cluster ion Beam Secondary Ion Mass Spectrometry (GCIB-SIMS). Angew Chem Int Ed Engl 60:11784–11788. https://doi.org/10.1002/anie.202102001
Riegman M, Sagie L, Galed C et al (2020) Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nat Cell Biol 22:1042–1048. https://doi.org/10.1038/s41556-020-0565-1
Fang X, Wang H, Han D et al (2019) Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A 116:2672–2680. https://doi.org/10.1073/pnas.1821022116
Tang L-J, Luo X-J, Tu H et al (2021) Ferroptosis occurs in phase of reperfusion but not ischemia in rat heart following ischemia or ischemia/reperfusion. Naunyn Schmiedebergs Arch Pharmacol 394:401–410. https://doi.org/10.1007/s00210-020-01932-z
Wang H, Liu C, Zhao Y, Gao G (2020) Mitochondria regulation in ferroptosis. Eur J Cell Biol 99:151058. https://doi.org/10.1016/j.ejcb.2019.151058
Liu Y, Shoji-Kawata S, Sumpter RM et al (2013) Autosis is a Na+, K+-ATPase–regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia–ischemia. Proc Natl Acad Sci U S A 110:20364–20371. https://doi.org/10.1073/pnas.1319661110
Liu Y, Levine B (2015) Autosis and autophagic cell death: the dark side of autophagy. Cell Death Differ 22:367–376. https://doi.org/10.1038/cdd.2014.143
Nah J, Zhai P, Huang C-Y et al (2020) Upregulation of Rubicon promotes autosis during myocardial ischemia/reperfusion injury. J Clin Invest 130:2978–2991. https://doi.org/10.1172/JCI132366
Nah J, Zablocki D, Sadoshima J (2020) Autosis a new target to prevent cell death. JACC Basic Transl Sci 5:857–869. https://doi.org/10.1016/j.jacbts.2020.04.014
Lin X, Xiao W, Xiao L, Liu M (2018) Molecular mechanisms of autophagy in cardiac ischemia/reperfusion injury (Review). Mol Med Report. https://doi.org/10.3892/mmr.2018.9028
Shi B, Ma M, Zheng Y et al (2019) mTOR and Beclin1: Two key autophagy-related molecules and their roles in myocardial ischemia/reperfusion injury. J Cellular Physiol 234:12562–12568. https://doi.org/10.1002/jcp.28125
Matsui Y, Takagi H, Qu X et al (2007) Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res 100:914–922. https://doi.org/10.1161/01.RES.0000261924.76669.36
Zhang T, Guo J, Gu J et al (2019) Protective role of mTOR in liver ischemia/reperfusion injury: involvement of inflammation and autophagy. Oxid Med Cell Longev 2019:7861290. https://doi.org/10.1155/2019/7861290
Zhao D, Yang J, Yang L (2017) Insights for oxidative stress and mTOR signaling in myocardial ischemia/reperfusion injury under diabetes. Oxid Med Cell Longev 2017:6437467. https://doi.org/10.1155/2017/6437467
Kong L, Xiong F, Sun N et al (2020) CaMKIIδ inhibition protects against myocardial ischemia/reperfusion injury: Role of Beclin-1-dependent autophagy. Eur J Pharmacol 886:173539. https://doi.org/10.1016/j.ejphar.2020.173539
Maejima Y, Isobe M, Sadoshima J (2016) Regulation of autophagy by Beclin 1 in the heart. J Mol Cell Cardiol 95:19–25. https://doi.org/10.1016/j.yjmcc.2015.10.032
Nakatogawa H (2020) Mechanisms governing autophagosome biogenesis. Nat Rev Mol Cell Biol 21:439–458. https://doi.org/10.1038/s41580-020-0241-0
Nah J, Zablocki D, Sadoshima J (2022) The role of autophagic cell death in cardiac disease. J Mol Cell Cardiol 173:16–24. https://doi.org/10.1016/j.yjmcc.2022.08.362
Schwartz LM (2021) Autophagic cell death during development—ancient and mysterious. Front Cell Dev Biol 9:656370. https://doi.org/10.3389/fcell.2021.656370
Denton D, Kumar S (2019) Autophagy-dependent cell death. Cell Death Differ 26:605–616. https://doi.org/10.1038/s41418-018-0252-y
Fernández ÁF, Liu Y, Ginet V et al (2020) Interaction between the autophagy protein Beclin 1 and Na+, K+-ATPase during starvation, exercise, and ischemia. JCI Insight. https://doi.org/10.1172/jci.insight.133282
Nah J, Fernández ÁF, Kitsis RN et al (2016) Does autophagy mediate cardiac myocyte death during stress? Circ Res 119:893–895. https://doi.org/10.1161/CIRCRESAHA.116.309765
Galluzzi L, Vitale I, Abrams JM et al (2012) Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 19:107–120. https://doi.org/10.1038/cdd.2011.96
Wu X, Liu Z, Yu X et al (2021) Autophagy and cardiac diseases: Therapeutic potential of natural products. Med Res Rev 41:314–341. https://doi.org/10.1002/med.21733
Zhao YG, Liu N, Miao G et al (2018) The ER contact proteins VAPA/B interact with multiple autophagy proteins to modulate autophagosome biogenesis. Curr Biol 28:1234-1245.e4. https://doi.org/10.1016/j.cub.2018.03.002
Nah J, Zablocki D, Sadoshima J (2021) The roles of the inhibitory autophagy regulator Rubicon in the heart: A new therapeutic target to prevent cardiac cell death. Exp Mol Med 53:528–536. https://doi.org/10.1038/s12276-021-00600-3
Sun Q, Westphal W, Wong KN et al (2010) Rubicon controls endosome maturation as a Rab7 effector. Proc Natl Acad Sci U S A 107:19338–19343. https://doi.org/10.1073/pnas.1010554107
Chang C, Young LN, Morris KL et al (2019) Bidirectional control of autophagy by BECN1 BARA domain dynamics. Mol Cell 73:339-353.e6. https://doi.org/10.1016/j.molcel.2018.10.035
Zhong Y, Wang QJ, Li X et al (2009) Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat Cell Biol 11:468–476. https://doi.org/10.1038/ncb1854
Kaur S, Changotra H (2020) The beclin 1 interactome: Modification and roles in the pathology of autophagy-related disorders. Biochimie 175:34–49. https://doi.org/10.1016/j.biochi.2020.04.025
Liu X, Zhang S, An L et al (2019) Loss of Rubicon ameliorates doxorubicin-induced cardiotoxicity through enhancement of mitochondrial quality. Int J Cardiol 296:129–135. https://doi.org/10.1016/j.ijcard.2019.07.074
Gatica D, Chiong M, Lavandero S, Klionsky DJ (2015) Molecular mechanisms of autophagy in the cardiovascular system. Circ Res 116:456–467. https://doi.org/10.1161/CIRCRESAHA.114.303788
Nah J, Sung E-A, Zhai P et al (2022) Tfeb-mediated transcriptional regulation of autophagy induces autosis during ischemia/reperfusion in the heart. Cells 11:258. https://doi.org/10.3390/cells11020258
Clausen MV, Hilbers F, Poulsen H (2017) The structure and function of the Na, K-ATPase isoforms in health and disease. Front Physiol 8:371. https://doi.org/10.3389/fphys.2017.00371
Gross NB, Abad N, Lichtstein D et al (2019) Endogenous Na+, K+-ATPase inhibitors and CSF [Na+] contribute to migraine formation. PLoS ONE 14:e0218041. https://doi.org/10.1371/journal.pone.0218041
PKA compartmentalization links cAMP signaling and autophagy | Cell Death & Differentiation. https://www.nature.com/articles/s41418-021-00761-8. Accessed 20 Feb 2024
Nirmala JG, Lopus M (2020) Cell death mechanisms in eukaryotes. Cell Biol Toxicol 36:145–164. https://doi.org/10.1007/s10565-019-09496-2
Di Malta C, Cinque L, Settembre C (2019) Transcriptional regulation of autophagy: mechanisms and diseases. Front Cell Dev Biol 7:114. https://doi.org/10.3389/fcell.2019.00114
Xiao L, Nie X, Cheng Y, Wang N (2021) Sodium-Glucose Cotransporter-2 inhibitors in vascular biology: cellular and molecular mechanisms. Cardiovasc Drugs Ther 35:1253–1267. https://doi.org/10.1007/s10557-021-07216-9
Neal B, Perkovic V, Mahaffey KW et al (2017) Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377:644–657. https://doi.org/10.1056/NEJMoa1611925
Verma S, Mazer CD, Fitchett D et al (2018) Empagliflozin reduces cardiovascular events, mortality and renal events in participants with type 2 diabetes after coronary artery bypass graft surgery: subanalysis of the EMPA-REG OUTCOME® randomised trial. Diabetologia 61:1712–1723. https://doi.org/10.1007/s00125-018-4644-9
Uthman L, Baartscheer A, Bleijlevens B et al (2018) Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia 61:722–726. https://doi.org/10.1007/s00125-017-4509-7
Bell RM, Yellon DM (2018) SGLT2 inhibitors: hypotheses on the mechanism of cardiovascular protection. Lancet Diabetes Endocrinol 6:435–437. https://doi.org/10.1016/S2213-8587(17)30314-5
Lytvyn Y, Bjornstad P, Udell JA et al (2017) Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation 136:1643–1658. https://doi.org/10.1161/CIRCULATIONAHA.117.030012
Packer M, Anker SD, Butler J et al (2017) Effects of sodium-glucose cotransporter 2 inhibitors for the treatment of patients with heart failure: proposal of a novel mechanism of action. JAMA Cardiol 2:1025–1029. https://doi.org/10.1001/jamacardio.2017.2275
Avogaro A, Fadini GP, Del Prato S (2020) Reinterpreting cardiorenal protection of renal sodium-glucose cotransporter 2 inhibitors via cellular life history programming. Diabetes Care 43:501–507. https://doi.org/10.2337/dc19-1410
Packer M (2020) Autophagy stimulation and intracellular sodium reduction as mediators of the cardioprotective effect of sodium-glucose cotransporter 2 inhibitors. Eur J Heart Fail 22:618–628. https://doi.org/10.1002/ejhf.1732
Packer M (2020) SGLT2 inhibitors produce cardiorenal benefits by promoting adaptive cellular reprogramming to induce a state of fasting mimicry: a paradigm shift in understanding their mechanism of action. Diabetes Care 43:508–511. https://doi.org/10.2337/dci19-0074
Packer M (2017) Activation and inhibition of sodium-hydrogen exchanger is a mechanism that links the pathophysiology and treatment of diabetes mellitus with that of heart failure. Circulation 136:1548–1559. https://doi.org/10.1161/CIRCULATIONAHA.117.030418
Jiang K, Xu Y, Wang D et al (2022) Cardioprotective mechanism of SGLT2 inhibitor against myocardial infarction is through reduction of autosis. Protein Cell 13:336–359. https://doi.org/10.1007/s13238-020-00809-4
Wang Y, Meyer JW, Ashraf M, Shull GE (2003) Mice with a null mutation in the NHE1 Na+-H+ exchanger are resistant to cardiac ischemia-reperfusion injury. Circ Res 93:776–782. https://doi.org/10.1161/01.RES.0000094746.24774.DC
Deng R, Jiang K, Chen F et al (2022) Novel cardioprotective mechanism for Empagliflozin in nondiabetic myocardial infarction with acute hyperglycemia. Biomed Pharmacother 154:113606. https://doi.org/10.1016/j.biopha.2022.113606
Wajima T, Beguier B, Yaguchi M (2004) Effects of cariporide (HOE642) on myocardial infarct size and ventricular arrhythmias in a rat ischemia/reperfusion model: comparison with other drugs. Pharmacology 70:68–73. https://doi.org/10.1159/000074670
Le Grand B, Pignier C, Létienne R et al (2009) Na+ currents in cardioprotection: better to be late. J Med Chem 52:4149–4160. https://doi.org/10.1021/jm900296e
Rodríguez-Sinovas A, García-Dorado D, Padilla F et al (2003) Pre-treatment with the Na+/H+ exchange inhibitor cariporide delays cell-to-cell electrical uncoupling during myocardial ischemia. Cardiovasc Res 58:109–117. https://doi.org/10.1016/s0008-6363(02)00840-4
Linz WJ, Busch AE (2003) NHE-1 inhibition: from protection during acute ischaemia/reperfusion to prevention/reversal of myocardial remodelling. Naunyn Schmiedebergs Arch Pharmacol 368:239–246. https://doi.org/10.1007/s00210-003-0808-2
Chang HB, Gao X, Nepomuceno R et al (2015) Na+/H+ exchanger in regulation of platelet activation and paradoxical effects of cariporide. Exp Neurol 272:11–16. https://doi.org/10.1016/j.expneurol.2014.12.023
Karmazyn M, Sostaric JV, Gan XT (2001) The myocardial Na+/H+ exchanger: a potential therapeutic target for the prevention of myocardial ischaemic and reperfusion injury and attenuation of postinfarction heart failure. Drugs 61:375–389. https://doi.org/10.2165/00003495-200161030-00006
He L, Chu Y, Yang J et al (2022) Activation of autophagic flux maintains mitochondrial homeostasis during cardiac ischemia/reperfusion injury. Cells 11:2111. https://doi.org/10.3390/cells11132111
Kriel J, Loos B (2019) The good, the bad and the autophagosome: exploring unanswered questions of autophagy-dependent cell death. Cell Death Differ 26:640–652. https://doi.org/10.1038/s41418-018-0267-4
Acknowledgements
This research was supported by grants from the National Natural Science Foundation of China (Grant Nos.82170260).
Funding
This article was funded by National Natural Science Foundation of China, 82170260, 82170260, 82170260, 82170260, 82170260, 82170260, 82170260.
Author information
Authors and Affiliations
Contributions
Xiaoting Yang contributed toward conceptualization, and writing-original draft preparation. Gang Zhou and Dong Zhang contributed toward data curation. Qingzhuo Yang and Yanfang Liu contributed toward visualization and investigation. Yi Li contributed toward production of matching images. Wu Hui contributed toward supervision. Xiaoting Yang contributed toward writing- reviewing and editing.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, X., Wu, H., Zhou, G. et al. Autosis: a new form of cell death in myocardial ischemia–reperfusion injury. Mol Cell Biochem (2024). https://doi.org/10.1007/s11010-024-04988-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-024-04988-0