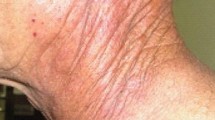
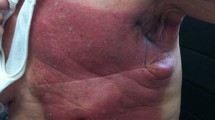
Abstract
Radiation-induced skin damage (RID) is the most prevalent, significant side effect of radiotherapy (RT). Nearly 95% of patients experience moderate to severe skin reactions after receiving radiation therapy. However, criteria for acute radiation dermatitis (ARD) treatment remain unavailable. Topical agents with anti-inflammatory properties may protect the skin and facilitate tissue regeneration in patients with RID. Many of these topical agents function through nuclear factor kappa B pathway regulation. They either reduce the levels of inflammatory factors or elicit anti-inflammatory properties of their own, thus preventing oxidative stress and inflammatory responses and thus enabling RID prevention and management. Herein, we explore the 25 topical agents investigated for RID prevention and management thus far and evaluate their mechanisms of action. These agents include 11 natural agents, 3 miscellaneous agents, 9 topical nonsteroidal agents, and 2 topical corticosteroids.


Similar content being viewed by others
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
Fuzissaki MA, Paiva CE, Oliveira MA, Lajolo Canto PP, Paiva Maia YC. The impact of radiodermatitis on breast cancer patients’ quality of life during radiotherapy: a prospective cohort study. J Pain Symptom Manage. 2019. https://doi.org/10.1016/j.jpainsymman.2019.03.017.
Yokota T, Zenda S, Ota I, Yamazaki T, Yamaguchi T, Ogawa T, et al. Phase 3 randomized trial of topical steroid versus placebo for prevention of radiation dermatitis in patients with head and neck cancer receiving chemoradiation. Int J Radiat Oncol Biol Phys. 2021;111(3):794–803. https://doi.org/10.1016/j.ijrobp.2021.05.133.
Yang X, Ren H, Guo X, Hu C, Fu J. Radiation-induced skin injury: pathogenesis, treatment, and management. Aging (Albany NY). 2020. https://doi.org/10.18632/aging.103932.
Najafi M, Motevaseli E, Shirazi A, Geraily G, Rezaeyan A, Norouzi F, et al. Mechanisms of inflammatory responses to radiation and normal tissues toxicity: clinical implications. Int J Radiat Biol. 2018;94(4):335–56. https://doi.org/10.1080/09553002.2018.1440092.
Wei J, Meng L, Hou X, Qu C, Wang B, Xin Y, et al. Radiation-induced skin reactions: mechanism and treatment. Cancer Manag Res. 2019. https://doi.org/10.2147/CMAR.S188655.
Behroozian T, Bonomo P, Patel P, Kanee L, Finkelstein S, van den Hurk C, et al. Multinational association of supportive care in cancer (MASCC) clinical practice guidelines for the prevention and management of acute radiation dermatitis: international delphi consensus-based recommendations. Lancet Oncol. 2023;24(4):e172–85. https://doi.org/10.1016/S1470-2045(23)00067-0.
Baharara H, Rahsepar S, Emami SA, Elyasi S, Mohammadpour AH, Ghavami V, et al. The efficacy of medicinal plant preparations in the alleviation of radiodermatitis in patients with breast cancer: a systematic review of clinical trials. Phytother Res. 2023. https://doi.org/10.1002/ptr.7894.
Karbasforooshan H, Hosseini S, Elyasi S, Fani Pakdel A, Karimi G. Topical silymarin administration for prevention of acute radiodermatitis in breast cancer patients: a randomized, double-blind, placebo-controlled clinical trial. Phytother Res. 2019;33(2):379–86. https://doi.org/10.1002/ptr.6231.
Gillessen A, Schmidt HH. Silymarin as supportive treatment in liver diseases: a narrative review. Adv Ther. 2020;37(4):1279–301. https://doi.org/10.1007/s12325-020-01251-y.
Cruceriu D, Balacescu O, Rakosy E. Calendula officinalis: potential roles in cancer treatment and palliative care. Integr Cancer Ther. 2018;17(4):1068–78. https://doi.org/10.1177/1534735418803766.
Koukourakis G, Pissakas G, Ganos CG, Sivolapenko G, Kardamakis D. Effectiveness and tolerability of natural herbal formulations in the prevention of radiation-Induced skin toxicity in patients undergoing radiotherapy. Int J Low Extrem Wounds. 2022;21(1):75–86. https://doi.org/10.1177/1534734620923912.
Kole AJ, Kole L, Moran MS. Acute radiation dermatitis in breast cancer patients: challenges and solutions. Breast Cancer (Dove Med Press). 2017. https://doi.org/10.2147/BCTT.S109763.
Rosenthal A, Israilevich R, Moy R. Management of acute radiation dermatitis: A review of the literature and proposal for treatment algorithm. J Am Acad Dermatol. 2019;81(2):558–67. https://doi.org/10.1016/j.jaad.2019.02.047.
Shahane K, Kshirsagar M, Tambe S, Jain D, Rout S, Ferreira MKM, et al. An updated review on the multifaceted therapeutic potential ofcalendula officinalis L. Pharmaceuticals (Basel). 2023;16(4):611. https://doi.org/10.3390/ph16040611.
Givol O, Kornhaber R, Visentin D, Cleary M, Haik J, Harats M. A systematic review of calendula officinalis extract for wound healing. Wound Repair Regen. 2019;27(5):548–61. https://doi.org/10.1111/wrr.12737.
Ferreira AS, Macedo C, Silva AM, Delerue-Matos C, Costa P, Rodrigues F. Natural products for the prevention and treatment of oral mucositis-a review. Int J Mol Sci. 2022;23(8):4385. https://doi.org/10.3390/ijms23084385.
Yang K, Kim SY, Park JH, Ahn WG, Jung SH, Oh D, et al. Topical application of phlorotannins from brown seaweed mitigates radiation dermatitis in a mouse model. Mar Drugs. 2020;18(8):377. https://doi.org/10.3390/md18080377.
Besednova NN, Andryukov BG, Zaporozhets TS, Kuznetsova TA, Kryzhanovsky SP, Ermakova SP, et al. Molecular targets of brown algae phlorotannins for the therapy of inflammatory processes of various origins. Mar Drugs. 2022;20(4):243. https://doi.org/10.3390/md20040243.
Maleki M, Mardani A, Manouchehri M, Ashghali Farahani M, Vaismoradi M, Glarcher M. Effect of chamomile on the complications of cancer: a systematic review. Integr Cancer Ther. 2023. https://doi.org/10.1177/15347354231164600.
Bhaskaran N, Shukla S, Srivastava JK, Gupta S. Chamomile: an anti-inflammatory agent inhibits inducible nitric oxide synthase expression by blocking RelA/p65 activity. Int J Mol Med. 2010;26(6):935–40. https://doi.org/10.3892/ijmm_00000545.
Sah A, Naseef PP, Kuruniyan MS, Jain GK, Zakir F, Aggarwal G. A comprehensive study of therapeutic applications of chamomile. Pharmaceuticals (Basel). 2022;15(10):1284. https://doi.org/10.3390/ph15101284.
Rafati M, Ghasemi A, Saeedi M, Habibi E, Salehifar E, Mosazadeh M, et al. Nigella sativa L. for prevention of acute radiation dermatitis in breast cancer: a randomized, double-blind, placebo-controlled, clinical trial. Complement Ther Med. 2019. https://doi.org/10.1016/j.ctim.2019.102205.
Nasiri N, Ilaghi Nezhad M, Sharififar F, Khazaneha M, Najafzadeh MJ, Mohamadi N. The therapeutic effects of nigella sativa on skin disease: A systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2022. https://doi.org/10.1155/2022/7993579.
Majdalawieh AF, Fayyad MW, Nasrallah GK. Anti-cancer properties and mechanisms of action of thymoquinone, the major active ingredient of Nigella sativa. Crit Rev Food Sci Nutr. 2017;57(18):3911–28. https://doi.org/10.1080/10408398.2016.1277971.
Hossen MJ, Yang WS, Kim D, Aravinthan A, Kim JH, Cho JY. Thymoquinone: An IRAK1 inhibitor with in vivo and in vitro anti-inflammatory activities. Sci Rep. 2017. https://doi.org/10.1038/srep42995.
Talebi M, Farkhondeh T, Samarghandian S. Biological and therapeutic activities of thymoquinone: Focus on the Nrf2 signaling pathway. Phytother Res. 2021;35(4):1739–53. https://doi.org/10.1002/ptr.6905.
Memarzia A, Khazdair MR, Behrouz S, Gholamnezhad Z, Jafarnezhad M, Saadat S, et al. Experimental and clinical reports on anti-inflammatory, antioxidant, and immunomodulatory effects of Curcuma longa and curcumin, an updated and comprehensive review. BioFactors. 2021;47(3):311–50. https://doi.org/10.1002/biof.1716.
Palatty PL, Azmidah A, Rao S, Jayachander D, Thilakchand KR, Rai MP, et al. Topical application of a sandal wood oil and turmeric based cream prevents radiodermatitis in head and neck cancer patients undergoing external beam radiotherapy: a pilot study. Br J Radiol. 2014;87(1038):20130490. https://doi.org/10.1259/bjr.20130490.
Ryan JL, Heckler CE, Ling M, Katz A, Williams JP, Pentland AP, et al. Curcumin for radiation dermatitis: a randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat Res. 2013;180(1):34–43. https://doi.org/10.1667/RR3255.1.
Ryan Wolf J, Gewandter JS, Bautista J, Heckler CE, Strasser J, Dyk P, et al. Utility of topical agents for radiation dermatitis and pain: a randomized clinical trial. Support Care Cancer. 2020;28(7):3303–11. https://doi.org/10.1007/s00520-019-05166-5.
Akbari S, Kariznavi E, Jannati M, Elyasi S, Tayarani-Najaran Z. Curcumin as a preventive or therapeutic measure for chemotherapy and radiotherapy induced adverse reaction: a comprehensive review. Food Chem Toxicol. 2020. https://doi.org/10.1016/j.fct.2020.111699.
Derosa G, Maffioli P, Simental-Mendia LE, Bo S, Sahebkar A. Effect of curcumin on circulating interleukin-6 concentrations: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2016. https://doi.org/10.1016/j.phrs.2016.07.004.
Han S, Xu J, Guo X, Huang M. Curcumin ameliorates severe influenza pneumonia via attenuating lung injury and regulating macrophage cytokines production. Clin Exp Pharmacol Physiol. 2018;45(1):84–93. https://doi.org/10.1111/1440-1681.12848.
Kao YS, Ma KS, Wu MY, Wu YC, Tu YK, Hung CH. Topical prevention of radiation dermatitis in head and neck cancer patients: a network meta-analysis. In Vivo. 2022. https://doi.org/10.21873/invivo.12851.
Cui Z, Xin M, Yin H, Zhang J, Han F. Topical use of olive oil preparation to prevent radiodermatitis: results of a prospective study in nasopharyngeal carcinoma patients. Int J Clin Exp Med. 2015;8(7):11000–6.
Chitapanarux I, Tovanabutra N, Chiewchanvit S, Sripan P, Chumachote A, Nobnop W, et al. Emulsion of olive oil and calcium hydroxide for the prevention of radiation dermatitis in hypofractionation post-mastectomy padiotherapy: a randomized controlled trial. Breast Care (Basel). 2019;14(6):394–400. https://doi.org/10.1159/000496062.
Robijns J, Becherini C, Caini S, Wolf JR, van den Hurk C, Beveridge M, et al. Natural and miscellaneous agents for the prevention of acute radiation dermatitis: a systematic review and meta-analysis. Support Care Cancer. 2023;31(3):195. https://doi.org/10.1007/s00520-023-07656-z.
Hendawy OM, Al-Sanea MM, Elbargisy RM, Rahman HU, Mohamed AAB, Kamal I, et al. Phenylboronic acid-grafted chitosan nanocapsules for effective delivery and controllable release of natural antioxidants: Olive oil and hydroxytyrosol. Pharmaceutics. 2022. https://doi.org/10.3390/pharmaceutics15010081.
Thanthong S, Nanthong R, Kongwattanakul S, Laebua K, Trirussapanich P, Pitiporn S, et al. Prophylaxis of radiation-induced dermatitis in patients with breast cancer using herbal creams: a prospective randomized controlled trial. Integr Cancer Ther. 2020. https://doi.org/10.1177/1534735420920714.
Mukherjee PK, Nema NK, Maity N, Sarkar BK. Phytochemical and therapeutic potential of cucumber. Fitoterapia. 2013. https://doi.org/10.1016/j.fitote.2012.10.003.
Vats M, Bhardwaj S, Chhabra A. Green synthesis of copper oxide nanoparticles using Cucumis sativus (cucumber) extracts and their bio-physical and biochemical characterization for cosmetic and dermatologic applications. Endocr Metab Immune Disord Drug Targets. 2021;21(4):726–33. https://doi.org/10.2174/1871530320666200705212107.
Charalambous M, Raftopoulos V, Paikousis L, Katodritis N, Lambrinou E, Vomvas D, et al. The effect of the use of thyme honey in minimizing radiation - induced oral mucositis in head and neck cancer patients: A randomized controlled trial. Eur J Oncol Nurs. 2018. https://doi.org/10.1016/j.ejon.2018.04.003.
Yang C, Gong G, Jin E, Han X, Zhuo Y, Yang S, et al. Topical application of honey in the management of chemo/radiotherapy-induced oral mucositis: A systematic review and network meta-analysis. Int J Nurs Stud. 2019. https://doi.org/10.1016/j.ijnurstu.2018.08.007.
Rao S, Hegde SK, Rao P, Dinkar C, Thilakchand KR, George T, et al. Honey mitigates radiation-induced oral mucositis in head and neck cancer patients without affecting the tumor response. Foods. 2017;6(9):77. https://doi.org/10.3390/foods6090077.
Hawley P, Hovan A, McGahan CE, Saunders D. A randomized placebo-controlled trial of manuka honey for radiation-induced oral mucositis. Support Care Cancer. 2014;22(3):751–61. https://doi.org/10.1007/s00520-013-2031-0.
Samarghandian S, Farkhondeh T, Samini F. Honey and health: a review of recent clinical research. Pharmacognosy Res. 2017;9(2):121–7. https://doi.org/10.4103/0974-8490.204647.
Ahmed S, Sulaiman SA, Baig AA, Ibrahim M, Liaqat S, Fatima S, et al. Honey as a potential natural antioxidant medicine: An insight into its molecular mechanisms of action. Oxid Med Cell Longev. 2018. https://doi.org/10.1155/2018/8367846.
Rekik DM, Ben Khedir S, Daoud A, Ksouda Moalla K, Rebai T, Sahnoun Z. Wound healing effect of lawsonia inermis. Skin Pharmacol Physiol. 2019;32(6):295–306. https://doi.org/10.1159/000501730.
Tungkasamit T, Chakrabandhu S, Samakgarn V, Kunawongkrit N, Jirawatwarakul N, Chumachote A, et al. Reduction in severity of radiation-induced dermatitis in head and neck cancer patients treated with topical aloe vera gel: a randomized multicenter double-blind placebo-controlled trial. Eur J Oncol Nurs. 2022. https://doi.org/10.1016/j.ejon.2022.102164.
Karbasizade S, Ghorbani F, Ghasemi Darestani N, Mansouri-Tehrani MM, Kazemi AH. Comparison of therapeutic effects of statins and aloe vera mouthwash on chemotherapy induced oral mucositis. Int J Physiol Pathophysiol Pharmacol. 2021;13(4):110–6.
Sahebjamee M, Mansourian A, Hajimirzamohammad M, Zadeh MT, Bekhradi R, Kazemian A, et al. Comparative efficacy of Aloe vera and benzydamine mouthwashes on radiation-induced oral mucositis: a triple-blind, randomised, controlled clinical trial. Oral Health Prev Dent. 2015;13(4):309–15. https://doi.org/10.3290/j.ohpd.a33091.
Wang T, Liao J, Zheng L, Zhou Y, Jin Q, Wu Y. Aloe vera for prevention of radiation-induced dermatitis: A systematic review and cumulative analysis of randomized controlled trials. Front Pharmacol. 2022. https://doi.org/10.3389/fphar.2022.976698.
Sanchez M, Gonzalez-Burgos E, Iglesias I, Gomez-Serranillos MP. Pharmacological update properties of aloe vera and its major active constituents. Molecules. 2020. https://doi.org/10.3390/molecules25061324.
Liu FW, Liu FC, Wang YR, Tsai HI, Yu HP. Aloin protects skin fibroblasts from heat stress-induced oxidative stress damage by regulating the oxidative defense system. PLoS ONE. 2015;10(12): e0143528. https://doi.org/10.1371/journal.pone.0143528.
Bala S, Chugh NA, Bansal SC, Garg ML, Koul A. Radiomodulatory effects of Aloe vera on hepatic and renal tissues of X-ray irradiated mice. Mutat Res. 2018. https://doi.org/10.1016/j.mrfmmm.2018.07.001.
Wahedi HM, Jeong M, Chae JK, Do SG, Yoon H, Kim SY. Aloesin from Aloe vera accelerates skin wound healing by modulating MAPK/Rho and Smad signaling pathways in vitro and in vivo. Phytomedicine. 2017. https://doi.org/10.1016/j.phymed.2017.02.005.
Kong M, Hwang DS, Lee JY, Yoon SW. The efficacy and safety of Jaungo, a traditional medicinal ointment, in preventing radiation dermatitis in patients with breast cancer: a prospective, single-Blinded, randomized pilot study. Evid Based Complement Alternat Med. 2016. https://doi.org/10.1155/2016/9481413.
Park JY, Kwak JH, Kang KS, Jung EB, Lee DS, Lee S, et al. Wound healing effects of deoxyshikonin isolated from Jawoongo: In vitro and in vivo studies. J Ethnopharmacol. 2017. https://doi.org/10.1016/j.jep.2016.10.031.
Kim EH, Kim W. Post treatment application of Jaungo after a combined therapy of carbon dioxide laser and trichloroacetic acid in a case of vulvar syringoma. J Pharmacopuncture. 2019;22(3):200–3. https://doi.org/10.3831/KPI.2019.22.027.
Franco P, Rampino M, Ostellino O, Schena M, Pecorari G, Garzino Demo P, et al. Management of acute skin toxicity with Hypericum perforatum and neem oil during platinum-based concurrent chemo-radiation in head and neck cancer patients. Med Oncol. 2017;34(2):30. https://doi.org/10.1007/s12032-017-0886-5.
Franco P, Potenza I, Moretto F, Segantin M, Grosso M, Lombardo A, et al. Hypericum perforatum and neem oil for the management of acute skin toxicity in head and neck cancer patients undergoing radiation or chemo-radiation: a single-arm prospective observational study. Radiat Oncol. 2014. https://doi.org/10.1186/s13014-014-0297-0.
Anderson PM, Lalla RV. Glutamine for amelioration of radiation and chemotherapy associated mucositis during cancer therapy. Nutrients. 2020. https://doi.org/10.3390/nu12061675.
de Sousa MA, de Lima Dantas JB, Medrado A, Lima HR, Martins GB, Carrera M. Nutritional supplements in the management of oral mucositis in patients with head and neck cancer: narrative literary review. Clin Nutr ESPEN. 2021. https://doi.org/10.1016/j.clnesp.2021.03.030.
Yarom N, Hovan A, Bossi P, Ariyawardana A, Jensen SB, Gobbo M, et al. Correction to: Systematic review of natural and miscellaneous agents, for the management of oral mucositis in cancer patients and clinical practice guidelines–part 1: vitamins, minerals and nutritional supplements. Support Care Cancer. 2021;29(7):4175–6. https://doi.org/10.1007/s00520-021-06141-9.
Konuk Sener D, Aydin M, Cangur S, Guven E. The effect of oral care with chlorhexidine, vitamin E and honey on mucositis in pediatric intensive care patients: A randomized controlled trial. J Pediatr Nurs. 2019. https://doi.org/10.1016/j.pedn.2019.02.001.
Pessoa AF, Florim JC, Rodrigues HG, Andrade-Oliveira V, Teixeira SA, Vitzel KF, et al. Oral administration of antioxidants improves skin wound healing in diabetic mice. Wound Repair Regen. 2016;24(6):981–93. https://doi.org/10.1111/wrr.12486.
Cinausero M, Aprile G, Ermacora P, Basile D, Vitale MG, Fanotto V, et al. New frontiers in the pathobiology and treatment of cancer regimen-related mucosal injury. Front Pharmacol. 2017. https://doi.org/10.3389/fphar.2017.00354.
Higgins MR, Izadi A, Kaviani M. Antioxidants and exercise performance: With a focus on vitamin E and C supplementation. Int J Environ Res Public Health. 2020;17(22):8452. https://doi.org/10.3390/ijerph17228452.
Jacobs C, Hutton B, Ng T, Shorr R, Clemons M. Is there a role for oral or intravenous ascorbate (vitamin C) in treating patients with cancer? A systematic review. Oncologist. 2015;20(2):210–23. https://doi.org/10.1634/theoncologist.2014-0381.
Sio TT, Le-Rademacher JG, Leenstra JL, Loprinzi CL, Rine G, Curtis A, et al. Effect of doxepin mouthwash or diphenhydramine-lidocaine-antacid mouthwash vs placebo on radiotherapy-related oral mucositis pain: The alliance a221304 randomized clinical trial. JAMA. 2019;321(15):1481–90. https://doi.org/10.1001/jama.2019.3504.
Shariati L, Amouheidari A, Naji Esfahani H, Abed A, Haghjooy Javanmard S, Laher I, et al. Protective effects of doxepin cream on radiation dermatitis in breast cancer: A single arm double-blind randomized clinical trial. Br J Clin Pharmacol. 2020;86(9):1875–81. https://doi.org/10.1111/bcp.14238.
Tao F, Zhu J, Duan L, Wu J, Zhang J, Yao K, et al. Anti-inflammatory effects of doxepin hydrochloride against LPS-induced C6-glioma cell inflammatory reaction by PI3K-mediated Akt signaling. J Biochem Mol Toxicol. 2020;34(2): e22424. https://doi.org/10.1002/jbt.22424.
Zabihi M, Hajhashemi V, Minaiyan M, Talebi A. Evaluation of the central and peripheral effects of doxepin on carrageenan-induced inflammatory paw edema in rat. Res Pharm Sci. 2017;12(4):337–45. https://doi.org/10.4103/1735-5362.212052.
Kondziołka J, Wilczyński S. Overview of the activei ingredients in cosmetic products for the care of skin that has been exposed to ionizing radiation - Analysis of their effectiveness in breast cancer radiotherapy. Clin Cosmet Investig Dermatol. 2021. https://doi.org/10.2147/ccid.s322228.
Pinnix C, Perkins GH, Strom EA, Tereffe W, Woodward W, Oh JL, et al. Topical hyaluronic acid vs. standard of care for the prevention of radiation dermatitis after adjuvant radiotherapy for breast cancer: single-blind randomized phase III clinical trial. Int J Radiat Oncol Biol Phys. 2012. https://doi.org/10.1016/j.ijrobp.2011.09.021.
Rahimi A, Mohamad O, Albuquerque K, Kim DWN, Chen D, Thomas K, et al. Novel hyaluronan formulation for preventing acute skin reactions in breast during radiotherapy: a randomized clinical trial. Support Care Cancer. 2020;28(3):1481–9. https://doi.org/10.1007/s00520-019-04957-0.
Fatima S, Hirakawa S, Marta GN, Caini S, Beveridge M, Bonomo P, et al. Topical non-steroidal agents for the prevention of radiation dermatitis: a systematic review and meta-analysis. Support Care Cancer. 2023;31(4):217. https://doi.org/10.1007/s00520-023-07677-8.
Presta G, Puliatti A, Bonetti L, Tolotti A, Sari D, Valcarenghi D. Effectiveness of hyaluronic acid gel (Jalosome soothing gel) for the treatment of radiodermatitis in a patient receiving head and neck radiotherapy associated with cetuximab: a case report and review. Int Wound J. 2019;16(6):1433–9. https://doi.org/10.1111/iwj.13210.
Lee CJ, Fang HF, Wang CY, Chou KR, Huang TW. Effect of hyaluronic acid on radiodermatitis in patients with breast cancer: a meta-analysis of randomized controlled trials. Support Care Cancer. 2022;30(5):3965–75. https://doi.org/10.1007/s00520-022-06828-7.
Juncan AM, Moisa DG, Santini A, Morgovan C, Rus LL, Vonica-Tincu AL, et al. Advantages of hyaluronic acid and its combination with other bioactive ingredients in cosmeceuticals. Molecules. 2021;26(15):4429. https://doi.org/10.3390/molecules26154429.
Ghasemi A, Ghashghai Z, Akbari J, Yazdani-Charati J, Salehifar E, Hosseinimehr SJ. Topical atorvastatin 1% for prevention of skin toxicity in patients receiving radiation therapy for breast cancer: a randomized, double-blind, placebo-controlled trial. Eur J Clin Pharmacol. 2019;75(2):171–8. https://doi.org/10.1007/s00228-018-2570-x.
Zhao H, Zhu W, Zhao X, Li X, Zhou Z, Zheng M, et al. Efficacy of Epigallocatechin-3-Gallate in preventing dermatitis in patients with breast cancer receiving postoperative radiotherapy: a double-blind, placebo-controlled, phase 2 randomized clinical trial. JAMA Dermatol. 2022;158(7):779–86. https://doi.org/10.1001/jamadermatol.2022.1736.
Zhao H, Zhu W, Jia L, Sun X, Chen G, Zhao X, et al. Phase I study of topical epigallocatechin-3-gallate (EGCG) in patients with breast cancer receiving adjuvant radiotherapy. Br J Radiol. 2016;89(1058):20150665. https://doi.org/10.1259/bjr.20150665.
Kim SR, Seong KJ, Kim WJ, Jung JY. Epigallocatechin gallate protects against hypoxia-induced inflammation in microglia via NF-kappaB suppression and Nrf-2/HO-1 activation. Int J Mol Sci. 2022;23(7):4004. https://doi.org/10.3390/ijms23074004.
Zhu W, Xu J, Ge Y, Cao H, Ge X, Luo J, et al. Epigallocatechin-3-gallate (EGCG) protects skin cells from ionizing radiation via heme oxygenase-1 (HO-1) overexpression. J Radiat Res. 2014;55(6):1056–65. https://doi.org/10.1093/jrr/rru047.
Musial C, Kuban-Jankowska A, Gorska-Ponikowska M. Beneficial properties of green tea catechins. Int J Mol Sci. 2020;21(5):1744. https://doi.org/10.3390/ijms21051744.
Yang HL, Lin MW, Korivi M, Wu JJ, Liao CH, Chang CT, et al. Coenzyme Q0 regulates NFkappaB/AP-1 activation and enhances Nrf2 stabilization in attenuation of LPS-induced inflammation and redox imbalance: Evidence from in vitro and in vivo studies. Biochim Biophys Acta. 2016;1859(2):246–61. https://doi.org/10.1016/j.bbagrm.2015.11.001.
Ronchetti D, Impagnatiello F, Guzzetta M, Gasparini L, Borgatti M, Gambari R, et al. Modulation of iNOS expression by a nitric oxide-releasing derivative of the natural antioxidant ferulic acid in activated RAW 264.7 macrophages. Eur J Pharmacol. 2006. https://doi.org/10.1016/j.ejphar.2005.12.034.
Chan RJ, Mann J, Tripcony L, Keller J, Cheuk R, Blades R, et al. Natural oil-based emulsion containing allantoin versus aqueous cream for managing radiation-induced skin reactions in patients with cancer: a phase 3, double-blind, randomized, controlled trial. Int J Radiat Oncol Biol Phys. 2014;90(4):756–64. https://doi.org/10.1016/j.ijrobp.2014.06.034.
Abtahi-Naeini B, Saffaei A, Sabzghabaee AM, Amiri R, Hosseini NS, Niknami E, et al. Topical sucralfate for treatment of mucocutaneous conditions: A systematic review on clinical evidences. Dermatol Ther. 2022;35(4): e15334. https://doi.org/10.1111/dth.15334.
Abbas H, Bensadoun RJ. Trolamine emulsion for the prevention of radiation dermatitis in patients with squamous cell carcinoma of the head and neck. Support Care Cancer. 2012;20(1):185–90. https://doi.org/10.1007/s00520-011-1110-3.
Simões FV, Santos VO, Silva RND, Silva RCD. Effectiveness of skin protectors and calendula officinalis for prevention and treatment of radiodermatitis: an integrative review. Rev Bras Enferm. 2020;73(suppl 5): e20190815. https://doi.org/10.1590/0034-7167-2019-0815.
Krausz AE, Adler BL, Landriscina A, Rosen JM, Musaev T, Nosanchuk JD, et al. Biafine topical emulsion accelerates excisional and burn wound healing in mice. Arch Dermatol Res. 2015;307(7):583–94. https://doi.org/10.1007/s00403-015-1559-x.
Geara FB, Eid T, Zouain N, Thebian R, Andraos T, Chehab C, et al. Randomized, prospective, open-label phase III trial comparing mebo ointment with biafine cream for the management of acute dermatitis during radiotherapy for breast cancer. Am J Clin Oncol. 2018;41(12):1257–62. https://doi.org/10.1097/COC.0000000000000460.
Tam S, Zhou G, Trombetta M, Caini S, Ryan Wolf J, van den Hurk C, et al. Topical corticosteroids for the prevention of severe radiation dermatitis: a systematic review and meta-analysis. Support Care Cancer. 2023;31(7):382. https://doi.org/10.1007/s00520-023-07820-5.
Spada F, Barnes TM, Greive KA. Comparative safety and efficacy of topical mometasone furoate with other topical corticosteroids. Australas J Dermatol. 2018;59(3):e168–74. https://doi.org/10.1111/ajd.12762.
Holmes CJ, Plichta JK, Gamelli RL, Radek KA. Burn injury alters epidermal cholinergic mediators and increases HMGB1 and Caspase 3 in autologous donor skin and burn margin. Shock. 2017;47(2):175–83. https://doi.org/10.1097/SHK.0000000000000752.
Liao Y, Feng G, Dai T, Long F, Tang J, Pu Y, et al. Randomized, self-controlled, prospective assessment of the efficacy of mometasone furoate local application in reducing acute radiation dermatitis in patients with head and neck squamous cell carcinomas. Medicine (Baltimore). 2019;98(52): e18230. https://doi.org/10.1097/MD.0000000000018230.
Behroozian T, Goldshtein D, Ryan Wolf J, van den Hurk C, Finkelstein S, Lam H, et al. MASCC clinical practice guidelines for the prevention and management of acute radiation dermatitis: part 1 systematic review. Clin Med. 2023. https://doi.org/10.1016/j.eclinm.2023.101886.
Erridge SC, McCabe M, Porter MK, Simpson P, Stillie AL. Prospective audit showing improved patient-assessed skin toxicity with use of betamethasone cream for those at high risk of radiation dermatitis. Radiother Oncol. 2016;121(1):143–7. https://doi.org/10.1016/j.radonc.2016.07.005.
Menon A, Prem SS, Kumari R. Topical betamethasone valerate as a prophylactic agent to prevent acute radiation dermatitis in head and neck malignancies: A randomized, open-Label, phase 3 trial. Int J Radiat Oncol Biol Phys. 2021;109(1):151–60. https://doi.org/10.1016/j.ijrobp.2020.08.040.
Uysal B, Gamsiz H, Dincoglan F, Demiral S, Sager O, Dirican B, et al. Comparative evaluation of topical corticosteroid and moisturizer in the prevention of radiodermatitis in breast cancer radiotherapy. Indian J Dermatol. 2020;65(4):279–83. https://doi.org/10.4103/ijd.IJD_607_18.
Acknowledgements
We would like to thank the native English speaking scientists of Elixigen Company (Huntington Beach, California) for editing our manuscript.
Funding
This work was supported by a grant from the Funding for Chinese Medicine Service System and Capacity Building (Key Project with Chinese Medicine Characteristics and Advantages, Ruikang Hospital, 2023).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical approval and informed consent statements
As this article is a review, there is no need for ethical approval and informed consent statements.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guangmei, D., Weishan, H., Wenya, L. et al. Evolution of radiation-induced dermatitis treatment. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03460-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12094-024-03460-1