Abstract
Background
The application of immune checkpoint inhibitors (ICIs) in treating patients with extensive-stage small-cell lung cancer (ES-SCLC) has brought us new hope, but the real-world outcome is relatively lacking. Our aim was to investigate the clinical use, efficacy, and survival benefit of ICIs in ES-SCLC from real-world data analysis.
Methods
A retrospective analysis of ES-SCLC patients was conducted between 2012 and 2022. Progression-free survival (PFS) and overall survival (OS) were assessed between groups to evaluate the value of ICIs at different lines of treatment. PFS1 was defined as the duration from initial therapy to disease progression or death. PFS2 was defined as the duration from the first disease progression to the second disease progression or death.
Results
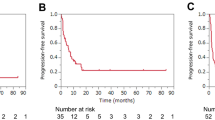
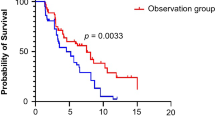
One hundred and eighty patients with ES-SCLC were included. We performed landmark analysis, which showed that compared to the second-line and subsequent-lines ICIs-combined therapy group (2SL-ICIs) and non-ICIs group, the first-line ICIs-combined therapy group (1L-ICIs) prolonged OS and PFS1. There was a trend toward prolonged OS in the 2SL-ICIs group than in the non-ICIs group, but the significance threshold was not met (median OS 11.94 months vs. 11.10 months, P = 0.14). A longer PFS2 was present in the 2SL-ICIs group than in the non-ICIs group (median PFS2 4.13 months vs. 2.60 months, P < 0.001).
Conclusion
First-line ICIs plus chemotherapy should be applied in clinical practice. If patients did not use ICIs plus chemotherapy in first-line therapy, the use of ICIs in the second line or subsequent lines of treatment could prolong PFS2.



Similar content being viewed by others
Data availability
All data and materials are real and available.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Oronsky B, Abrouk N, Caroen S, Lybeck M, Guo X, Wang X, et al. A 2022 update on extensive stage small-cell lung cancer (SCLC). J Cancer. 2022;13(9):2945–53.
Zugazagoitia J, Paz-Ares L. Extensive-stage small-cell lung cancer: first-line and second-line treatment options. J Clin Oncol. 2022;40(6):671–80.
Li L, Liu T, Liu Q, Mu S, Tao H, Yang X, et al. Rechallenge of immunotherapy beyond progression in patients with extensive-stage small-cell lung cancer. Front Pharmacol. 2022;13: 967559.
Ma X, Wang S, Zhang Y, Wei H, Yu J. Efficacy and safety of immune checkpoint inhibitors (ICIs) in extensive-stage small cell lung cancer (SCLC). J Cancer Res Clin Oncol. 2021;147(2):593–606.
Iams WT, Porter J, Horn L. Immunotherapeutic approaches for small-cell lung cancer. Nat Rev Clin Oncol. 2020;17(5):300–12.
Melosky B, Cheema PK, Brade A, McLeod D, Liu G, Price PW, et al. Prolonging survival: the role of immune checkpoint inhibitors in the treatment of extensive-stage small cell lung cancer. Oncologist. 2020;25(11):981–92.
Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–39.
Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–9.
Wang J, Zhou C, Yao W, Wang Q, Min X, Chen G, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(6):739–47.
Cheng Y, Han L, Wu L, Chen J, Sun H, Wen G, et al. Effect of first-line serplulimab vs placebo added to chemotherapy on survival in patients with extensive-stage small cell lung cancer: the ASTRUM-005 randomized clinical trial. JAMA. 2022;328(12):1223–32.
Yang Y, Ai X, Xu H, Yang G, Yang L, Hao X, et al. Treatment patterns and outcomes of immunotherapy in extensive-stage small-cell lung cancer based on real-world practice. Thorac Cancer. 2022;13(23):3295–303.
Pujol JL, Greillier L, Audigier-Valette C, Moro-Sibilot D, Uwer L, Hureaux J, et al. A randomized non-comparative phase II study of anti-programmed cell death-ligand 1 atezolizumab or chemotherapy as second-line therapy in patients with small cell lung cancer: results from the IFCT-1603 trial. J Thorac Oncol. 2019;14(5):903–13.
Spigel DR, Vicente D, Ciuleanu TE, Gettinger S, Peters S, Horn L, et al. Second-line nivolumab in relapsed small-cell lung cancer: CheckMate 331(☆). Ann Oncol. 2021;32(5):631–41.
Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csoszi T, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38(21):2369–79.
Quinn C, Garrison LP, Pownell AK, Atkins MB, de Pouvourville G, Harrington K, et al. Current challenges for assessing the long-term clinical benefit of cancer immunotherapy: a multi-stakeholder perspective. J Immunother Cancer. 2020;8(2):e000648.
Michielin O, Lalani AK, Robert C, Sharma P, Peters S. Defining unique clinical hallmarks for immune checkpoint inhibitor-based therapies. J Immunother Cancer. 2022;10(1):e003024.
Borghaei H, Gettinger S, Vokes EE, Chow LQM, Burgio MA, de Castro CJ, et al. Five-year outcomes from the randomized, phase III trials CheckMate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol. 2021;39(7):723–33.
Motzer RJ, Escudier B, McDermott DF, Arén Frontera O, Melichar B, Powles T, et al. Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J Immunother Cancer. 2020;8(2):e000891
Regan MM, Jegede OA, Mantia CM, Powles T, Werner L, Motzer RJ, et al. Treatment-free survival after immune checkpoint inhibitor therapy versus targeted therapy for advanced renal cell carcinoma: 42-month results of the checkmate 214 trial. Clin Cancer Res. 2021;27(24):6687–95.
Mishra MV, Louie AV, Gondi V, Slotman B. The evolving role of radiotherapy in the management of small cell lung cancer. J Thorac Dis. 2018;10(Suppl 21):S2545–54.
Sun A, Durocher-Allen LD, Ellis PM, Ung YC, Goffin JR, Ramchandar K, Darling G. Guideline for the initial management of small cell lung cancer (limited and extensive stage) and the role of thoracic radiotherapy and first-line chemotherapy. Clin Oncol (R Coll Radiol). 2018;30(10):658–66.
Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer. 2023;22(1):40.
Maddison P, Newsom-Davis J, Mills KR, Souhami RL. Favourable prognosis in Lambert-Eaton myasthenic syndrome and small-cell lung carcinoma. Lancet. 1999;353(9147):117–8.
Reck M, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24(1):75–83.
Wu J, Waxman DJ. Immunogenic chemotherapy: Dose and schedule dependence and combination with immunotherapy. Cancer Lett. 2018;419:210–21.
Slotman BJ, van Tinteren H, Praag JO, Knegjens JL, El Sharouni SY, Hatton M, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet. 2015;385(9962):36–42.
Acknowledgements
The authors also appreciate the Ethics Committee of Shandong Provincial Qianfoshan Hospital, Shandong University (No. 2022-S607).
Funding
This research was funded by Shandong Natural Science Foundation (ZR202108070028 and ZR2022MH103).
Author information
Authors and Affiliations
Contributions
All authors are involved in the creation of manuscripts by making specific contributions.
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts of interest to report.
Ethical approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The retrospective study was approved by the Evaluation Committee of Shandong Provincial Qianfoshan Hospital prior to being performed (No. 2022-S607). Individual consent for this retrospective analysis was waived. Participating hospitals notified and agreed to carry out research.
Consent to publish
Individual consent for this retrospective analysis was waived. Participating hospitals notified and agreed to carry out research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mi, S., Yang, Y., Liu, X. et al. Effect of immune checkpoint inhibitors at different treatment time periods on prognosis of patients with extensive-stage small-cell lung cancer. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03471-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12094-024-03471-y