Abstract
Purpose of Review
Immune-mediated necrotizing myopathy (IMNM), characterized by acute or subacute onset, severe weakness, and elevated creatine kinase levels, poses diagnostic and therapeutic challenges. This article provides a succinct overview of IMNM, including clinical features, diagnostic strategies, and treatment approaches.
Recent Findings
Recent insights highlight the different clinical presentations and therapeutic options of IMNM stratified by autoantibody positivity and type. Additionally, recent findings call into question the reported link between statin use and IMNM.
Summary
This review synthesizes current knowledge on IMNM, emphasizing its distinct clinical features and challenging management. The evolving understanding of IMNM underscores the need for a comprehensive diagnostic approach that utilizes a growing range of modalities. Early and aggressive immunomodulatory therapy remains pivotal. Ongoing research aims to refine diagnostic tools and therapeutic interventions for this challenging muscle disorder, underscoring the importance of advancing our understanding to enhance patient outcomes.

Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bohan A, Peter JB. Polymyositis and dermatomyositis 1. N Engl J Med. 1975;292(7):344–7.
Miller FW, et al. Polymyositis: an overdiagnosed entity. Neurology. 2004;63(2):402–402.
Watanabe Y, et al. Clinical features and prognosis in anti-SRP and anti-HMGCR necrotising myopathy. J Neurol Neurosurg Psychiatry. 2016;87(10):1038–44.
Nakao Y, et al. A Novel antibody which precipitates 7.5s Rna is isolated from a patient with autoimmune-disease. Biochem Biophys Res Commun. 1982;109(4):1332–8.
Allenbach Y, et al. Immune-mediated necrotizing myopathy: clinical features and pathogenesis. Nat Rev Rheumatol. 2020;16(12):689–701.
Miller T, et al. Myopathy with antibodies to the signal recognition particle: clinical and pathological features. J Neurol Neurosurg Psychiatry. 2002;73(4):420–8.
Christopher-Stine L, et al. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum. 2010;62(9):2757–66.
Mammen AL, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63(3):713–21.
Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343(6257):425–30.
Morikawa S, et al. Analysis of the global RNA expression profiles of skeletal muscle cells treated with statins. J Atheroscler Thromb. 2005;12(3):121–31.
Werner JL, et al. Antibody levels correlate with creatine kinase levels and strength in anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Rheum. 2012;64(12):4087–93.
Allenbach Y, et al. Anti-HMGCR autoantibodies in European patients with autoimmune necrotizing myopathies. Medicine. 2014;93(3):150–7.
Benveniste O, et al. Correlation of anti-signal recognition particle autoantibody levels with creatine kinase activity in patients with necrotizing myopathy. Arthritis Rheum. 2011;63(7):1961–71.
Bergua C, et al. In vivo pathogenicity of IgG from patients with anti-SRP or anti-HMGCR autoantibodies in immune-mediated necrotising myopathy. Ann Rheum Dis. 2019;78(1):131–9.
Meyer A, et al. Incidence and prevalence of inflammatory myopathies: a systematic review. Rheumatology (Oxford). 2015;54(1):50–63.
•Shelly S, et al. Incidence and prevalence of immune-mediated necrotizing myopathy in adults in Olmsted County, Minnesota. Muscle Nerve. 2022;65:5, 541–546. This study identifies local prevalance of IMNM.
Kassardjian CD, et al. Clinical features and treatment outcomes of necrotizing autoimmune myopathy. JAMA Neurol. 2015;72(9):996–1003.
Liang WC, et al. Pediatric necrotizing myopathy associated with anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase antibodies. Rheumatology. 2017;56(2):287–93.
Wei J, Ketner E, Mammen AL. Increased risk of statin-associated autoimmune myopathy among American Indians. Arthritis Rheumatol. 2022;74(9):1602–3.
Close RM, et al. Potential implications of six American Indian patients with myopathy, statin exposure and anti-HMGCR antibodies. Rheumatology. 2021;60(2):692–8.
Pinal-Fernandez I, et al. Longitudinal course of disease in a large cohort of myositis patients with autoantibodies recognizing the signal recognition particle. Arthritis Care Res. 2017;69(2):263–70.
Pinal-Fernandez I, et al. Longitudinal course of disease in a large cohort of myositis patients with autoantibodies recognizing the signal recognition particle. Arthritis Care Res (Hoboken). 2017;69(2):263–70.
Mohassel P, et al. Anti-HMGCR myopathy may resemble limb-girdle muscular dystrophy. Neurol Neuroimmunol Neuroinflamm. 2019;6(1):e523.
•Hiebeler, M., et al., Slowly progressive limb-girdle weakness and hyperckemia - limb girdle muscular dystrophy or anti-3-hydroxy-3-methylglutaryl-CoA-reductase-myopathy? J Neuromuscul Dis. 2022;9:(5):607–614. This case report highlights diagnostic challenges in differentiating LGMD from slowly progressing forms of anti-HMGCR IMNM.
Suzuki S, Nishikawa A, Kuwana M, Nishimura H, Watanabe Y, Nakahara J, Hayashi YK, Suzuki N, Nishino I. Inflammatory myopathy with anti-signal recognition particle antibodies: case series of 100 patients. Orphanet J Rare Dis. 2015;10:61. https://doi.org/10.1186/s13023-015-0277-y.
Trallero-Araguas E, et al. Usefulness of anti-p155 autoantibody for diagnosing cancer-associated dermatomyositis: a systematic review and meta-analysis. Arthritis Rheum. 2012;64(2):523–32.
Fiorentino DF, et al. Most patients with cancer-associated dermatomyositis have antibodies to nuclear matrix protein NXP-2 or transcription intermediary factor 1gamma. Arthritis Rheum. 2013;65(11):2954–62.
••Allenbach Y, et al. 224th ENMC International Workshop:: clinico-sero-pathological classification of immune-mediated necrotizing myopathies Zandvoort, The Netherlands, 14–16 October 2016. Neuromuscul Disord. 2018. 28(1):87–99. International panel which provided consensus characterization of IMNM clinicopathological, serological and therapeutic findings.
Allenbach Y, et al. High risk of cancer in autoimmune necrotizing myopathies: usefulness of myositis specific antibody. Brain. 2016;139(Pt 8):2131–5.
Tiniakou E, Mammen AL. Idiopathic inflammatory myopathies and malignancy: a comprehensive review. Clin Rev Allergy Immunol. 2017;52(1):20–33.
Kadoya M, et al. Cancer association as a risk factor for anti-HMGCR antibody-positive myopathy. Neurol Neuroimmunol Neuroinflamm. 2016;3(6):e290.
•Shelly S, et al. Cancer and immune-mediated necrotizing myopathy: a longitudinal referral case-controlled outcomes evaluation. Rheumatology (Oxford). 2022;62:(1):281–289. This study of malignancy in IMNM with 5 year follow up did not find increased incidence compared to age matched controls.
•Mecoli CA, et al. Subsets of idiopathic inflammatory myositis enriched for contemporaneous cancer relative to the general population. Arthritis Rheumatol. 2023;75:(4):620–629. Contrary to the prior reference, this study with similar follow up showed an increased incidence of malignancy in IMNM
Manousakis G. Inflammatory myopathies. Continuum (Minneap Minn). 2022;28(6):1643–62.
Pinal-Fernandez I, Casal-Dominguez M, Mammen AL. Immune-mediated necrotizing myopathy. Curr Rheumatol Rep. 2018;20(4):21.
Ganga HV, Slim HB, Thompson PD. A systematic review of statin-induced muscle problems in clinical trials. Am Heart J. 2014;168(1):6–15.
Cholesterol Treatment Trialists, C. Effect of statin therapy on muscle symptoms: an individual participant data meta-analysis of large-scale, randomised, double-blind trials. Lancet. 2022;400(10355):832–45.
Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97(8A):52C-60C.
Mammen AL. Statin-associated autoimmune myopathy. N Engl J Med. 2016;374(7):664–9.
Vladutiu GD, Isackson PJ. SLCO1B1 variants and statin-induced myopathy. N Engl J Med. 2009;360(3):304.
Grable-Esposito P, et al. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve. 2010;41(2):185–90.
Caughey GE, et al. Association of statin exposure with histologically confirmed idiopathic inflammatory myositis in an australian population. JAMA Intern Med. 2018;178(9):1224–9.
Mohassel P, Mammen AL. Anti-HMGCR myopathy. J Neuromuscul Dis. 2018;5(1):11–20.
Alshehri A, et al. Myopathy with anti-HMGCR antibodies: perimysium and myofiber pathology. Neurol Neuroimmunol Neuroinflamm. 2015;2(4):e124.
Dalakas MC. Inflammatory myopathies: update on diagnosis, pathogenesis and therapies, and COVID-19-related implications. Acta Myol. 2020;39(4):289–301.
Triplett JD, et al. Anti-SRP associated necrotizing autoimmune myopathy presenting with asymptomatically elevated creatine kinase. Muscle Nerve. 2019;59(3):E17–9.
•Chow KL, et al. HMGCR autoantibody testing: two tiers required. Pathology. 2022;54:(1):129–131. Characterization of Muscle MRI findings during the clinical and treatment course of IMNM.
Pinal-Fernandez I, et al. Thigh muscle MRI in immune-mediated necrotising myopathy: extensive oedema, early muscle damage and role of anti-SRP autoantibodies as a marker of severity. Ann Rheum Dis. 2017;76(4):681–7.
Landon-Cardinal O, et al. Severe axial and pelvifemoral muscle damage in immune-mediated necrotizing myopathy evaluated by whole-body MRI. Neuromuscul Disord. 2019;29:S43–S43.
Fionda L, et al. Muscle MRI in immune-mediated necrotizing myopathy (IMNM): implications for clinical management and treatment strategies. J Neurol. 2023;270(2):960–74.
Oh EK, et al. Clinical and radiological features of korean patients with anti-HMGCR myopathy. J Clin Neurol. 2023;19(5):460–8.
Sener U, et al. Needle electromyography and histopathologic correlation in myopathies. Muscle Nerve. 2019;59(3):315–20.
Paganoni S, Amato A. Electrodiagnostic evaluation of myopathies. Phys Med Rehabil Clin N Am. 2013;24(1):193–207.
Joyce NC, Oskarsson B, Jin LW. Muscle biopsy evaluation in neuromuscular disorders. Phys Med Rehabil Clin N Am. 2012;23(3):609–31.
Allenbach Y, et al. Necrosis in anti-SRP(+) and anti-HMGCR(+)myopathies: role of autoantibodies and complement. Neurology. 2018;90(6):e507–17.
Chung T, et al. The composition of cellular infiltrates in anti-HMG-CoA reductase-associated myopathy. Muscle Nerve. 2015;52(2):189–95.
de Souza JM, Hoff LS, Shinjo SK. Intravenous human immunoglobulin and/or methylprednisolone pulse therapies as a possible treat-to-target strategy in immune-mediated necrotizing myopathies. Rheumatol Int. 2019;39(7):1201–12.
Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med. 2015;373(17):1680–2.
Lim J, et al. Intravenous immunoglobulins as first-line treatment in idiopathic inflammatory myopathies: a pilot study. Rheumatology (Oxford). 2021;60(4):1784–92.
Garcia-Rosell M, et al. Signal recognition antibody-positive myopathy and response to intravenous immunoglobulin G (IVIG). J Clin Rheumatol. 2013;19(4):214–7.
Valiyil R, et al. Rituximab therapy for myopathy associated with anti-signal recognition particle antibodies: a case series. Arthritis Care Res (Hoboken). 2010;62(9):1328–34.
Landon-Cardinal O, et al. Rituximab in the treatment of refractory anti-HMGCR immune-mediated necrotizing myopathy. J Rheumatol. 2019;46(6):623–7.
Ramanathan S, et al. Clinical course and treatment of anti-HMGCR antibody-associated necrotizing autoimmune myopathy. Neurol Neuroimmunol Neuroinflamm. 2015;2(3):e96.
Xiong A, et al. Rituximab in the treatment of immune-mediated necrotizing myopathy: a review of case reports and case series. Ther Adv Neurol Disord. 2021;14:1756286421998918.
Tiniakou E, et al. Use of proprotein convertase subtilisin/kexin type 9 inhibitors in statin-associated immune-mediated necrotizing myopathy: a case series. Arthritis Rheumatol. 2019;71(10):1723–6.
Zhang H, et al. Plasma exchange therapy in refractory inflammatory myopathy with anti-signal recognition particle antibody: a case series. Rheumatology (Oxford). 2022;61(6):2625–30.
Touat M, et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology. 2018;91(10):e985–94.
Kostine M, et al. EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Ann Rheum Dis. 2021;80(1):36–48.
Tiniakou E, et al. More severe disease and slower recovery in younger patients with anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Rheumatology (Oxford). 2017;56(5):787–94.
••Wang JX, et al. Outcome predictors of immune-mediated necrotizing myopathy-a retrospective, multicentre study. Rheumatology (Oxford). 2022;61:(9):3824–3829. This study described outcomes associated with various clinical characteristics and treatment approaches in IMNM.
Knauss S, et al. PD1 pathway in immune-mediated myopathies: pathogenesis of dysfunctional T cells revisited. Neurol Neuroimmunol Neuroinflamm. 2019;6(3):e558.
••Mammen AL, et al. Zilucoplan in immune-mediated necrotising myopathy: a phase 2, randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2023;5:(2):e67-e76. The first phase 2 clinical trial in patients with IMNM.
Aggarwal R, Lundberg IE, Song YW, et al. POS0839 randomized, double-blind, placebo controlled trial to evaluate efficacy and safety of SC abatacept in adults with active idiopathic inflammatory myopathy. Annals of the Rheumatic Diseases 2022;81:711.
Van Thillo A, et al. Physical therapy in adult inflammatory myopathy patients: a systematic review. Clin Rheumatol. 2019;38(8):2039–51.
Acknowledgements
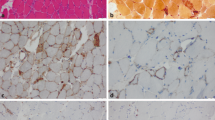
The authors wish to acknowledge Michael L. Miller, MD, PhD for contributing histopathological images and captions.
Author information
Authors and Affiliations
Contributions
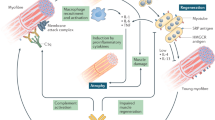
C.K. and F.M. wrote the main manuscript text and table. C.K. prepared the figure.
Corresponding author
Ethics declarations
Competing of Interest
The authors declare no competing interests
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koumas, C., Michelassi, F. Immune-Mediated Necrotizing Myopathies: Current Landscape. Curr Neurol Neurosci Rep 24, 141–150 (2024). https://doi.org/10.1007/s11910-024-01337-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-024-01337-y